Operation of Submerged Anaerobic Membrane Bioreactors at 20 °C: Effect of Solids Retention Time on Flux, Mixed Liquor Characteristics and Performance
Abstract
:1. Introduction
2. Materials and Methods
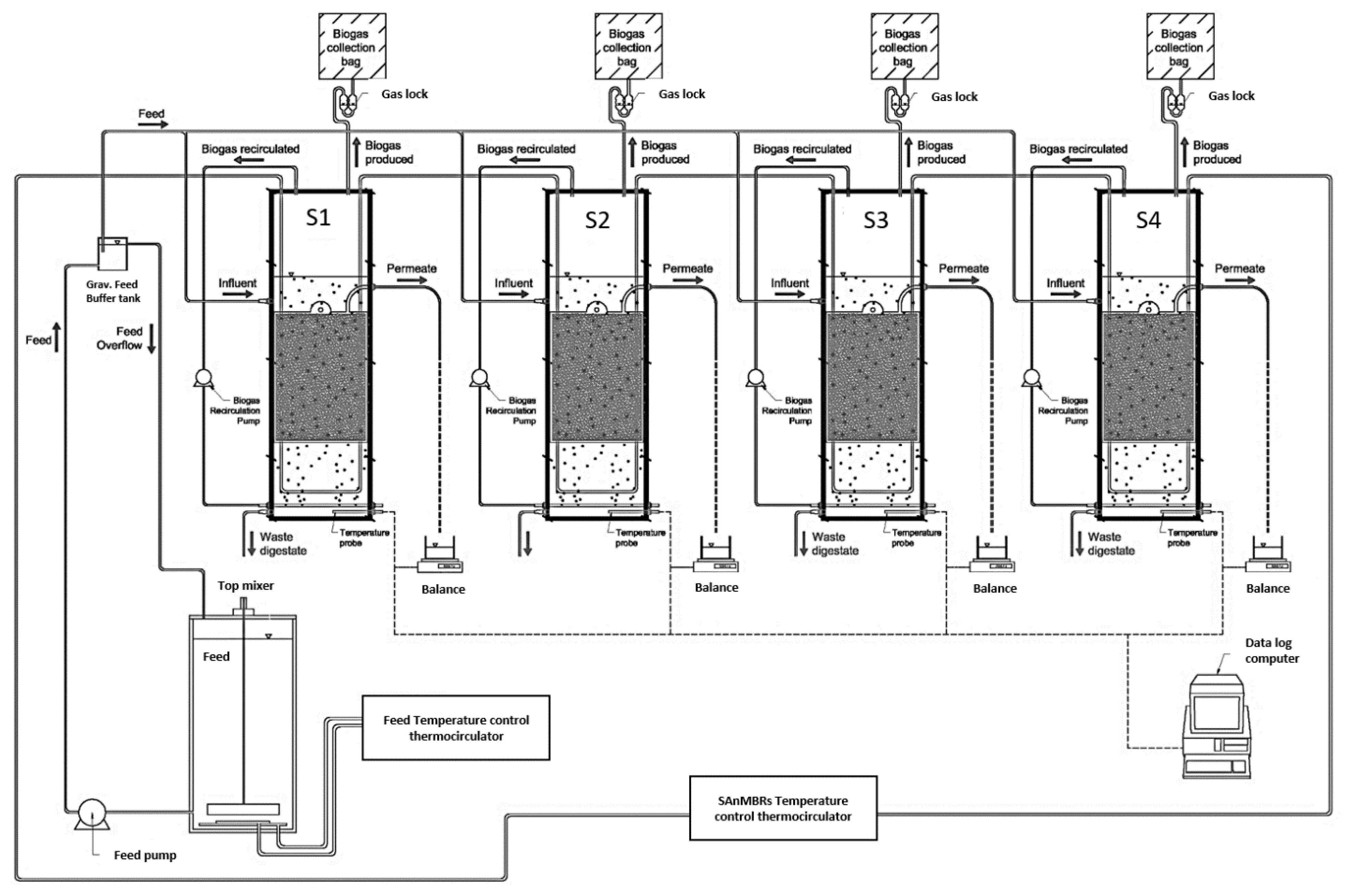
2.1. SAnMBR Design and Operation
2.2. Inoculum and Substrate
2.3. Performance and Stability
3. Results and Discussion
3.1. Operational Performance
3.1.1. Membrane Flux, TMP, MLSS, HRT and OLR
3.1.2. COD Removal Rates and TE Requirements
3.1.3. COD Balances and Dissolved Methane
3.1.4. Specific Methane Productivity
3.2. Membrane Performance and Fouling Phenomena
3.3. Mixed Liquor Characteristics
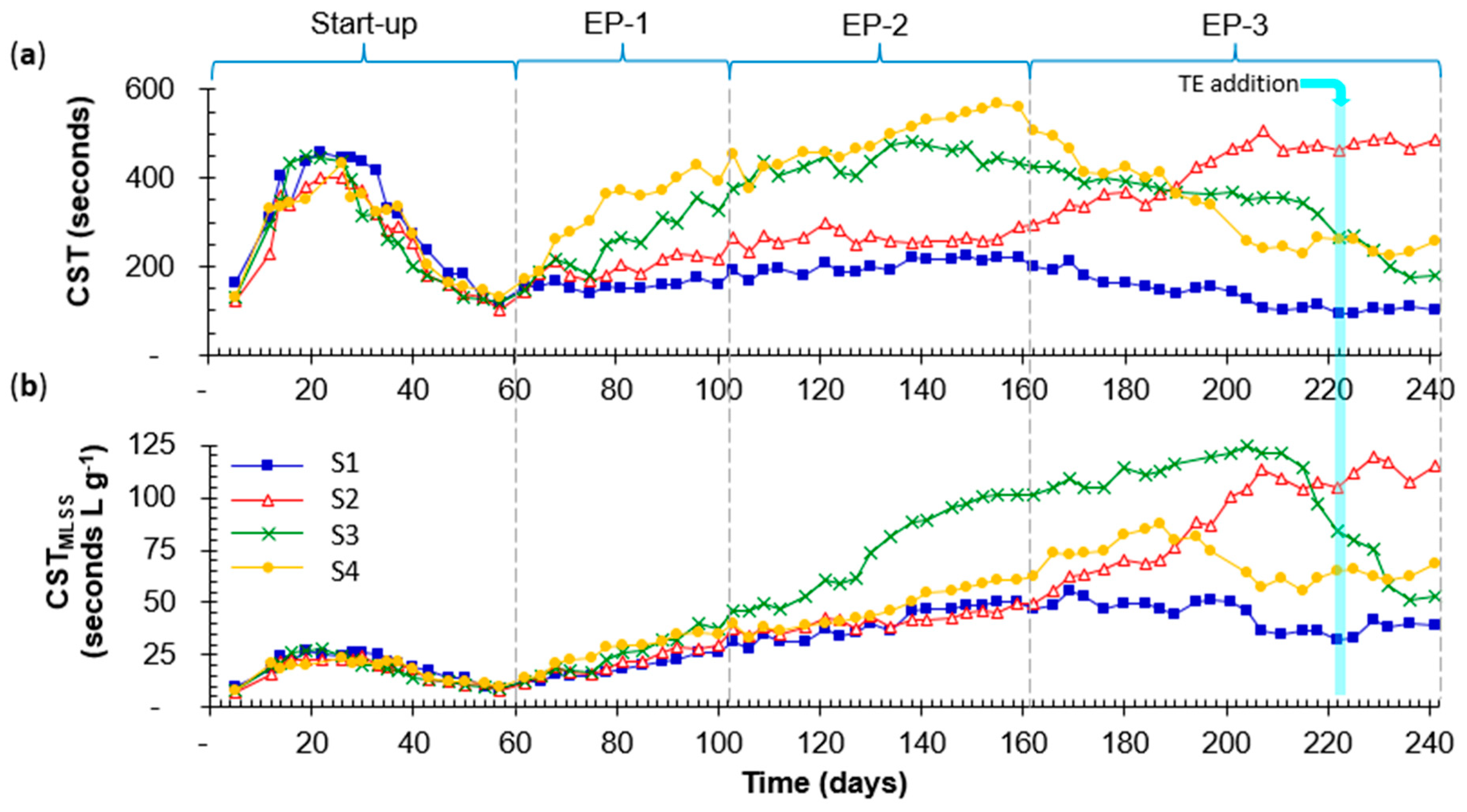
3.3.1. Capillary Suction Time
3.3.2. Frozen Image Centrifugation
3.3.3. Extracellular Polymeric Substances
3.3.4. Biomass Growth and Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Parameter | SRT | TMP | Membrane Flux (J) * | OLR | HRT | Feed COD | Effluent COD | COD Removal Rate | |
|---|---|---|---|---|---|---|---|---|---|
| Phase | Reactor | (days) | (kPa) | (L m−2 h−1) | (g COD L−1 day−1) | (hours) | (mg L−1) | (mg L−1) | (%) |
| Start-up (day 0–59, total 60 days) | A | 90 | 7.1 → 2.1 → 2.5 | 16.1 → 6.7 | 0.50–1.45 | 5.8 → 12.5 | 305 → 507 | 99 → 200 → 59 | 74% → 53% → 89% |
| B | 90 | 7.1 → 2.1 → 2.5 | 16.1 → 6.4 | 0.50–1.47 | 5.8 → 13.1 | 305 → 507 | 100 → 222 → 51 | 74% → 55% → 90% | |
| C | 90 | 7.1 → 2.1 → 2.5 | 16.1 → 6.3 | 0.50–1.49 | 5.8 → 13.3 | 305 → 507 | 83 → 213 → 39 | 79% → 49% → 93% | |
| D | 90 | 7.1 → 2.1 → 2.5 | 16.1 → 6.4 | 0.50–1.46 | 5.8 → 13.1 | 305 → 507 | 83 → 224 → 62 | 72% → 42% → 88% | |
| EP-1 (day 60–111, total 52 days) | A | 30 | 2.5 → 3.2 → 1.8 | 6.7 → 5.3 → 4.1 | 0.98 ± 0.03 | 12.5 → 15.8 → 20.2 | 567 → 860 | 59 → 144 | 88% ± 1% |
| B | 45 | 2.5 → 5.1 → 2.9 | 6.4 → 5.1 → 4.1 | 0.94 ± 0.04 | 13.1 → 16.5 → 20.3 | 567 → 860 | 51 → 65 | 91% ± 1% | |
| C | 60 | 2.5 → 6.7 → 4.3 → 6.3 | 6.3 → 4.2 → 3.7 | 1.00 → 0.90 | 13.3 → 17.7 → 22.5 | 567 → 860 | 41 → 36 | 92% ± 2% | |
| D | 90 | 2.5 → 3.2 → 2.2 | 6.4 → 5.3 → 4.1 | 0.97 ± 0.02 | 13.1 →16.2 → 20.4 | 567 → 860 | 61 → 21 | 88% → 97% | |
| EP-2 (day 112–160, total 48 days) | A | 30 | 1.8 ± 0.5 | 4.1 ± 0.0 | 0.98 ± 0.01 | 20.6 ± 0.2 | 843 ± 26 | 144 → 125 | 85% ± 2% |
| B | 45 | 2.8 ± 0.6 | 4.1 ± 0.0 | 0.99 ± 0.01 | 20.5 ± 0.2 | 843 ± 26 | 65 → 70 | 93% ± 1% | |
| C | 30 | 6.3 → 9.8 | 3.7 → 3.0 | 0.90 → 0.72 | 22.5 → 28.5 | 843 ± 26 | 36 → 77 | 95% → 91% | |
| D | 90 | 2.2 → 9.8 | 4.1 → 3.2 | 0.97 → 0.76 | 20.4 → 26.7 | 843 ± 26 | 21 → 44 | 96% ± 1% | |
| EP-3 (day 161–242, total 82 days) | A | 20 | 1.9 → 2.2 | 4.0 ± 0.1 | 0.98 ± 0.02 | 21.0 ± 0.4 | 853 ± 21 | 136 → 228 → 68 | 84% → 74% → 92% |
| B | 45 | 2.8 → 9.8 | 4.1 → 3.2 | 0.99 → 0.78 | 20.5 → 26.3 | 853 ± 21 | 70→ 84 → 22 | 92% → 92% → 97% | |
| C | 30 | 9.8 → 3.8 | 3.0 → 2.7 → 4.0 | 0.72 → 1.02 | 28.5 → 31.5 → 21.9 | 853 ± 21 | 77 → 217 → 39 | 91% → 75% → 96% | |
| D | 30 | 9.8 → 6.5 | 3.2 → 4.1 | 0.76 → 0.99 | 26.7 → 21.1 | 853 ± 21 | 44 → 91 → 34 | 95% → 89%→ 96% | |
| Parameter | SRT | CH4 in Biogas * | SMP ** | MLSS | MLVSS | CST | pH | |
|---|---|---|---|---|---|---|---|---|
| Phase | Reactor | (days) | (%) | (L CH4 g−1 COD Removed) | (g L−1) | (g L−1) | (seconds) | |
| Start-up (day 0–59, 60 days) | A | 90 | 88% → 93% | 0.00 → 0.35 → 0.21 | 16.2 → 12.7 | 12.5 →10.3 | 162 → 461 → 117 | 7.0 → 6.2 → 6.8 |
| B | 90 | 81% → 92% | 0.00 → 0.29 → 0.20 | 16.2 → 12.5 | 12.6 → 10.2 | 122 → 402 → 101 | 7.0 → 6.2 → 6.8 | |
| C | 90 | 76% → 93% | 0.00 → 0.42 → 0.22 | 16.2 → 12.4 | 13.3 → 10.0 | 130 → 449 → 117 | 7.0 → 6.2 → 6.8 | |
| D | 90 | 87% → 93% | 0.00 → 0.42 → 0.21 | 16.2 → 12.7 | 12.7 → 10.4 | 132 → 435 → 131 | 7.0 → 6.2 → 6.9 | |
| EP-1 (day 60–111, 52 days) | A | 30 | 92% ± 1% | 0.21 ± 0.01 | 12.7 → 6.2 | 10.3 → 5.4 | 117 → 198 | 6.8 ± 0.0 |
| B | 45 | 91% ± 1% | 0.22 ± 0.02 | 12.5 → 7.3 | 10.2 → 6.2 | 101 → 252 | 6.8 ± 0.0 | |
| C | 60 | 92% ± 2% | 0.23 ± 0.02 | 12.4 → 8.7 | 10.0 → 7.4 | 117 → 438 | 6.8 ± 0.0 | |
| D | 90 | 92% ± 2% | 0.23 ± 0.02 | 12.7 → 11.7 | 10.4 → 9.8 | 131 → 430 | 6.8 ± 0.0 | |
| EP-2 (day 112–160, 48 days) | A | 30 | 90% ± 1% | 0.22 ± 0.01 | 6.2 → 4.4 | 5.4 → 3.8 | 198 →215 | 6.7 ± 0.0 |
| B | 45 | 90% ± 1% | 0.24 ± 0.01 | 7.3 → 6.0 | 6.2 → 5.2 | 252 →291 | 6.7 ± 0.1 | |
| C | 30 | 90% ± 1% | 0.23 ± 0.01 | 8.7 → 4.3 | 7.4 → 3.7 | 407 → 482 → 433 | 6.7 ± 0.0 | |
| D | 90 | 90% ± 1% | 0.26 ± 0.02 | 11.7 → 9.3 | 9.8 → 8.1 | 430 → 569 | 6.8 ± 0.0 | |
| EP-3 (day 161–242, 82 days) | A | 20 | 90% ± 1% | 0.23 ± 0.01 | 4.4 → 2.6 | 3.8 → 2.2 | 222→ 101 | 6.7 ± 0.0 |
| B | 45 | 90% ± 1% | 0.25 ± 0.01 | 6.0 → 4.2 | 5.2 → 3.7 | 291 → 488 | 6.8 ± 0.0 | |
| C | 30 | 90% ± 1% | 0.23 ± 0.01 | 4.3 → 2.8 → 3.4 | 3.7 → 2.9 | 433 → 182 | 6.8 ± 0.0 | |
| D | 30 | 90% ± 1% | 0.24 ± 0.01 | 9.3 → 6.4 → 3.8 | 8.1 → 3.3 | 569 → 240 | 6.8 ± 0.0 | |

References
- Kong, Z.; Wu, J.; Rong, C.; Wang, T.; Li, L.; Luo, Z.; Ji, J.; Hanaoka, T.; Sakemi, S.; Ito, M. Large pilot-scale submerged anaerobic membrane bioreactor for the treatment of municipal wastewater and biogas production at 25 °C. Bioresour. Technol. 2021, 319, 124123. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Cao, L.; Love, N.G.; Raskin, L.; Skerlos, S.J. Navigating wastewater energy recovery strategies: A life cycle comparison of anaerobic membrane bioreactor and conventional treatment systems with anaerobic digestion. Environ. Sci. Technol. 2014, 48, 5972–5981. [Google Scholar] [CrossRef]
- Stuckey, D.C. Recent developments in anaerobic membrane reactors. Bioresour. Technol. 2012, 122, 137–148. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, S.; Li, Y.-y.; Wen, W.; Wang, X.C.; Chen, R. Application of anaerobic membrane bioreactors to municipal wastewater treatment at ambient temperature: A review of achievements, challenges, and perspectives. Bioresour. Technol. 2018, 267, 756–768. [Google Scholar] [CrossRef]
- McKeown, R.M.; Hughes, D.; Collins, G.; Mahony, T.; O’Flaherty, V. Low-temperature anaerobic digestion for wastewater treatment. Curr. Opin. Biotechnol. 2012, 23, 444–451. [Google Scholar] [CrossRef]
- Lim, K.; Evans, P.J.; Parameswaran, P. Long-term performance of a pilot-scale gas-sparged anaerobic membrane bioreactor under ambient temperatures for holistic wastewater treatment. Environ. Sci. Technol. 2019, 53, 7347–7354. [Google Scholar] [CrossRef] [PubMed]
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, H.; van Lier, J.B. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Love, N.G.; Skerlos, S.J.; Raskin, L. Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: A critical review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, S.; Astals, S.; Peces, M.; Cardete, M.; Fernández, I.; Mata-Alvarez, J.; Dosta, J. Advances in anaerobic membrane bioreactor technology for municipal wastewater treatment: A 2020 updated review. Renew. Sustain. Energy Rev. 2020, 130, 109936. [Google Scholar] [CrossRef]
- Anjum, F.; Khan, I.M.; Kim, J.; Aslam, M.; Blandin, G.; Heran, M.; Lesage, G. Trends and progress in AnMBR for domestic wastewater treatment and their impacts on process efficiency and membrane fouling. Environ. Technol. Innov. 2020, 101204. [Google Scholar] [CrossRef]
- Dvořák, L.; Gómez, M.; Dolina, J.; Černín, A. Anaerobic membrane bioreactors—a mini review with emphasis on industrial wastewater treatment: Applications, limitations and perspectives. Desalination Water Treat. 2016, 57, 19062–19076. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Psychrophilic anaerobic membrane bioreactor treatment of domestic wastewater. Water Res. 2013, 47, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Nie, Y.; Wakahara, S.; Komori, D.; Li, Y.-Y. Investigation on the response of anaerobic membrane bioreactor to temperature decrease from 25 °C to 10 °C in sewage treatment. Bioresour. Technol. 2017, 243, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Bae, J. Current status of the pilot-scale anaerobic membrane bioreactor treatments of domestic wastewaters: A critical review. Bioresour. Technol. 2018, 247, 1038–1046. [Google Scholar] [CrossRef]
- Tan, T.W.; Ng, H.Y.; Ong, S.L. Effect of mean cell residence time on the performance and microbial diversity of pre-denitrification submerged membrane bioreactors. Chemosphere 2008, 70, 387–396. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- WEF. WEF Manual and Practice No. 36; McGraw-Hill Education UK: London, UK, 2011. [Google Scholar]
- Chen, R.; Nie, Y.; Hu, Y.; Miao, R.; Utashiro, T.; Li, Q.; Xu, M.; Li, Y.-Y. Fouling behaviour of soluble microbial products and extracellular polymeric substances in a submerged anaerobic membrane bioreactor treating low-strength wastewater at room temperature. J. Membr. Sci. 2017, 531, 1–9. [Google Scholar] [CrossRef]
- Ng, H.Y.; Tan, T.W.; Ong, S.L. Membrane fouling of submerged membrane bioreactors: Impact of mean cell residence time and the contributing factors. Environ. Sci. Technol. 2006, 40, 2706–2713. [Google Scholar] [CrossRef]
- Jinsong, Z.; Chuan, C.H.; Jiti, Z.; Fane, A. Effect of sludge retention time on membrane bio-fouling intensity in a submerged membrane bioreactor. Sep. Sci. Technol. 2006, 41, 1313–1329. [Google Scholar] [CrossRef]
- Baek, S.H.; Pagilla, K.R.; Kim, H.-J. Lab-scale study of an anaerobic membrane bioreactor (AnMBR) for dilute municipal wastewater treatment. Biotechnol. Bioprocess Eng. 2010, 15, 704–708. [Google Scholar] [CrossRef]
- Yeo, H.; Lee, H.-S. The effect of solids retention time on dissolved methane concentration in anaerobic membrane bioreactors. Environ. Technol. 2013, 34, 2105–2112. [Google Scholar] [CrossRef]
- Dong, Q.; Parker, W.; Dagnew, M. Influence of SRT and HRT on bioprocess performance in anaerobic membrane bioreactors treating municipal wastewater. Water Environ. Res. 2016, 88, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Parker, W.; Dagnew, M. Long term performance of membranes in an anaerobic membrane bioreactor treating municipal wastewater. Chemosphere 2016, 144, 249–256. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Zhou, Y.; Stuckey, D.C. Effect of operating conditions on speciation and bioavailability of trace metals in submerged anaerobic membrane bioreactors. Bioresour. Technol. 2017, 243, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, A.; Calimlioglu, B.; Sahinkaya, E. Impact of SRT on the efficiency and microbial community of sequential anaerobic and aerobic membrane bioreactors for the treatment of textile industry wastewater. Chem. Eng. J. 2017, 314, 378–387. [Google Scholar] [CrossRef]
- Ji, J.; Chen, Y.; Hu, Y.; Ohtsu, A.; Ni, J.; Li, Y.; Sakuma, S.; Hojo, T.; Chen, R.; Li, Y.-Y. One-year operation of a 20-L submerged anaerobic membrane bioreactor for real domestic wastewater treatment at room temperature: Pursuing the optimal HRT and sustainable flux. Sci. Total Environ. 2021, 775, 145799. [Google Scholar] [CrossRef] [PubMed]
- Dereli, R.K.; Grelot, A.; Heffernan, B.; van der Zee, F.P.; van Lier, J.B. Implications of changes in solids retention time on long term evolution of sludge filterability in anaerobic membrane bioreactors treating high strength industrial wastewater. Water Res. 2014, 59, 11–22. [Google Scholar] [CrossRef]
- Dereli, R.K.; van der Zee, F.P.; Heffernan, B.; Grelot, A.; van Lier, J.B. Effect of sludge retention time on the biological performance of anaerobic membrane bioreactors treating corn-to-ethanol thin stillage with high lipid content. Water Res. 2014, 49, 453–464. [Google Scholar] [CrossRef]
- Szabo-Corbacho, M.A.; Pacheco-Ruiz, S.; Míguez, D.; Hooijmans, C.M.; García, H.A.; Brdjanovic, D.; van Lier, J.B. Impact of solids retention time on the biological performance of an AnMBR treating lipid-rich synthetic dairy wastewater. Environ. Technol. 2021, 42, 597–608. [Google Scholar] [CrossRef]
- Pacheco-Ruiz, S.; Heaven, S.; Banks, C.J. Effect of mean cell residence time on transmembrane flux, mixed-liquor characteristics and overall performance of a submerged anaerobic membrane bioreactor. Environ. Technol. 2017, 38, 1263–1274. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Submerged anaerobic membrane bioreactor for low-strength wastewater treatment: Effect of HRT and SRT on treatment performance and membrane fouling. Water Res. 2011, 45, 705–713. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Performance of submerged anaerobic membrane bioreactor at different SRTs for domestic wastewater treatment. J. Biotechnol. 2013, 164, 82–90. [Google Scholar] [CrossRef]
- Baudry, M.; Zhou, T.; Van Gaelen, P.; Smets, I.; Pacheco-Ruiz, S. Protocol to evaluate and correlate membrane performance and mixed-liquor characteristics of full-scale and pilot-scale AnMBRs. In Proceedings of the 16th IWA World Conference on Anaerobic Digestion, Delft, The Netherlands, 23–27 June 2019. [Google Scholar]
- Pacheco-Ruiz, S.; Heaven, S.; Banks, C.J. Development and testing of a fully gravitational submerged anaerobic membrane bioreactor for wastewater treatment. Environ. Technol. 2015, 36, 2328–2339. [Google Scholar] [CrossRef] [Green Version]
- Henze, M.; Comeau, Y. Wastewater Characterization. In Biological Wastewater Treatment: Principles, Modelling and Design; Henze, M., Loosdrecht, M.C.M.V., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2011. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering—Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- SCA. The Determination of Chemical Oxygen Demand in Waters and Effluents; Standing Committee of Analysts; Environment Agency: Bristol, UK, 2007.
- Walker, M.; Zhang, Y.; Heaven, S.; Banks, C. Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes. Bioresour. Technol. 2009, 100, 6339–6346. [Google Scholar] [CrossRef] [Green Version]
- Walsh, K.P.; McLaughlan, R.G. Bubble extraction of dissolved gases from groundwater samples. Water Air Soil Pollut. 1999, 115, 525–534. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Domínguez, L.; Rodríguez, M.; Prats, D. Effect of different extraction methods on bound EPS from MBR sludges. Part I: Influence of extraction methods over three-dimensional EEM fluorescence spectroscopy fingerprint. Desalination 2010, 261, 19–26. [Google Scholar] [CrossRef]
- Liang, Z.; Li, W.; Yang, S.; Du, P. Extraction and structural characteristics of extracellular polymeric substances (EPS), pellets in autotrophic nitrifying biofilm and activated sludge. Chemosphere 2010, 81, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Chabaliná, L.D.; Pastor, M.R.; Rico, D.P. Characterization of soluble and bound EPS obtained from 2 submerged membrane bioreactors by 3D-EEM and HPSEC. Talanta 2013, 115, 706–712. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Frølund, B.; Griebe, T.; Nielsen, P. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 755–761. [Google Scholar] [CrossRef]
- Balcıoğlu, G.; Yilmaz, G.; Goender, Z.B. Evaluation of anaerobic membrane bioreactor (AnMBR) treating confectionery wastewater at long-term operation under different organic loading rates: Performance and membrane fouling. Chem. Eng. J. 2021, 404, 126261. [Google Scholar] [CrossRef]
- Yu, D.; Li, C.; Wang, L.; Zhang, J.; Liu, J.; Wei, Y. Multiple effects of trace elements on methanogenesis in a two-phase anaerobic membrane bioreactor treating starch wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.D.M.; Lafita, C.; Gabaldón, C.; Spanjers, H.; van Lier, J.B. Trace metals supplementation in anaerobic membrane bioreactors treating highly saline phenolic wastewater. Bioresour. Technol. 2017, 234, 106–114. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.; Carretero, L.; Gatti, M.; Martí, N.; Borrás, L.; Ribes, J.; Seco, A. Reliable method for assessing the COD mass balance of a submerged anaerobic membrane bioreactor (SAMBR) treating sulphate-rich municipal wastewater. Water Sci. Technol. 2012, 66, 494–502. [Google Scholar] [CrossRef]
- Jin, B.; Wilén, B.-M.; Lant, P. Impacts of morphological, physical and chemical properties of sludge flocs on dewaterability of activated sludge. Chem. Eng. J. 2004, 98, 115–126. [Google Scholar] [CrossRef]
- Pollice, A.; Laera, G.; Saturno, D.; Giordano, C. Effects of sludge retention time on the performance of a membrane bioreactor treating municipal sewage. J. Membr. Sci. 2008, 317, 65–70. [Google Scholar] [CrossRef]
- Astals, S.; Esteban-Gutiérrez, M.; Fernández-Arévalo, T.; Aymerich, E.; García-Heras, J.; Mata-Alvarez, J. Anaerobic digestion of seven different sewage sludges: A biodegradability and modelling study. Water Res. 2013, 47, 6033–6043. [Google Scholar] [CrossRef]
- Astals, S.; Venegas, C.; Peces, M.; Jofre, J.; Lucena, F.; Mata-Alvarez, J. Balancing hygienization and anaerobic digestion of raw sewage sludge. Water Res. 2012, 46, 6218–6227. [Google Scholar] [CrossRef]
- Takashima, M.; Tanaka, Y. Acidic thermal post-treatment for enhancing anaerobic digestion of sewage sludge. J. Environ. Chem. Eng. 2014, 2, 773–779. [Google Scholar] [CrossRef]
- Odriozola, M.; Lousada-Ferreira, M.; Spanjers, H.; van Lier, J.B. Effect of sludge characteristics on optimal required dosage of flux enhancer in anaerobic membrane bioreactors. J. Membr. Sci. 2021, 619, 118776. [Google Scholar] [CrossRef]
- Lockyear, C.; White, M. The WRc Thickenability Test Using a Low-Speed Centrifuge; WRc Medmenham Laboratory: Stevenage, UK, 1979. [Google Scholar]
- Hoyland, G.; Dee, A.; Day, M. Optimum design of sewage sludge consolidation tanks. Water Environ. J. 1989, 3, 505–516. [Google Scholar] [CrossRef]
- Lockyear, C. Predicting Full-Scale Batch Thickener Performance Using the Frozen-Image Centrifuge. Effl. Water Treat. J. 1981, 21, 560–563. [Google Scholar]
- Suhartini, S.; Heaven, S.; Banks, C.J. Comparison of mesophilic and thermophilic anaerobic digestion of sugar beet pulp: Performance, dewaterability and foam control. Bioresour. Technol. 2014, 152, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabraiz, S.; Petropoulos, E.; Shamurad, B.; Quintela-Baluja, M.; Mohapatra, S.; Acharya, K.; Charlton, A.; Davenport, R.J.; Dolfing, J.; Sallis, P.J. Temperature and immigration effects on quorum sensing in the biofilms of anaerobic membrane bioreactors. J. Environ. Manag. 2021, 293, 112947. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Yu, G.; Liu, J.; Zhou, Z. Relationship between sludge characteristics and membrane flux determination in submerged membrane bioreactors. J. Membr. Sci. 2006, 284, 87–94. [Google Scholar] [CrossRef]
- Kouzeli-Katsiri, A. Characterization of wastewater sludges end user’s view. In Monitoring of Water Quality; Elsevier Science: Amsterdam, The Netherlands, 1998; pp. 75–88. [Google Scholar]
- Spinosa, L. Technological characterization of sewage sludge. Waste Manag. Res. 1985, 3, 389–398. [Google Scholar] [CrossRef]
- Spinosa, L.; Doshi, P. Re-conceptualizing Sludge Management: Focusing on Institutional and Governance Aspects. J. Environ. Sci. Eng. 2020, 9, 98–107. [Google Scholar]
- Nilusha, R.T.; Yu, D.; Zhang, J.; Wei, Y. Effects of Solids Retention Time on the Anaerobic Membrane Bioreactor with Yttria-Based Ceramic Membrane Treating Domestic Wastewater at Ambient Temperature. Membranes 2020, 10, 196. [Google Scholar] [CrossRef]
- Laspidou, C.S.; Rittmann, B.E. A unified theory for extracellular polymeric substances, soluble microbial products, and active and inert biomass. Water Res. 2002, 36, 2711–2720. [Google Scholar] [CrossRef]
- Ramesh, A.; Lee, D.-J.; Hong, S. Soluble microbial products (SMP) and soluble extracellular polymeric substances (EPS) from wastewater sludge. Appl. Microbiol. Biotechnol. 2006, 73, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Guo, Z.; Liang, Z.; Hou, X.; Li, Z.; Mu, D.; Ge, C.; Zhang, C.; Jin, C. Long-term investigation into the membrane fouling behavior in anaerobic membrane bioreactors for municipal wastewater treatment operated at two different temperatures. Membranes 2020, 10, 231. [Google Scholar] [CrossRef]
- Ji, J.; Ni, J.; Ohtsu, A.; Isozumi, N.; Hu, Y.; Du, R.; Chen, Y.; Qin, Y.; Kubota, K.; Li, Y.-Y. Important effects of temperature on treating real municipal wastewater by a submerged anaerobic membrane bioreactor: Removal efficiency, biogas, and microbial community. Bioresour. Technol. 2021, 336, 125306. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Nie, Y.; Ji, J.; Utashiro, T.; Li, Q.; Komori, D.; Li, Y.-Y. Submerged anaerobic membrane bioreactor (SAnMBR) performance on sewage treatment: Removal efficiencies, biogas production and membrane fouling. Water Sci. Technol. 2017, 76, 1308–1317. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Ruiz, S.; Heaven, S.; Banks, C.J. Operation of Submerged Anaerobic Membrane Bioreactors at 20 °C: Effect of Solids Retention Time on Flux, Mixed Liquor Characteristics and Performance. Processes 2021, 9, 1525. https://doi.org/10.3390/pr9091525
Pacheco-Ruiz S, Heaven S, Banks CJ. Operation of Submerged Anaerobic Membrane Bioreactors at 20 °C: Effect of Solids Retention Time on Flux, Mixed Liquor Characteristics and Performance. Processes. 2021; 9(9):1525. https://doi.org/10.3390/pr9091525
Chicago/Turabian StylePacheco-Ruiz, Santiago, Sonia Heaven, and Charles J. Banks. 2021. "Operation of Submerged Anaerobic Membrane Bioreactors at 20 °C: Effect of Solids Retention Time on Flux, Mixed Liquor Characteristics and Performance" Processes 9, no. 9: 1525. https://doi.org/10.3390/pr9091525







