Nano Iron Oxide-PCL Composite as an Improved Soft Tissue Scaffold
Abstract
:1. Introduction
2. Results
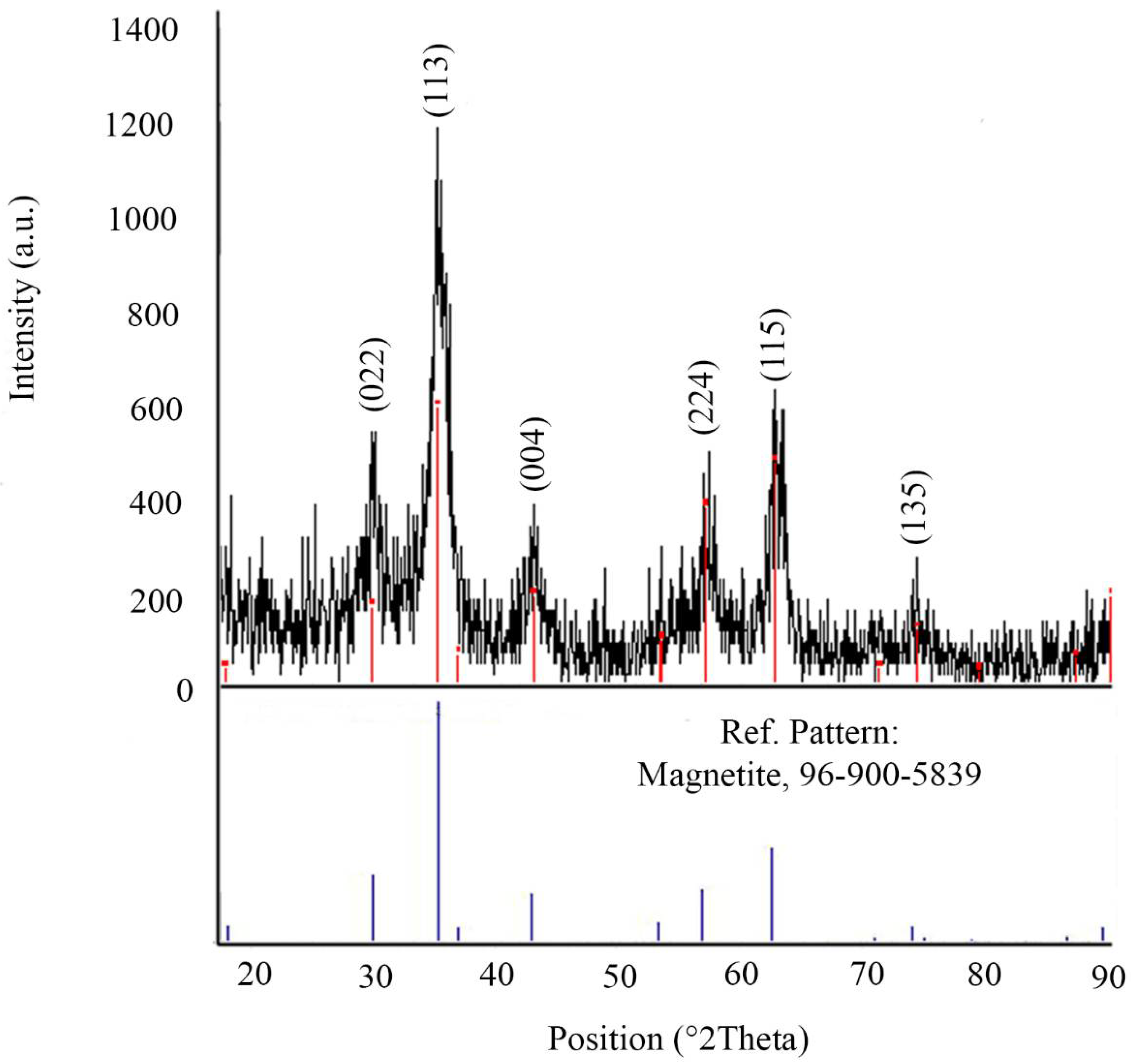
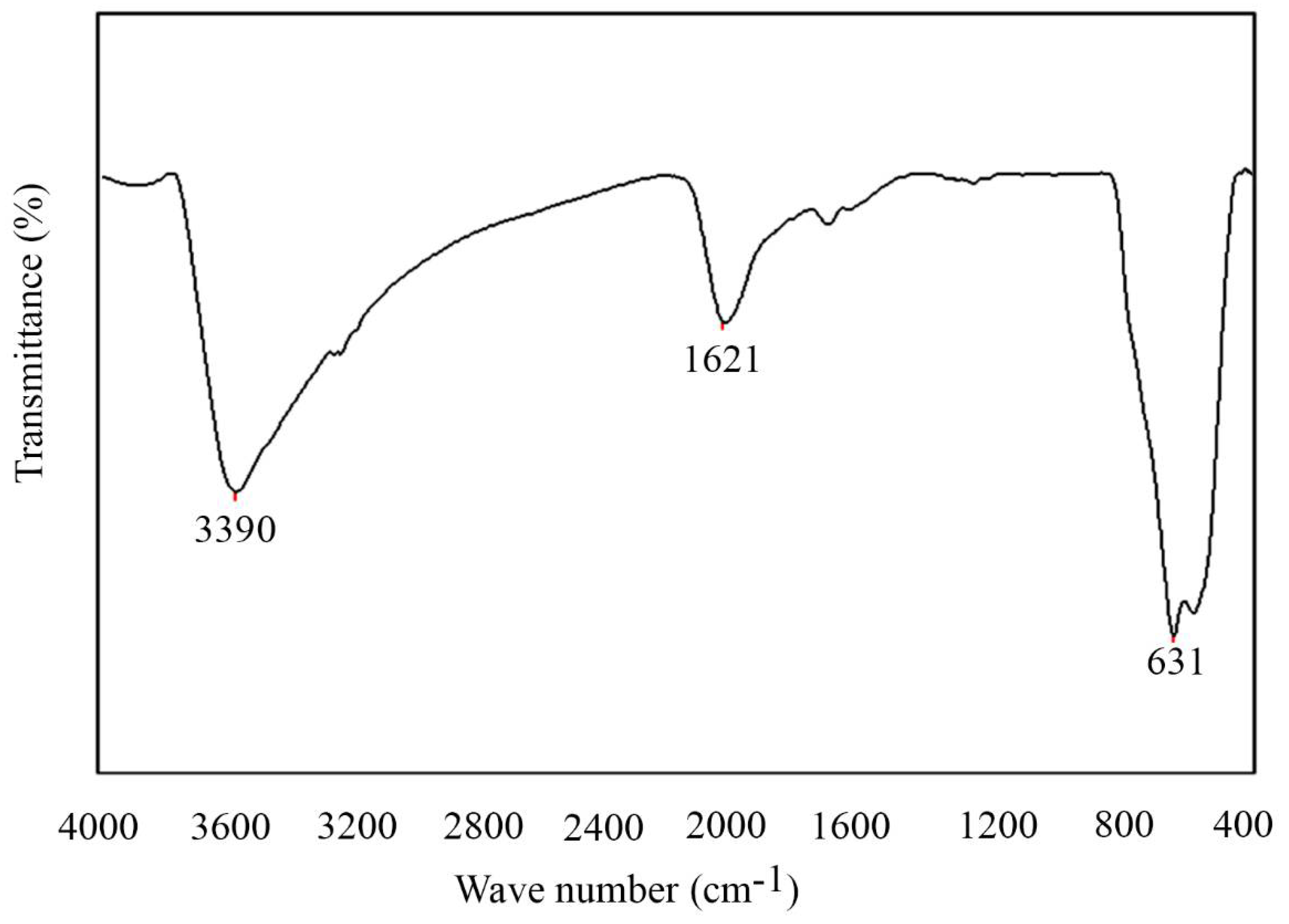
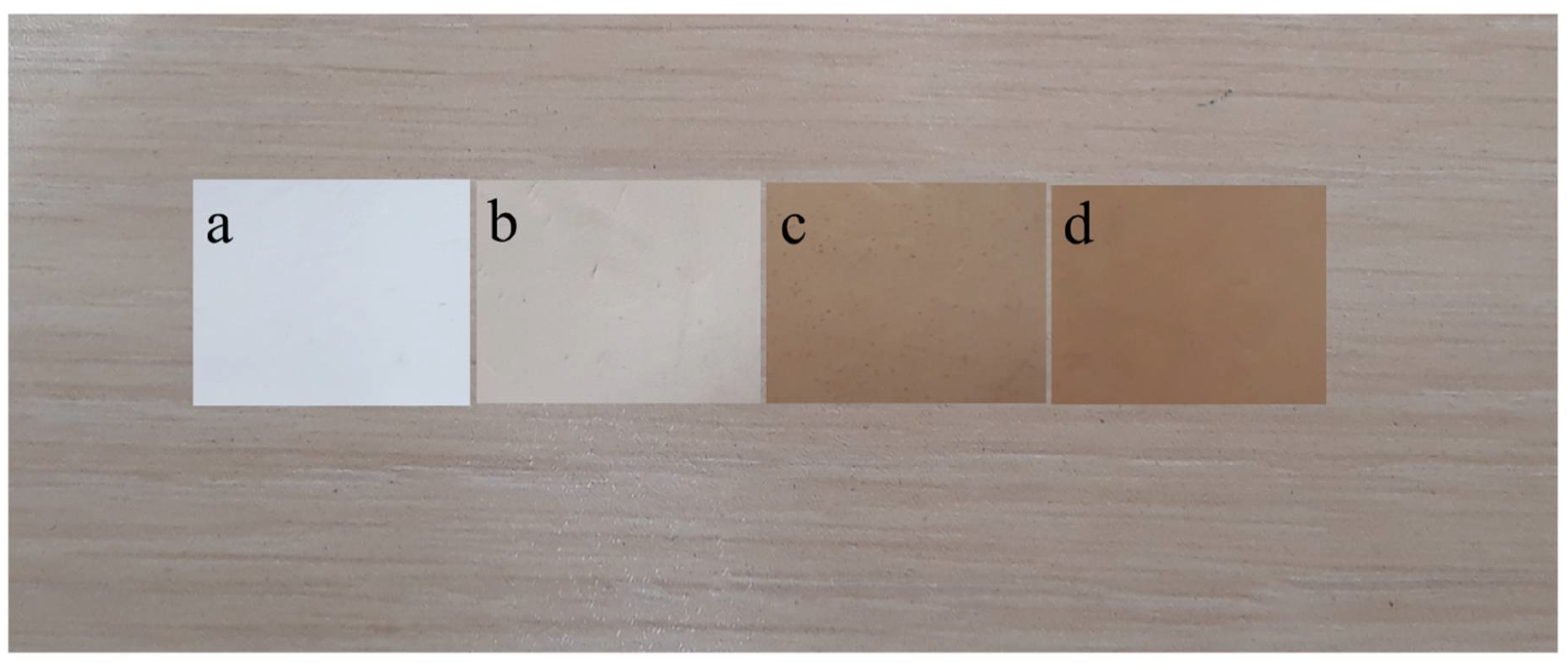
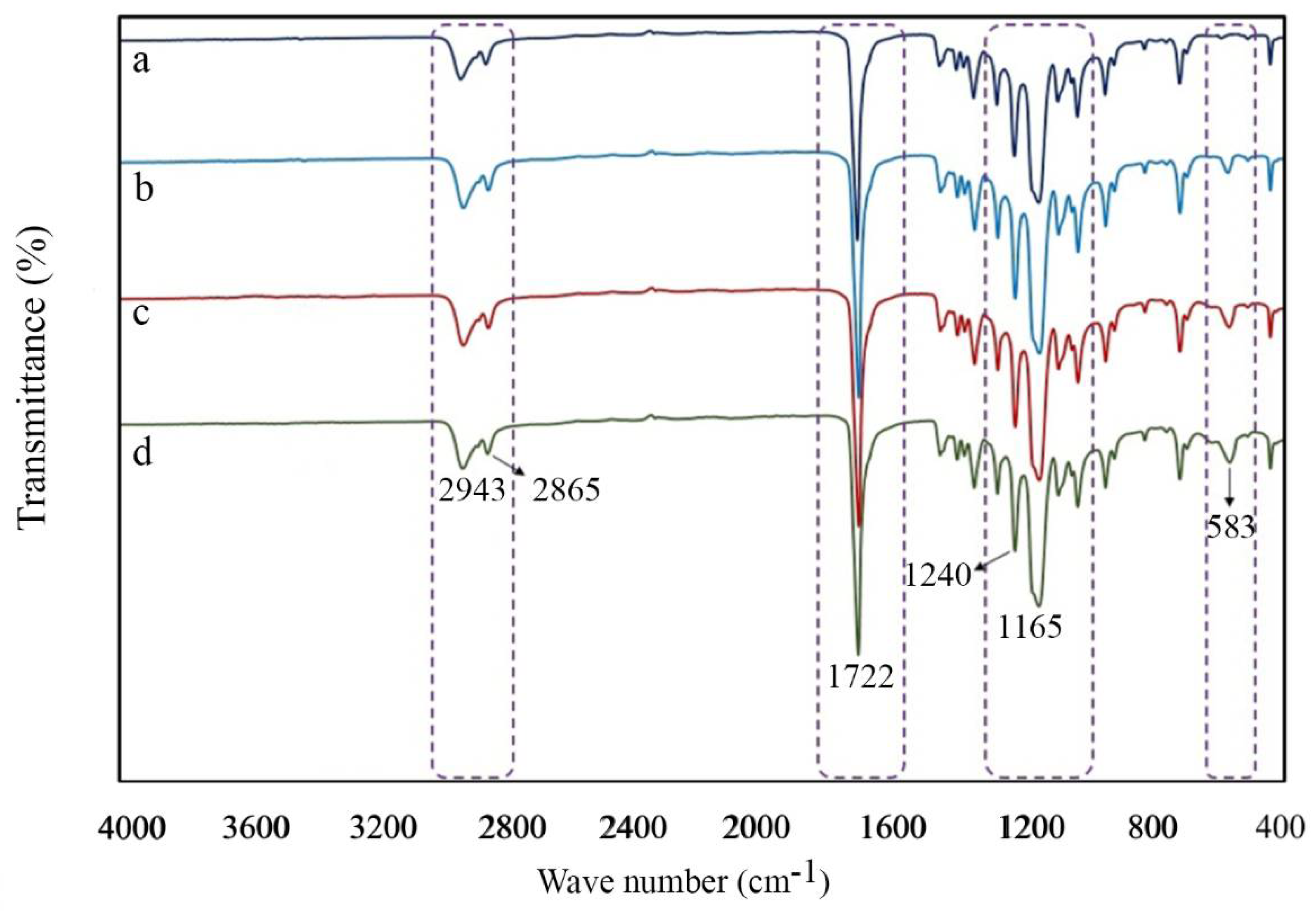
2.1. Characterization of Nanoparticles
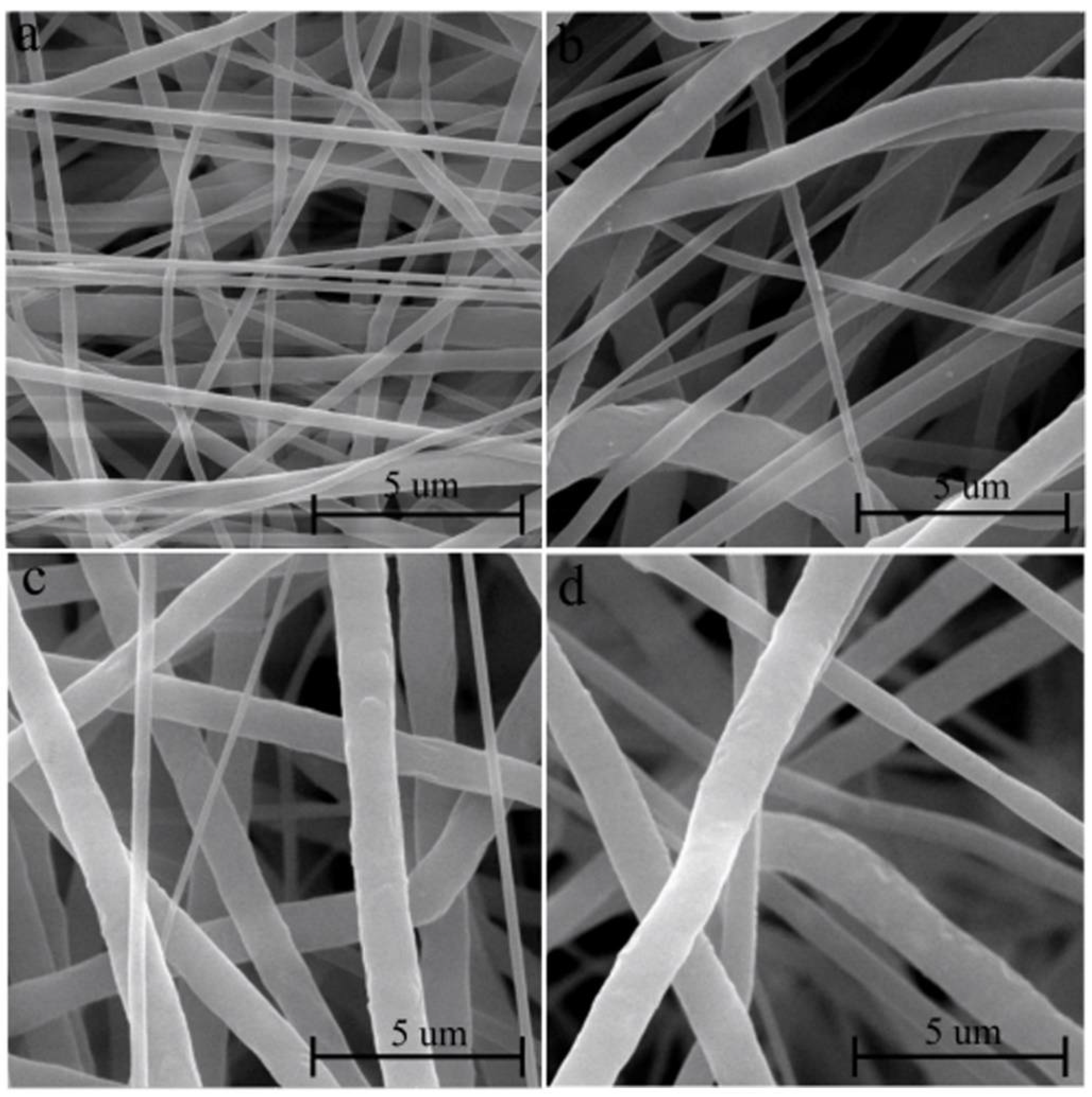
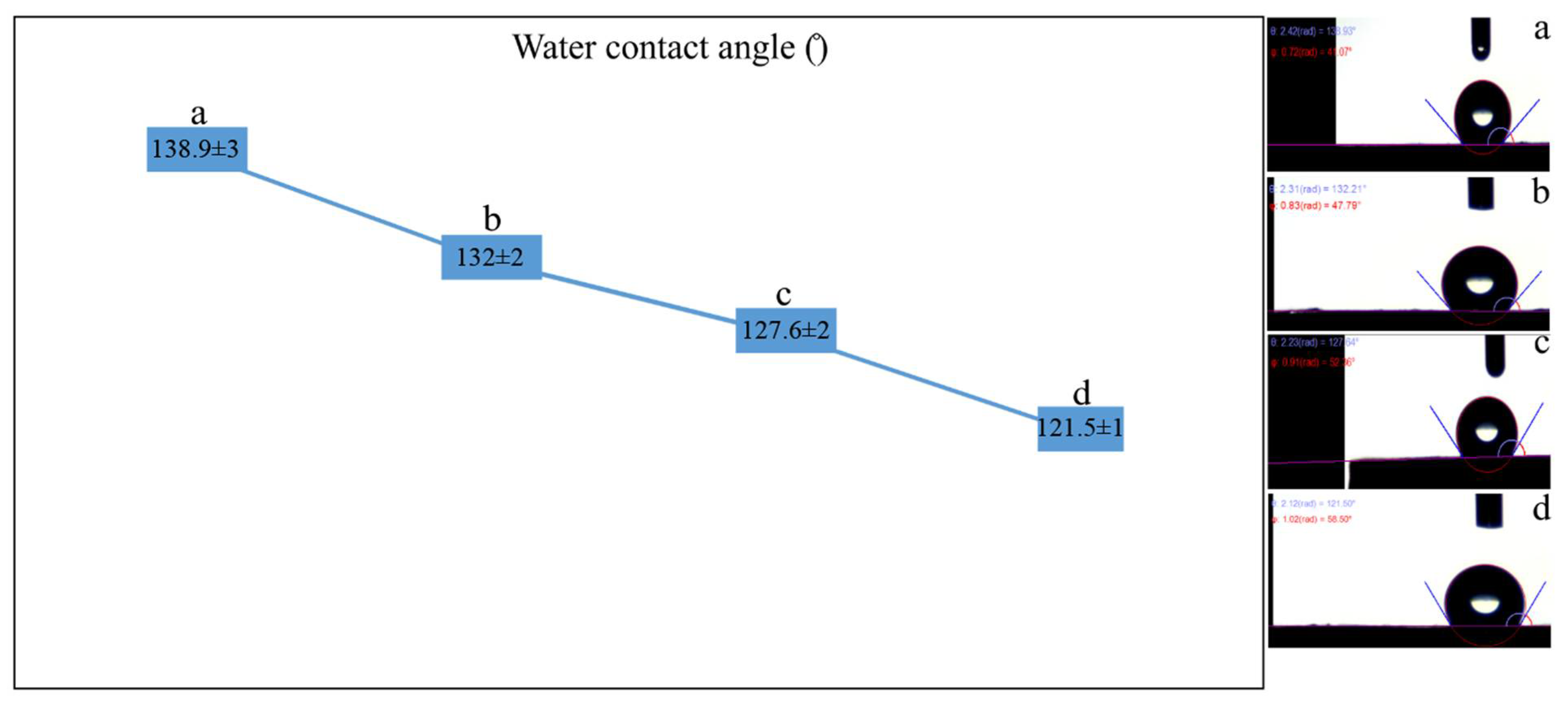
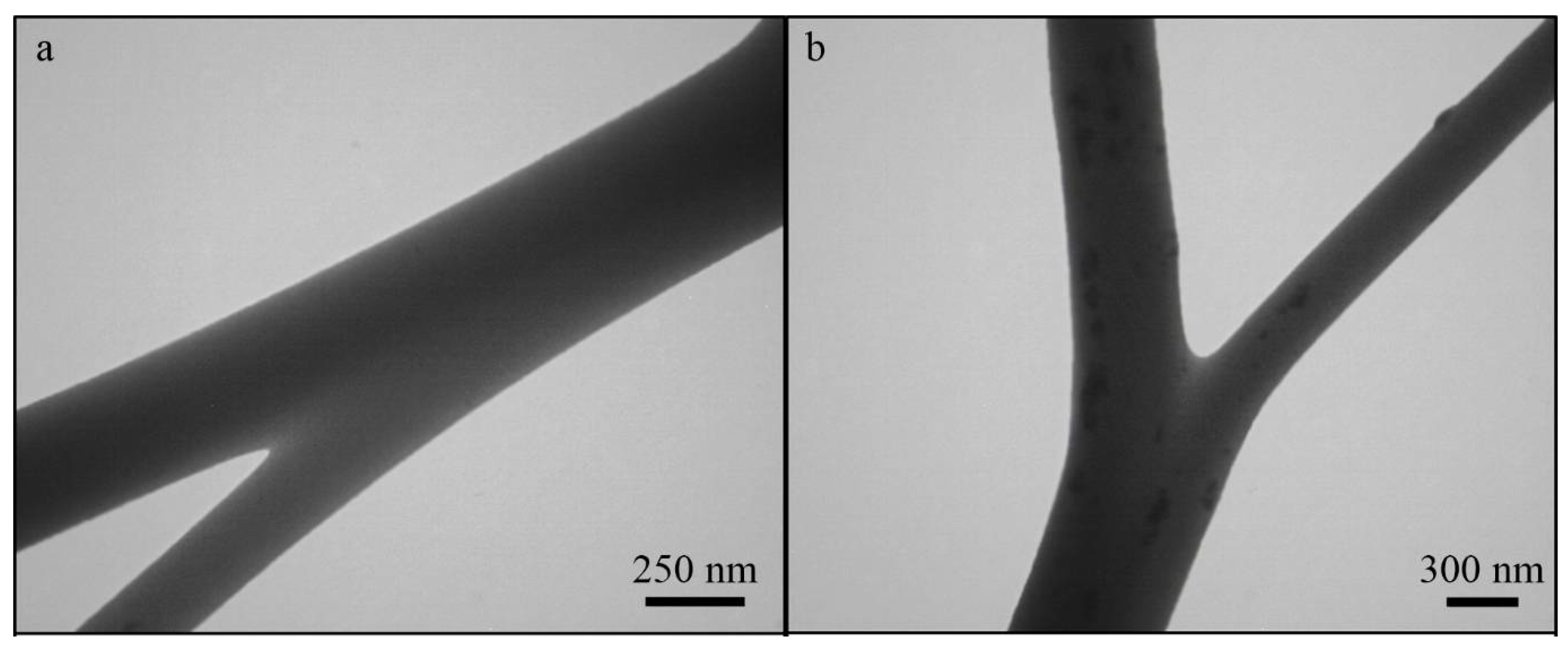
2.2. Characterization of Scaffolds
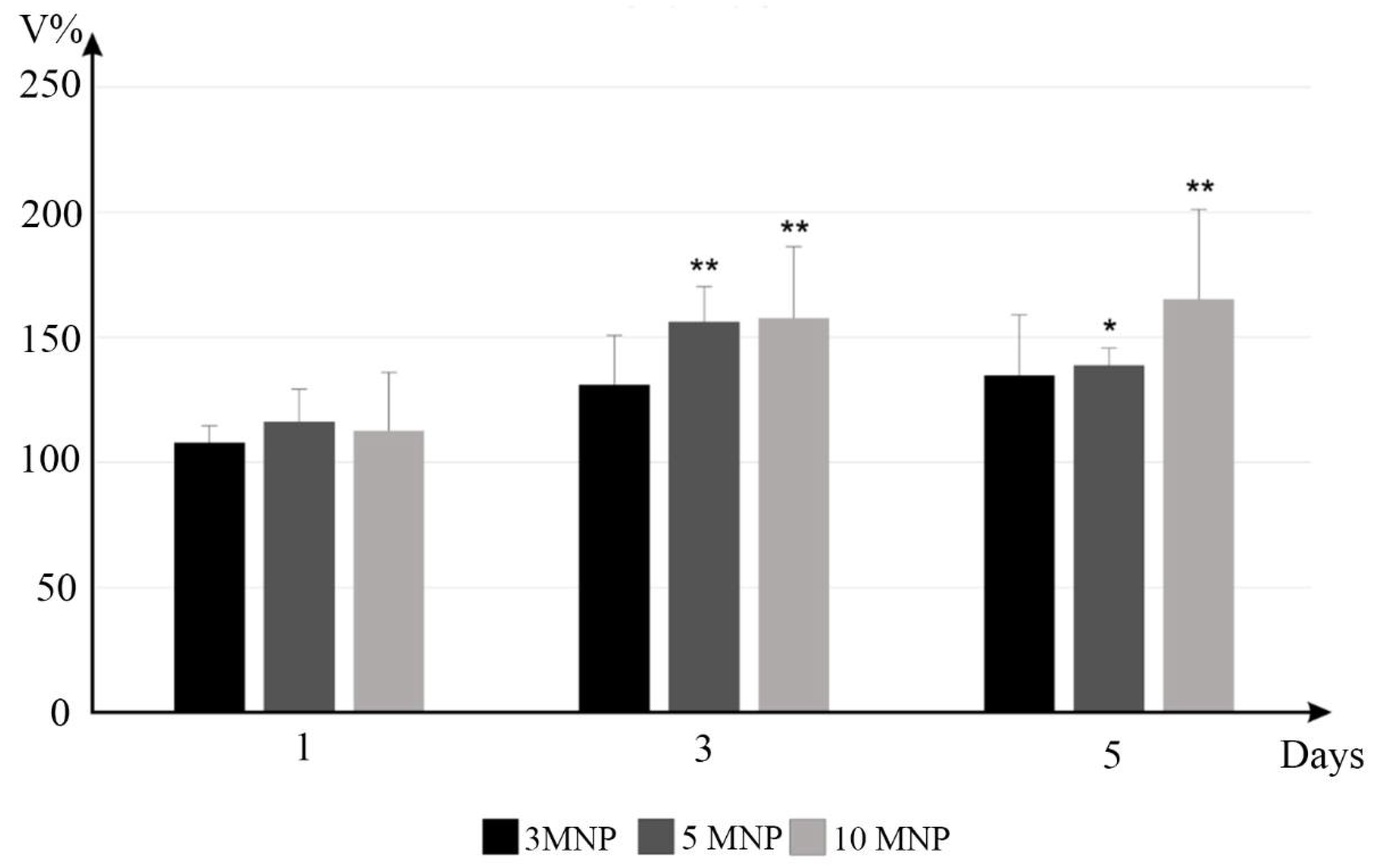
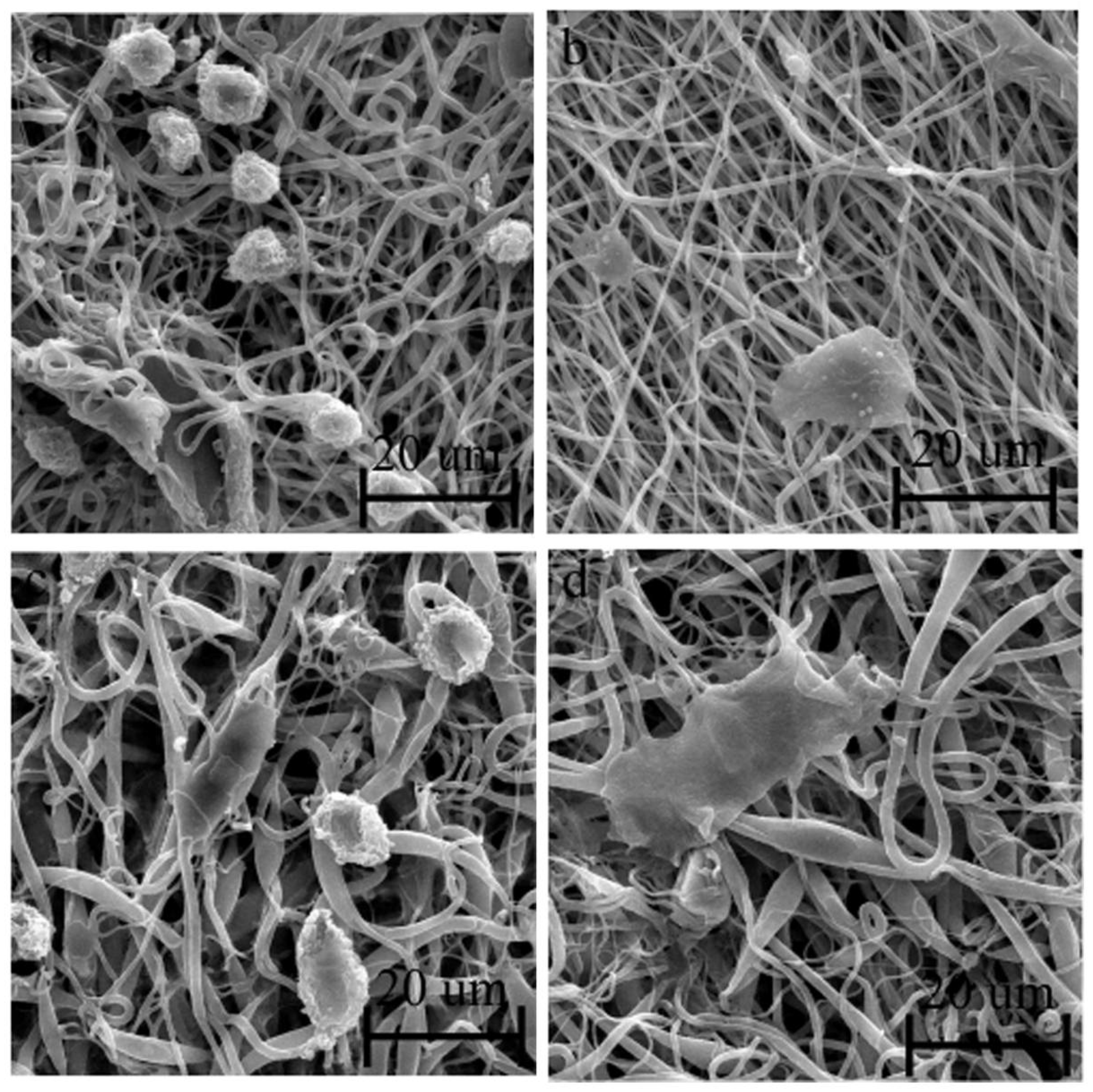
2.3. In Vitro Studies
3. Discussion
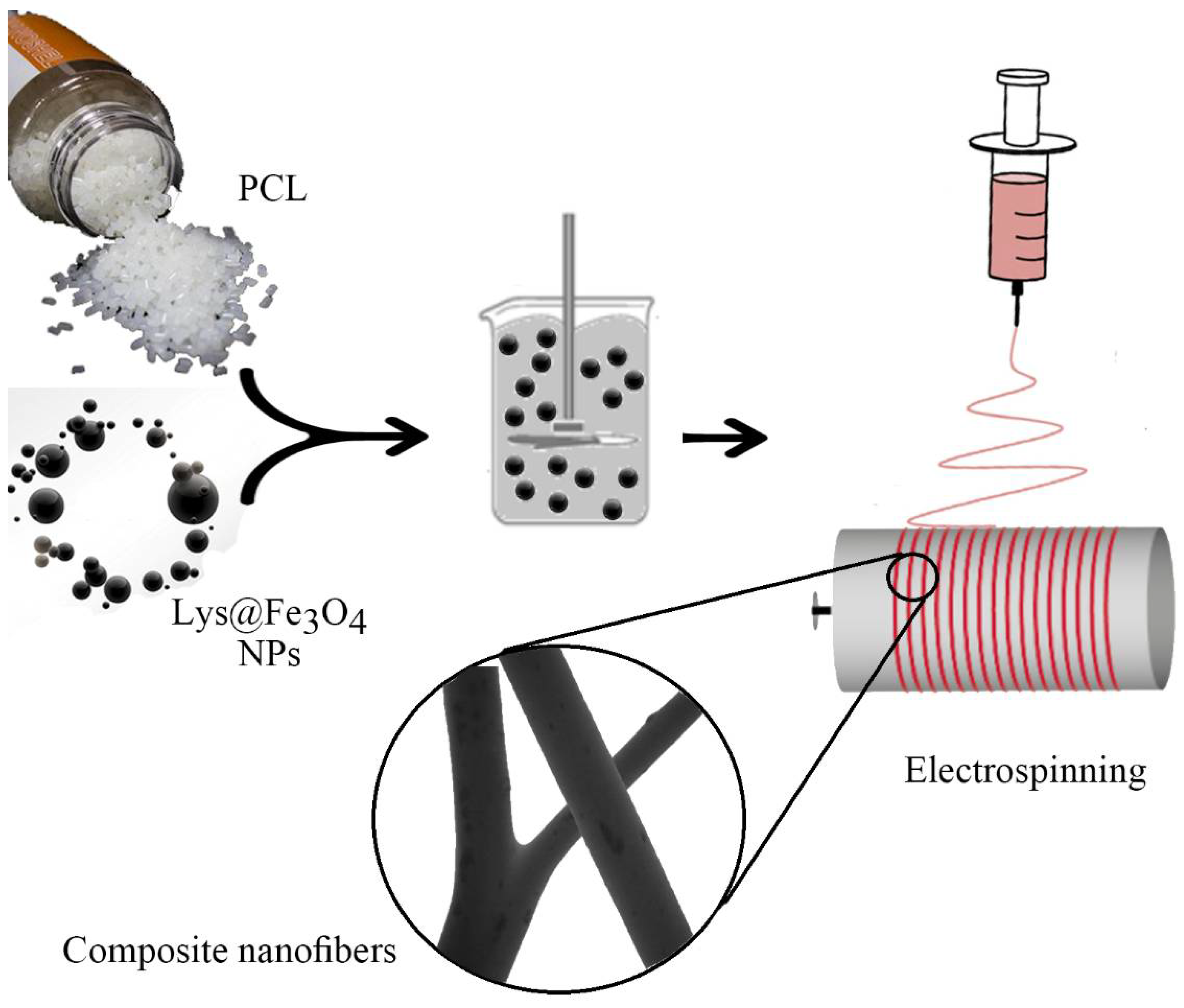
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Lys Coated Magnetite Nanoparticles
4.3. Characterization of Lys Coated Magnetite Nanoparticles
4.4. Preparation of PCL Nanofibers
4.5. Characterization of Fibers
4.6. Cells Cultured and In Vitro Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asvar, Z.; Mirzaei, E.; Azarpira, N.; Geramizadeh, B.; Fadaie, M. Evaluation of electrospinning parameters on the tensile strength and suture retention strength of polycaprolactone nanofibrous scaffolds through surface response methodology. J. Mech. Behav. Biomed. Mater. 2017, 75, 369–378. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef]
- Hammond, J.S.; Beckingham, I.J.; Shakesheff, K.M. Scaffolds for liver tissue engineering. Expert Rev. Med. Devices 2006, 3, 21–27. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Patel, K.D.; Lee, J.H.; Lee, E.J.; Kim, J.H.; Kim, T.H.; Kim, H.W. Potential of magnetic nanofiber scaffolds with mechanical and biological properties applicable for bone regeneration. PLoS ONE 2014, 9, e91584. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, D.; Güreş, D.; Tecim, T.; Genç, R.; Bölgen, N. Magnetic nanoparticle-loaded electrospun poly (ε-caprolactone) nanofibers for drug delivery applications. Appl. Nanosci. 2018, 8, 1461–1469. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Daňková, J.; Buzgo, M.; Vejpravova, J.; Kubíčková, S.; Sovková, V.; Vysloužilová, L.; Mantlíková, A.; Nečas, A.; Amler, E. Highly efficient mesenchymal stem cell proliferation on poly-ε-caprolactone nanofibers with embedded magnetic nanoparticles. Int. J. Nanomed. 2015, 10, 7307–7317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manea, L.; Hristian, L.; Leon, A.; Popa, A. Recent advances of basic materials to obtain electrospun polymeric nanofibers for medical applications. IOP Conf. Ser. Mater. Sci. Eng. 2016, 145, 032006. [Google Scholar] [CrossRef]
- Soares, R.M.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- De Santis, R.; Gloria, A.; Russo, T.; d’Amora, U.; Zeppetelli, S.; Dionigi, C.; Sytcheva, A.; Herrmannsdörfer, T.; Dediu, V.; Ambrosio, L. A basic approach toward the development of nanocomposite magnetic scaffolds for advanced bone tissue engineering. J. Appl. Polym. Sci. 2011, 122, 3599–3605. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, J.; Pang, X.; Zhao, M.; Wang, B.; Yang, L.; Wan, H.; Wu, J.; Fu, S. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C 2017, 73, 537–543. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, B.; Zhang, W.; Yan, C.; Yao, Q.; Shao, C.; Yu, F.; Li, F.; Fu, Y. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp. Ther. Med. 2019, 17, 3717–3726. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Woo, D.G.; Sun, B.K.; Chung, H.M.; Im, S.J.; Choi, Y.M.; Park, K.; Huh, K.M.; Park, K.H. In vitro and in vivo test of PEG/PCL-based hydrogel scaffold for cell delivery application. J. Control. Release 2007, 124, 51–59. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Chen, Y.R.; Zhang, J.Y.; Jiang, D.; Yuan, F.Z.; Mao, Z.M.; Yang, F.; Jiang, W.B.; Wang, X.; Yu, J.K. Facile Strategy on Hydrophilic Modification of Poly(ε-caprolactone) Scaffolds for Assisting Tissue-Engineered Meniscus Constructs In Vitro. Front. Pharmacol. 2020, 11, 471. [Google Scholar] [CrossRef]
- Kim, J.-J.; Singh, R.K.; Seo, S.-J.; Kim, T.-H.; Kim, J.-H.; Lee, E.-J.; Kim, H.-W. Magnetic scaffolds of polycaprolactone with functionalized magnetite nanoparticles: Physicochemical, mechanical, and biological properties effective for bone regeneration. RSC Adv. 2014, 4, 17325–17336. [Google Scholar] [CrossRef]
- Ballesteros, C.A.; Correa, D.S.; Zucolotto, V. Polycaprolactone nanofiber mats decorated with photoresponsive nanogels and silver nanoparticles: Slow release for antibacterial control. Mater. Sci. Eng. C 2020, 107, 110334. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound healing performance of PCL/Chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Bhattarai, D.P.; Park, J.; Park, C.H.; Kim, C.S. Polyaniline-coated titanium oxide nanoparticles and simvastatin-loaded poly (ε-caprolactone) composite nanofibers scaffold for bone tissue regeneration application. Colloids Surf. B 2020, 192, 111007. [Google Scholar] [CrossRef]
- Gounani, Z.; Pourianejad, S.; Asadollahi, M.A.; Meyer, R.L.; Rosenholm, J.M.; Arpanaei, A. Polycaprolactone-gelatin nanofibers incorporated with dual antibiotic-loaded carboxyl-modified silica nanoparticles. J. Mater. Sci. 2020, 55, 17134–17150. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Yousefi, A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B 2014, 102, 1304–1315. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Kouhpayeh, A.; Davaran, S.; Barar, J.; Ghasemi, Y. Impacts of amine functionalized iron oxide nanoparticles on HepG2 cell line. Curr. Nanosci. 2015, 11, 113–119. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Varma, V.; Yang, S.; Ghasemi, Y.; Berenjian, A. Synthesis and application of amine functionalized iron oxide nanoparticles on menaquinone-7 fermentation: A step towards process intensification. Nanomaterials 2015, 6, 1. [Google Scholar] [CrossRef]
- Raee, M.J.; Ebrahiminezhad, A.; Gholami, A.; Ghoshoon, M.B.; Ghasemi, Y. Magnetic immobilization of recombinant E. coli producing extracellular asparaginase: An effective way to intensify downstream process. Sep. Sci. Technol. 2018, 53, 1397–1404. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Impact of amino-acid coating on the synthesis and characteristics of iron-oxide nanoparticles (IONs). Bull. Korean Chem. Soc. 2012, 33, 3957–3962. [Google Scholar] [CrossRef] [Green Version]
- Raee, M.J.; Ebrahiminezhad, A.; Ghoshoon, M.B.; Gholami, A.; Ghasemi, Y. Synthesis and characterization of L-lysin coated iron oxide nanoparticles as appropriate choices for cell immobilization and magnetic separation. Nanosci. Nanotechnology-Asia 2019, 9, 462–466. [Google Scholar] [CrossRef]
- Durmus, Z.; Kavas, H.; Toprak, M.S.; Baykal, A.; Altincekic, T.G.; Aslan, A.; Bozkurt, A.; Cosgun, S. l-lysine coated iron oxide nanoparticles: Synthesis, structural and conductivity characterization. J. Alloy. Compd. 2009, 484, 371–376. [Google Scholar] [CrossRef]
- Patel, D.; Chang, Y.; Lee, G.H. Amino acid functionalized magnetite nanoparticles in saline solution. Curr. Appl. Phys. 2009, 9, S32–S34. [Google Scholar] [CrossRef]
- Bortolassi, A.C.C.; Nagarajan, S.; de Araújo Lima, B.; Guerra, V.G.; Aguiar, M.L.; Huon, V.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Efficient nanoparticles removal and bactericidal action of electrospun nanofibers membranes for air filtration. Mater. Sci. Eng. C 2019, 102, 718–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, Y.; He, X.; Li, H.; Wei, B.; Yang, W. Electrospinning on a plucked string. J. Mater. Sci. 2019, 54, 901–910. [Google Scholar] [CrossRef]
- Petras, D.; Slobodian, P.; Pavlínek, V.; Sáha, P.; Kimmer, D. The effect of pvac solution viscosity on diameter of pvac nanofibres prepared by technology of electrospinning. AIP Conf. Proc. 2011, 1375, 312–319. [Google Scholar]
- Bagheri, M.; Mahmoodzadeh, A. Polycaprolactone/Graphene Nanocomposites: Synthesis, Characterization and Mechanical Properties of Electrospun Nanofibers. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1566–1577. [Google Scholar] [CrossRef]
- Ramírez-Cedillo, E.; Ortega-Lara, W.; Rocha-Pizaña, M.R.; Gutierrez-Uribe, J.A.; Elías-Zúñiga, A.; Rodríguez, C.A. Electrospun Polycaprolactone Fibrous Membranes Containing Ag, TiO₂ and Na₂Ti₆O(13) Particles for Potential Use in Bone Regeneration. Membranes 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-M.; Zhang, Y.; Ramakrishna, S.; Lim, C. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Berenjian, A.; Taghizadeh, S.; Ghasemi, Y.; Taherpour, A.; Sarmah, A.K.; Ebrahiminezhad, A. One-put green synthesis of multifunctional silver iron core-shell nanostructure with antimicrobial and catalytic properties. Ind. Crop. Prod. 2019, 130, 230–236. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, W.; Li, L.; Zheng, Y. Fabrication and characterization of three-dimensional nanofiber membrance of PCL–MWCNTs by electrospinning. Mater. Sci. Eng. C 2010, 30, 1014–1021. [Google Scholar] [CrossRef]
- Daniel, C.; Zhovner, D.; Guerra, G. Thermal stability of nanoporous crystalline and amorphous phases of poly (2, 6-dimethyl-1, 4-phenylene) oxide. Macromolecules 2013, 46, 449–454. [Google Scholar] [CrossRef]
- Lian, H.; Meng, Z. Melt electrospinning vs. solution electrospinning: A comparative study of drug-loaded poly (ε-caprolactone) fibres. Mater. Sci. Eng. C 2017, 74, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.B.; Gardella, L.; Forouharshad, M.; Pellegrino, T.; Monticelli, O. Star poly (ε-caprolactone)-based electrospun fibers as biocompatible scaffold for doxorubicin with prolonged drug release activity. Colloids Surf. B 2018, 161, 488–496. [Google Scholar] [CrossRef]
- Rychter, M.; Baranowska-Korczyc, A.; Milanowski, B.; Jarek, M.; Maciejewska, B.M.; Coy, E.L.; Lulek, J. Cilostazol-loaded poly (ε-Caprolactone) electrospun drug delivery system for cardiovascular applications. Pharm. Res. 2018, 35, 32. [Google Scholar] [CrossRef] [Green Version]
- Diao, H.J.; Low, W.C.; Milbreta, U.; Lu, Q.R.; Chew, S.Y. Nanofiber-mediated microRNA delivery to enhance differentiation and maturation of oligodendroglial precursor cells. J. Control. Release 2015, 208, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Liao, I.-C.; Chen, S.; Liu, J.B.; Leong, K.W. Sustained viral gene delivery through core-shell fibers. J. Control. Release 2009, 139, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, F.; Karumidze, N.; Kusradze, I.; Goderdzishvili, M.; Teixeira, P.; Gouveia, I.C. Immobilization of bacteriophage in wound-dressing nanostructure. Nanomedicine 2017, 13, 2475–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]

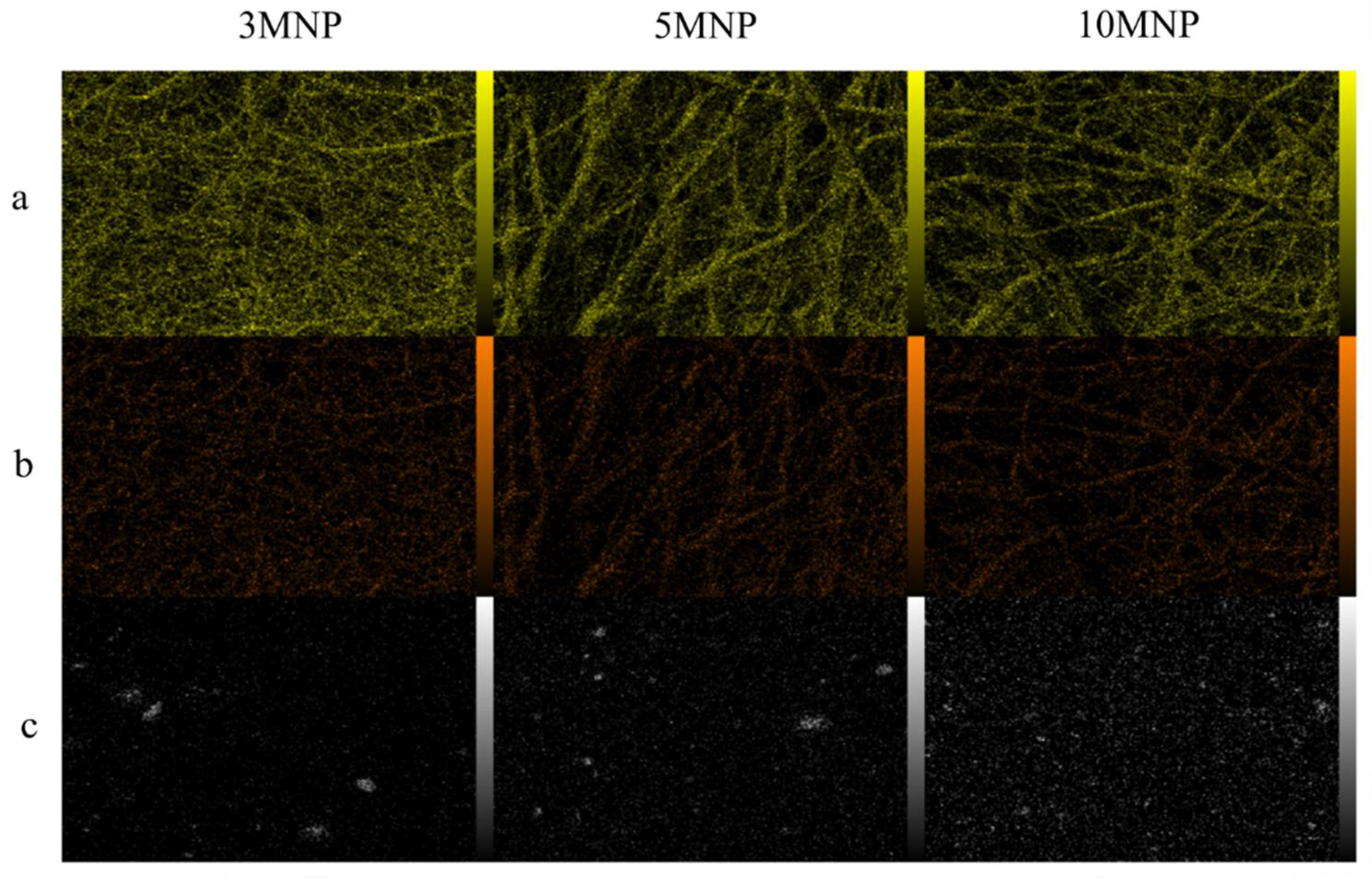



| Composite | 3MNP | 5MNP | 10MNP | ||||
|---|---|---|---|---|---|---|---|
| Elements | A% | W% | A% | W% | A% | W% | |
| Carbon | 73.19 | 66.93 | 72.34 | 65.53 | 72.88 | 65.77 | |
| Oxygen | 26.66 | 32.47 | 27.29 | 32.93 | 26.58 | 31.95 | |
| Iron | 0.15 | 0.6 | 0.36 | 1.53 | 0.54 | 2.27 | |
| Samples | F (MPa) | Emod (MPa) | Elongationpp (%) | Elongationbp (%) |
|---|---|---|---|---|
| PCL | 1.98 ± 0.32 | 2.95 ± 0.66 | 49.41 ± 14 | 57.64 ± 16 |
| 3MNP | 2.21 ± 0.37 | 2.38 ± 0.75 | 72.96 ± 17 | 117.63 ± 27 |
| 5MNP | 3.38 ± 0.53 | 4.42 ± 0.9 | 59.85 ± 12 | 93.87 ± 23 |
| 10MNP | 4.9 ± 0.62 | 6.41 ± 1.2 | 62.71 ± 13 | 81.65 ± 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaei, V.; Mirzaei, E.; Taghizadeh, S.-M.; Berenjian, A.; Ebrahiminezhad, A. Nano Iron Oxide-PCL Composite as an Improved Soft Tissue Scaffold. Processes 2021, 9, 1559. https://doi.org/10.3390/pr9091559
Rezaei V, Mirzaei E, Taghizadeh S-M, Berenjian A, Ebrahiminezhad A. Nano Iron Oxide-PCL Composite as an Improved Soft Tissue Scaffold. Processes. 2021; 9(9):1559. https://doi.org/10.3390/pr9091559
Chicago/Turabian StyleRezaei, Vahid, Esmaeil Mirzaei, Seyedeh-Masoumeh Taghizadeh, Aydin Berenjian, and Alireza Ebrahiminezhad. 2021. "Nano Iron Oxide-PCL Composite as an Improved Soft Tissue Scaffold" Processes 9, no. 9: 1559. https://doi.org/10.3390/pr9091559
APA StyleRezaei, V., Mirzaei, E., Taghizadeh, S.-M., Berenjian, A., & Ebrahiminezhad, A. (2021). Nano Iron Oxide-PCL Composite as an Improved Soft Tissue Scaffold. Processes, 9(9), 1559. https://doi.org/10.3390/pr9091559







