Estimating the Methane Potential of Energy Crops: An Overview on Types of Data Sources and Their Limitations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Overview on Types of Testing Methods Applied
- Biochemical methane potential (BMP) tests and other long-retention batch assays;
- Short-retention batch tests;
- Continuous/semi-continuous tests;
- Theoretical calculations.
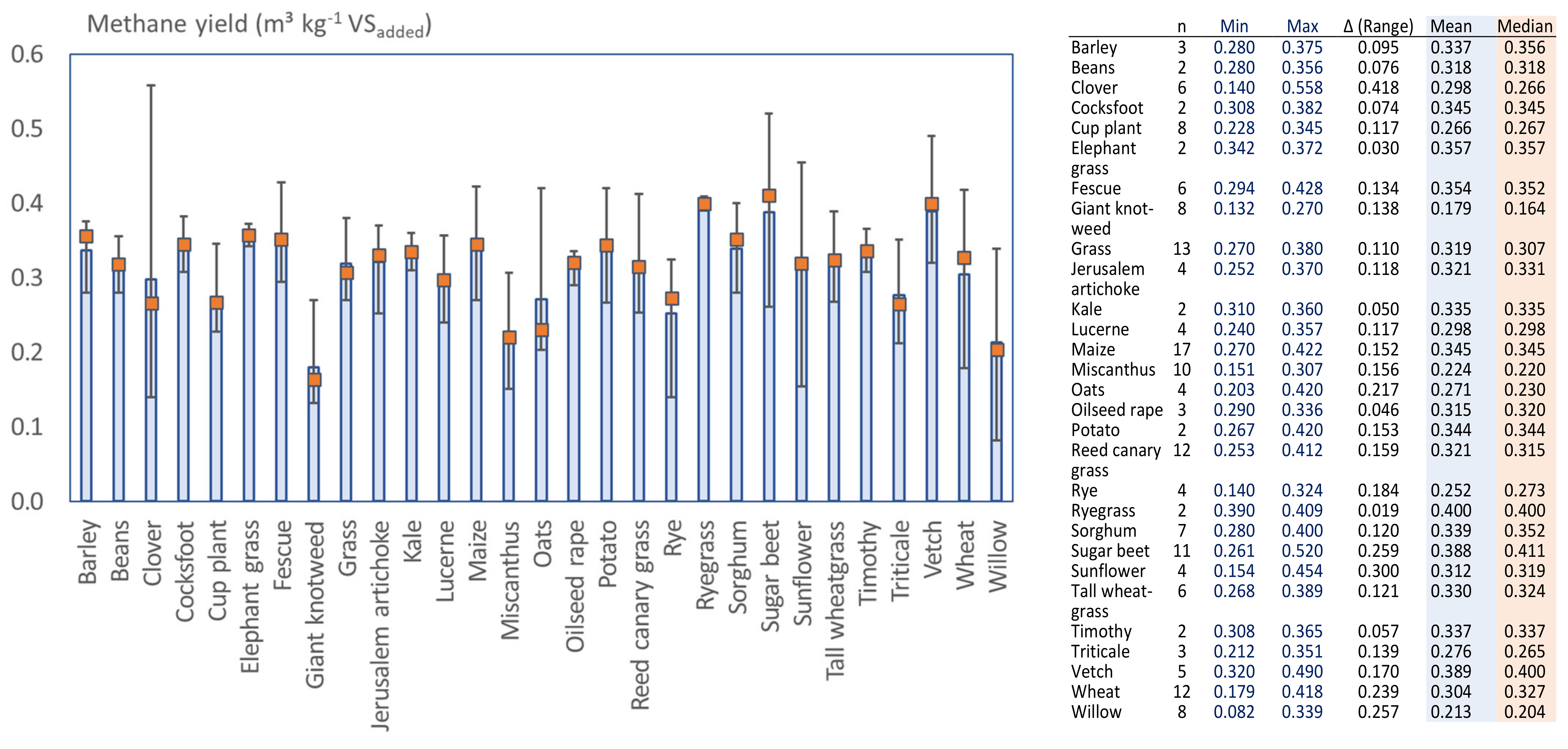
3.2. Methane Potentials Obtained in BMP and Long Retention Batch Tests
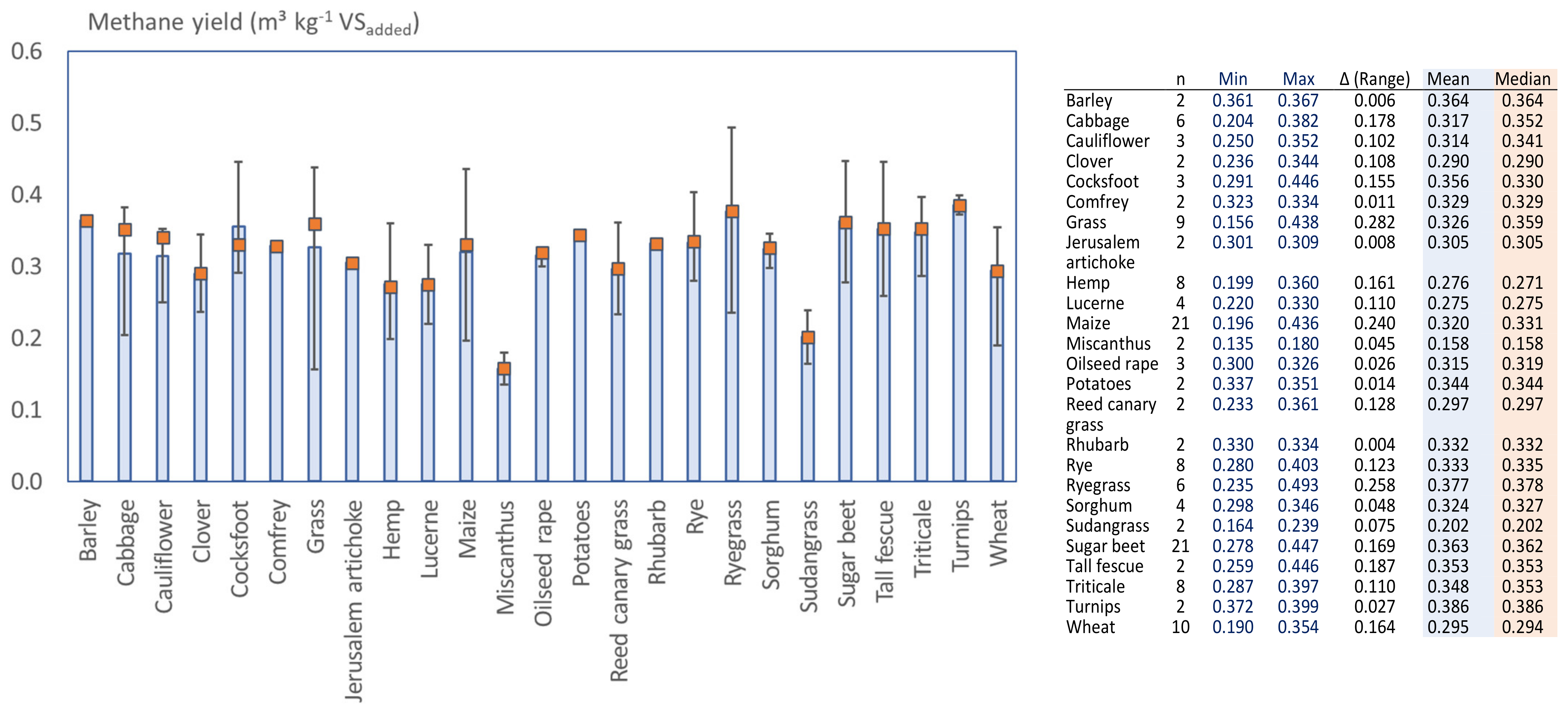
3.3. Methane Production Obtained from Short Retention Batch Tests
3.4. Methane Production from Continuous/Semi-Continuous Digestion Processes
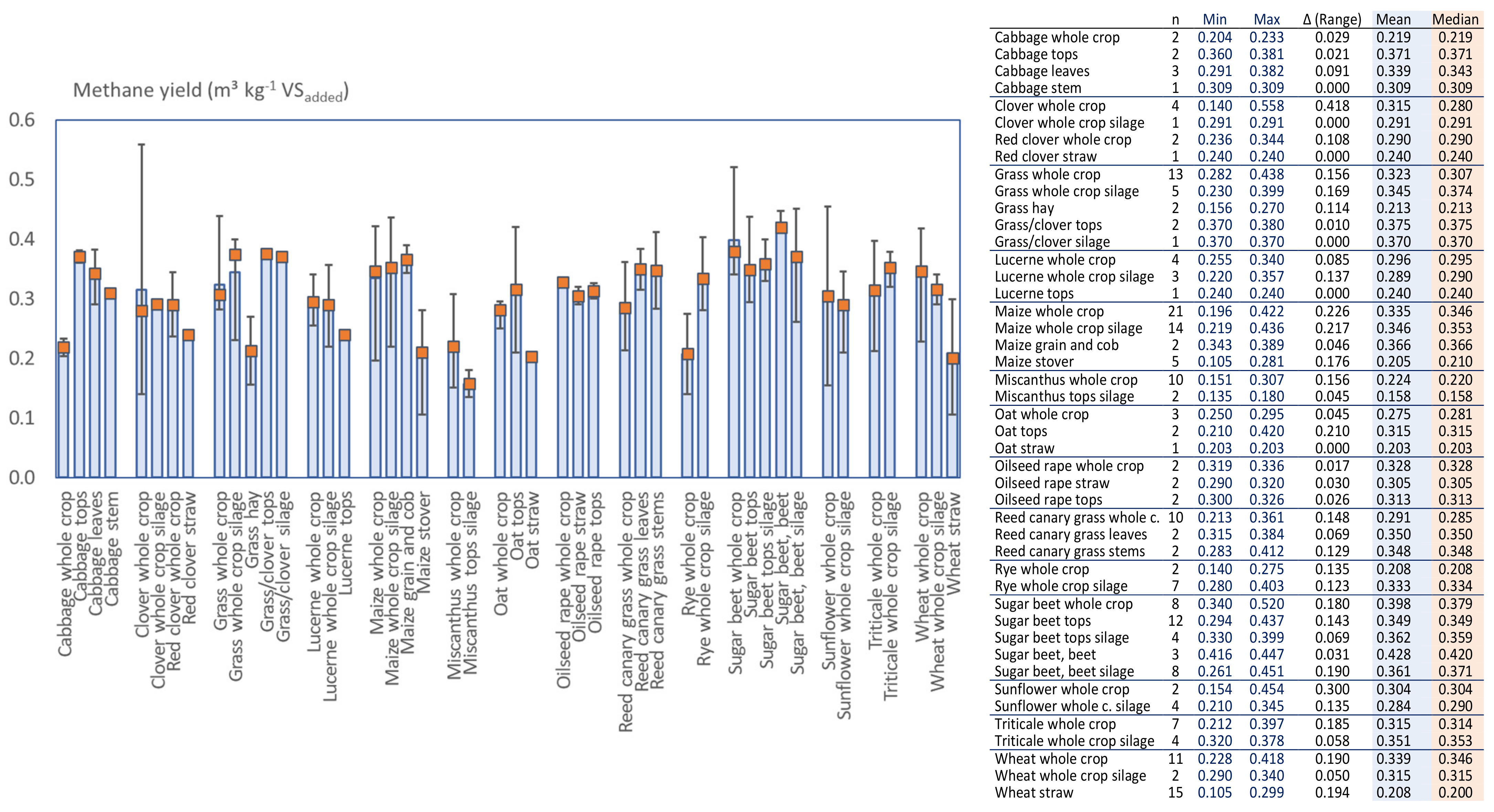
3.5. Variations of the Reported Methane Potential for Various Crops
3.6. Methane Production Based on Elemental/Biochemical Composition
nNH3 + sH2S
3.7. Results Published without Methods
4. Discussion
4.1. Issues Potentially Affecting the Precision of Methane Potential Reported in Literature
4.2. Limitations Regarding Literature Data from Experiments Other Than BMP Tests
4.3. Transparency of Published Data
4.4. Alternatives to Relying on Literature Data
4.5. Which Crop Material Has the Highest Methane Potential?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Literature Data from Different Types of Assays
| Reference | Crops | Crop Parts | Methane Yield (m3 kg−1 VSadded) | Comments |
|---|---|---|---|---|
| Amon et al. [51] | Maize | Whole crop | 0.359–0.422 | Range of varieties |
| Maize | Whole crop | 0.343–0.407 | Range of harvest times | |
| Wheat | Whole crop | 0.228–0.343 | Range of harvest times | |
| Rye | Whole crop | 0.140–0.275 | Range of harvest times | |
| Sunflowers | Whole crop | 0.154–0.454 | Range of harvest times | |
| Triticale | Whole crop | 0.212–0.265 | Range of harvest times | |
| Bauer et al. [53] | Barley | Whole crop | 0.375 | Silage |
| Lucerne | Whole crop | 0.357 | Silage | |
| Maize | Whole crop | 0.345 | Silage | |
| Sorghum | Whole crop | 0.362 | Silage | |
| Sunflower | Whole crop | 0.345 | Silage | |
| Wheat | Straw | 0.276 | ||
| Chiumenti et al. [61] | Grass | Whole crop | 0.308–0.340 | |
| Cornell et al. [72] | Maize | Whole crop | 0.33 | Ensiled |
| Feng et al. [41] | Fescue | Whole crop | 0.294–0.310 | Ensiled |
| Gallegos et al. [50] | Wheat | Straw | 0.179–0.244 | Ensiled and chopped |
| Garcia et al. [71] | Barley | Whole crop | 0.280 | |
| Maize | Whole crop | 0.289 | ||
| Millet | Whole crop | 0.253 | ||
| Sorghum | Whole crop | 0.313 | ||
| Triticale | Whole crop | 0.351 | ||
| Gunaseelan [32] | Cabbage | Stems | 0.309 | |
| Leaves | 0.291 | |||
| Carrot | Leaves | 0.241 | ||
| Petiole | 0.309 | |||
| Elephant grass | Lamina | 0.372 | ||
| Sheath | 0.342 | |||
| Garden beet | Leaves | 0.231 | ||
| Pea | Pods (seeds removed) | 0.390 | ||
| Potato | Peels | 0.267 | ||
| Sudangrass | Whole crop | 0.256 | ||
| Turnip | Leaves | 0.314 | ||
| Haag et al. [57] | Cup plant | Whole crop | 0.228–0.261 | Several varieties, dried and ground |
| Heidarzadeh Vazifehkhoran et al. [54] | Sugar beet | Beet | 0.337–0.420 | Silage in open silos |
| Beet | 0.411–0.451 | Silage in closed silos | ||
| Jerger et al. [35] | Sorghum | Tops | 0.28–0.40 | Range of cultivars |
| Jurado et al. [44] | Miscanthus | Whole crop | 0.249 | Milled |
| Wheat | Straw | 0.200 | Milled | |
| Willow | Woody component | 0.082 | Milled | |
| Kakuk et al. [45] | Willow | Leaves | 0.187–0.339 | Green (< 1 year), various harvest dates and species |
| Stems | 0.149–0.252 | |||
| Stems | 0.195–0.212 | Woody (> year) | ||
| Kandel et al. [40] | Fescue | Whole crop | 0.401–0.428 | Various harvest dates |
| Kandel et al. [39] | Reed canary grass | Leaf | 0.315–0.384 | Various harvest patterns |
| Reed canary grass | Stem | 0.283–0.412 | Various harvest patterns | |
| Kaparaju et al. [67] | Clover | Whole crop | 0.14–0.21 | |
| Grass hay | Whole crop | 0.27 | ||
| Oats | Whole crop | 0.25 | ||
| Lehtomäki and Björnsson [64] | Grass/clover | Whole crop | 0.37 | Silage |
| Sugar beet | Leaves and beets | 0.45 | ||
| Willow | Whole crop | 0.29 | ||
| Lehtomäki et al. [65] | Giant knotweed | Tops | 0.17–0.27 | Range of harvest dates |
| Grass/clover mix | Tops | 0.37–0.38 | ||
| Jerusalem artichoke | Tops | 0.36–0.37 | ||
| Lupine | Whole crop | 0.3 | ||
| Marrow kale | Tops | 0.31–0.36 | ||
| Nettle | Tops | 0.31–0.32 | ||
| Oat | Tops | 0.21–0.42 | ||
| Oilseed rape | Straw | 0.32 | ||
| Red clover | Straw | 0.24 | ||
| Reed canary grass | Whole crop | 0.28–0.34 | ||
| Sugar beet | 0.34–0.43 | |||
| Vetch–oat mixture | Tops | 0.32–0.49 | ||
| Whole crop | 0.4–0.41 | |||
| Lehtomäki et al. [68] | Grass | Whole crop | 0.306 | |
| Oat | Straw | 0.203 | ||
| Sugar beet | Tops | 0.353 | ||
| Li et al. [79] | Miscanthus | Whole crop | 0.182 | Particle sizes 20 and 30 mm |
| Machmüller et al. [52] | Clover | Whole crop | 0.291 | Silage |
| Maize | Grain and cob | 0.343 | ||
| Whole crop | 0.338 | Silage | ||
| Rye | Whole crop | 0.324 | Silage | |
| Sugar beet | Beet | 0.261 | Silage | |
| Sunflower | Whole crop | 0.293 | Silage | |
| Mast et al. [58] | Cup plant | Whole crop | 0.232–0.275 | Various dates, dried and milled |
| Giant knotweed | Whole crop | 0.146–0.158 | Various dates, dried and milled | |
| Energy dock | Whole crop | 0.187–0.297 | Various dates, dried and milled | |
| Tall wheatgrass | Whole crop | 0.311–0.376 | Various dates, dried and milled | |
| Mittweg et al. [27] | Maize | Whole crop | 0.346–0.362 | |
| Cobs | 0.389 | |||
| Nges et al. [78] | Miscanthus | Whole crop | 0.151–0.238 | Particle sizes 0.5–20 mm |
| Parawira et al. [69] | Potato | Tuber waste | (0.42) | Value for VS degraded |
| Sugar beet | Leaves | (0.52) | Value for VS degraded | |
| Peng et al. [76] | Miscanthus | Whole crop | 0.172–0.267 | Range of types and growth stages |
| Petersson et al. [37] | Faba bean | Straw | (0.28) calculated | Reported: 18.9 g (100g DM)−1 |
| Oilseed rape | Straw | (0.29) | 18.8 g (100g DM)−1 | |
| Winter rye | Straw | (0.27) | 18.2 g (100g DM)−1 | |
| Pouech et al. [63] | Barley | Whole crop | 0.356 | |
| Clover | Whole crop | 0.350–0.558 | Range of harvest times | |
| Forage sorghum | Whole crop | 0.295 | ||
| Grain sorghum | Whole crop | 0.372 | ||
| Lucerne | Whole crop | 0.340 | ||
| Maize | Whole crop | 0.397 | ||
| Oilseed rape | Whole crop | 0.336 | ||
| Ryegrass | Whole crop | 0.390–0.409 | Fresh and ensiled samples | |
| Sweet sorghum | Whole crop | 0.352 | ||
| Wheat | Whole crop | 0.384–0.418 | Range of harvest times | |
| Rincón et al. [73] | Wheat | Whole crop | 0.311–0.360 | Various harvest dates |
| Rincón et al. [74] | Wheat | Whole crop | 0.346–0.361 | Spring and winter planting |
| Ruf and Emmerling [59] | Cup plant | Whole crop | 0.236–0.282 | 2 years, poorly drained, dried |
| Giant knotweed | Whole crop | 0.189–0.222 | 2 years, poorly drained, dried | |
| Jerusalem artichoke | Whole crop | 0.252–0.301 | 2 years, poorly drained, dried | |
| Maize | Whole crop | 0.282–0.347 | 2 years, poorly drained, dried | |
| Reed canary grass | Whole crop | 0.277–0.290 | 2 years, poorly drained, dried | |
| Tall wheatgrass | Whole crop | 0.268–0.302 | 2 years, poorly drained, dried | |
| Schmidt et al. [62] | Cup plant | Whole crop | 0.272–0.345 | Three sites, range of harvest dates |
| Giant knotweed | Whole crop | 0.132–0.147 | Three sites, range of harvest dates | |
| Reed canary grass | Whole crop | 0.315–0.355 | Two sites, range of harvest dates | |
| Tall wheatgrass | Whole crop | 0.336–0.389 | Two sites, range of harvest dates | |
| Virginia mallow | Whole crop | 0.213–0.315 | Three sites, range of harvest dates | |
| Seppälä et al. [66] | Cocksfoot | Whole crop | 0.308–0.382 | Range of sites and harvest dates |
| Fescue | Whole crop | 0.296–0.394 | Range of sites and harvest dates | |
| Timothy | Whole crop | 0.308–0.365 | Range of sites and harvest dates | |
| Reed Canary Grass | Whole crop | 0.253–0.351 | Range of sites and harvest dates | |
| Thomas et al. [80] | Miscanthus | Whole crop | 0.166–0.202 | Various species |
| Virkajärvi et al. [77] | Grass | Whole crop | 0.302–0.307 | Various fertiliser strategies |
| Grass/clover mix | Whole crop | 0.285–0.292 | Various fertiliser strategies | |
| Wahid et al. [42] | Lucerne | Whole crop | 0.255 | |
| Wahid et al. [43] | Miscanthus | Whole crop | 0.302–0.307 | Various harvest times |
| Zauner and Küntzel [36] | Horse bean | Tops | 0.356 | |
| Lucerne | Tops | 0.240 | ||
| Maize | Whole crop | 0.270–0.298 | Various growth stages | |
| Mixed grass | Whole crop | 0.298–0.315 | Various fermentation periods | |
| Sugar beet | Tops | 0.294 | ||
| Vetch | Tops | 0.323 |
| Reference | Crops | Crop Parts | Methane Yield (m3 kg−1 VSadded) | Comments |
|---|---|---|---|---|
| Allen et al. [106] | Energy beet | Whole crop | 0.375 | |
| Fodder beet | Whole crop | 0.333 | ||
| Grass | Whole crop | 0.368 | ||
| Whole crop | 0.374–0.399 | Silage, various harvests | ||
| Whole crop | 0.156 | Hay | ||
| Maize | Whole crop | 0.354 | Fresh | |
| 0.394 | Ensiled | |||
| Oilseed rape | Whole crop | 0.319 | ||
| Potatoes | Whole crop | 0.337–0.351 | Various species | |
| Spring barley | Whole crop | 0.361 | ||
| Spring wheat | Whole crop | 0.340 | ||
| Sugar beet | Whole crop | 0.344 | ||
| Triticale | Whole crop | 0.314 | ||
| Turnips | Whole crop | 0.399 | ||
| Winter barley | Whole crop | 0.367 | ||
| Winter oats | Whole crop | 0.281 | ||
| Winter wheat | Whole crop | 0.354 | ||
| Badger et al. [89] | Kale | Tops | 0.296 | (Methane values calculated from biogas and indicated percentage of methane) |
| Maize | Tops | 0.342 | ||
| Oats | Whole crop | 0.295 | ||
| Sugar beet | Tops | 0.297 | ||
| Wheat | Straw | 0.255 | ||
| Bruni et al. [103] | Maize | Whole crop | 0.313–0.401 | Range of varieties and harvest dates |
| Gissen et al. [102] | Hemp | Whole crop | 0.260–0.292 | Various fertiliser strategies |
| Maize | Whole crop | 0.327–0.382 | Various fertiliser strategies | |
| Sugar beet | Root | 0.416–0.420 | Various fertiliser strategies | |
| Tops | 0.362–0.367 | Various fertiliser strategies | ||
| Triticale | Whole crop | 0.397 | ||
| Herrmann et al. [98] | Maize | Whole crop | 0.331–0.378 | All crops as silage; range of silage periods |
| Sorghum | Whole crop | 0.317–0.346 | ||
| Forage rye | Whole crop | 0.293–0.346 | ||
| Triticale | Whole crop | 0.340–0.365 | ||
| Herrmann et al. [99] | Maize | Whole crop | 0.323–0.362 | All crops as silage; range of chopping length and silage periods |
| Sorghum | Whole crop | 0.298–0.336 | ||
| Forage rye | Whole crop | 0.334–0.403 | ||
| Triticale | Whole crop | 0.320–0.378 | ||
| Winter rye | Whole crop | 0.321–0.336 | ||
| Herrmann et al. [100] | Sugar beet | Whole crop | 0.350–0.399 | Silage |
| Sunflowers | Whole crop | 0.210–0.286 | Silage | |
| Winter wheat | Whole crop | 0.269–0.328 | Silage | |
| Kaiser et al. [96] | Grass | Whole crop | 0.282–0.438 | Fresh |
| Hemp | Tops | 0.250–0.360 | Ensiled, various cuts | |
| Lucerne | Whole crop | 0.260–0.330 | ||
| Maize | Whole crop | 0.219–0.436 | Silage | |
| Miscanthus | Tops | 0.135–0.180 | Ensiled | |
| Red clover | Whole crop | 0.236–0.344 | Fresh and ensiled | |
| Ryegrass | Whole crop | 0.220–0.290 0.235–0.395 | Fresh and ensiled | |
| Sudangrass | Tops | 0.164–0.239 | Fresh and ensiled | |
| Sugar beet | Beet | 0.278–0.328 | Silage | |
| Tops | 0.335–0.395 | |||
| Kakuk et al. [114] | Maize | Stover | 0.210–0.281 | Various particles sizes and loadings |
| Kolbl et al. [108] | Forage turnip | Whole crop | 0.372 | Milled |
| Maize | Whole crop | 0.236 | Ensiled | |
| Triticale | Whole crop | 0.287 | Chopped | |
| Kreuger et al. [104] | Hemp | Whole crop | 0.199–0.270 | Range of harvest dates |
| Kreuger et al. [101] | Hemp | Whole crop | 0.301 | Fresh frozen |
| Whole crop | 0.272 | Ensiled | ||
| Maize | Whole crop | 0.363 | Fresh frozen | |
| Whole crop | 0.367 | Ensiled | ||
| Sugar beet | Beet | 0.447 | Low-sugar variety, fresh frozen | |
| Beet | 0.405 | Low-sugar variety, ensiled | ||
| Tops | 0.437 | Low-sugar variety, fresh frozen | ||
| Tops | 0.367 | Low-sugar variety, ensiled | ||
| Mähnert et al. [97] | Cocksfoot | Whole crop | 0.33 | Fresh and ensiled |
| Meadow foxtail | Whole crop | 0.31 | Fresh | |
| Ryegrass | Whole crop | 0.36 | Fresh and ensiled | |
| Nges et al. [107] | Wheat | Straw | 0.297 | Dried and ground |
| Nizami and Murphy [92] | Ryegrass | Whole crop | 0.483–0.493 | Fresh |
| Prade et al. [105] | Grass | Whole crop | 0.230–0.330 | Ensiled, range of harvest dates |
| Rye | Whole crop | 0.280–0.350 | Ensiled, range of harvest dates | |
| Wheat | Whole crop | 0.290–0.340 | Ensiled, range of harvest dates | |
| Raposo et al. [93] | Maize | Whole crop | 0.196–0.233 | Range of inoculum–substrate ratios |
| Spence et al. [7] | Grass | Whole crop | 0.359 | |
| Ryegrass | Whole crop | 0.294 | ||
| Triticale | Whole crop | 0.380 | ||
| Wheat | Whole crop | 0.283 | ||
| Svensson et al. [91] | Sugar beet | Tops | 0.33 | Ensiled |
| Wheat | Straw | 0.19 | ||
| Tilvikiene et al. [95] | Cocksfoot | Whole crop | 0.291–0.446 | Various fertiliser and harvest times |
| Reed canary grass | Whole crop | 0.233–0.361 | Various fertiliser and harvest times | |
| Tall fescue | Whole crop | 0.259–0.446 | Various fertiliser and harvest times | |
| Yan et al. [94] | Broccoli | Whole crop | ||
| Cabbage | Whole crop | 0.204 | Ground | |
| Cauliflower | Whole crop | 0.250 | Ground | |
| Leek | Whole crop | 0.183 | Ground | |
| Purple cabbage | Whole crop | 0.233 | Ground | |
| Zhao et al. [109] | Maize | Stover | 0.250 | Dried and ground |
| Zubr [90] | Cauliflower | Leaves | 0.341–0.352 | |
| Comfrey | Tops | 0.323–0.334 | ||
| Jerusalem artichoke | Tops | 0.301–0.309 | ||
| Oilseed rape | Tops | 0.300–0.326 | Fresh and ensiled | |
| Rhubarb | Tops | 0.330–0.334 | ||
| Sugar beet | Tops | 0.316–0.345 | ||
| White cabbage | Tops | 0.360–0.381 | ||
| Leaves | 0.343–0.382 |
| Reference | Crops | Crop Parts | Methane Yield (m3 kg−1 VSadded) | Comments |
|---|---|---|---|---|
| Haag et al. [57] | Cup plant | Whole crop | 0.220–0.244 | Various varieties, ensiled |
| Heeg et al. [82] | Wheat | Straw | 0.105–0.173 | Two-phase, meso- and thermophilic |
| Kakuk et al. [114] | Maize | Stover | 0.105–0.177 | Wet and dry digestion |
| Lehtomäki and Björnsson [64] | Grass | Whole crop | 0.39 | Silage |
| Sugar beet | Beets and tops | 0.38 | ||
| Willow | Shoots | 0.16 | Chopped | |
| Nges and Björnsson [112] | Sugar beet | Roots and tops | 0.343–0.383 | Various loading rates |
| Nges et al. [107] | Wheat | Straw | 0.250-0.299 | Dried and ground, different loading |
| Nizami and Murphy [92] | Ryegrass | Whole crop | 0.363–0.451 | Range of loading rates |
| Pohl et al. [81] | Wheat | Straw | 0.127–0.180 | Two-phase, meso- and thermophilic |
| Pohl et al. [117] | Wheat | Straw | 0.144–0.207 | Two-phase, meso- and thermophilic |
| Rincón et al. [116] | Winter wheat | Whole crop | 0.334 | Ensiled |
| Scherer et al. [111] | Fodder beet | Beet | 0.401 | |
| Veluchamy et al. [115] | Maize | Whole crop | 0.360–0.410 | Ensiled |
| Zhu et al. [113] | Potato | Tubers | 0.387 | |
| The following values are not included further because they are not available (cannot be derived) on a VS basis. | ||||
| Methane yield (m3 kg−1 TSadded) | ||||
| Stewart et al. [110] | Grass | Whole crop | 0.217–0.292 | |
| Grass/clover mix | Whole crop | 0.278 | ||
| Kale | Tops | 0.179–0.304 | Fresh and ensiled | |
| Lucerne | Whole crop | 0.248–0.390 | ||
| Maize | Tops | 0.231 | ||
| Oats | Whole crop | 0.227–0.257 | ||
| Wheat | Straw | 0.245 | ||
| Barley | Straw | 0.128–0.162 | Chopped and ground | |
| Potato | Tuber waste | 0.350–0.410 | ||
Appedix B. Calculated Methane Yields
| Reference | Crops | Crop Parts | Methane Yield (m3 kg−1 VSadded) | Comments |
|---|---|---|---|---|
| Linke et al. [121] | Barley | Whole crop | 0.420 | |
| Fodder beet | Leaves | 0.430 | ||
| Beet | 0.411 | |||
| Forage rye | Whole crop | 0.431 | ||
| Grass | Whole crop | 0.433 | ||
| Hemp | Whole crop | 0.409 | ||
| Lucerne | Whole crop | 0.432 | ||
| Maize | Whole crop | 0.422 | ||
| Rye | Grain | 0.419 | ||
| Straw | 0.409 | |||
| LfL [123] | Barley | Straw | 0.196 | |
| Beans | Whole crop | 0.277 | Green | |
| Whole crop | 0.286 | Silage | ||
| Clover grass | Whole crop | 0.284–0.325 | Various harvest dates | |
| Grass | Whole crop | 0.296–0.333 | Various harvest dates; Wet silage | |
| Whole crop | 0.296–0.340 | Various harvest dates; Wilted silage | ||
| Grass hay | Whole crop | 0.250–0.307 | Various harvest dates | |
| Lucerne | Whole crop | 0.265–0.308 | Various harvest dates | |
| Maize | Whole crop | 0.287–0.328 | Various harvest dates | |
| Meadow grass | Whole crop | 0.290–0.322 | Various harvest dates | |
| Mustard | Whole crop | 0.358–0.368 | Various harvest dates | |
| Oat | Straw | 0.197 | ||
| Grain | 0.322 | |||
| Oilseed rape | Whole crop | 0.341–0.356 | Various harvest dates; Green | |
| Whole crop | 0.361–0.376 | Various harvest dates; Silage | ||
| Oilseed rape | Straw | 0.129 | ||
| Seed | 0.504 | |||
| Pasture grass | Whole crop | 0.307–0.322 | Various harvest dates | |
| Red clover | Whole crop | 0.278–0.316 | Various harvest dates | |
| Rye | Straw | 0.179 | ||
| Grain | 0.365 | |||
| Whole crop | 0.261 | Silage | ||
| Ryegrass | Whole crop | 0.287–0.320 | Various harvest dates | |
| Sainfoin | Whole crop | 0.267–0.292 | Various harvest dates | |
| Sugar beet | Tops | 0.321–0.331 | Various harvest dates | |
| Wheat | Straw | 0.187 | ||
| Grain | 0.370 | |||
| Whole crop | 0.262 | Silage |
Appendix C. Methane Values Reported without Methods
| Reference | Crops | Crop Parts | Methane Yield (m3 kg−1 VSadded) | Comments |
|---|---|---|---|---|
| Koettner [125] | Milled grain | 0.37 | ||
| Grass | 0.32 | Silage | ||
| Maize | 0.32 | Silage | ||
| Meadow grass | 0.32 | |||
| Rape seed cake | 0.46 | |||
| Vegetable residues | 0.38 | |||
| KTBL [126,128] | Fodder beet | Beet | 0.364–0.496 | |
| Tops | 0.312 | Silage | ||
| Hemp | Whole crop | 0.143 | ||
| Maize | Whole crop | 0.304 | ||
| Rape seed cake | 0.439 | |||
| Rye | Whole crop | 0.313–0.319 | Fresh and ensiled | |
| Sugar beet | Beet | 0.357 | ||
| Tops | 0.312 | Silage | ||
| Wheat | Grain | 0.33 | ||
| Straw | 0.138 | |||
| Weiland [129] | Barley | Whole crop | 0.36 | Silage |
| Clover | Whole crop | 0.35 | Silage | |
| Fodder beet | Beet and leaf | 0.456 | Silage | |
| Kale | Whole crop | 0.255 | Silage | |
| Lucerne | Whole crop | 0.41 | Silage | |
| Maize | Whole crop | 0.41 | Silage | |
| Oilseed rape | Whole crop | 0.34 | Silage | |
| Potato | Tubers | 0.276 | Silage | |
| Ryegrass | Whole crop | 0.41 | Silage | |
| Wheat | Whole crop | 0.39 | Silage |
References
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Achinas, S.; Euverink, G.J.W. Rambling facets of manure-based biogas production in Europe: A briefing. Renew. Sustain. Energy Rev. 2020, 119, 109566. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total Environ. 2020, 728, 138224. [Google Scholar] [CrossRef]
- Jeung, J.H.; Chung, W.J.; Chang, S.W. Evaluation of anaerobic co-digestion to enhance the efficiency of livestock manure anaerobic digestion. Sustainability 2019, 11, 7170. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Kumar Khanal, S. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from anaerobic digestion as an energy vector: Current upgrading development. Energies 2021, 14, 2742. [Google Scholar] [CrossRef]
- Spence, A.; Blanco Madrigal, E.; Patil, R.; Bajón Fernández, Y. Evaluation of anaerobic digestibility of energy crops and agricultural by-products. Bioresour. Technol. Rep. 2019, 5, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to energy: A focus on the impact of substrate type in biogas production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Frigon, J.C.; Guiot, S.R. Biomethane production from starch and lignocellulosic crops: A comparative review. Biofuels Bioprod. Biorefin. 2010, 4, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Gunaseelan, V.N. Anaerobic digestion of biomass for methane production: A review. Biomass Bioenergy 1997, 13, 83–114. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, E. Biogas production from different lignocellulosic biomass sources: Advances and perspectives. 3 Biotech 2018, 8, 233. [Google Scholar] [CrossRef]
- Dar, R.A.; Parmar, M.; Dar, E.A.; Sani, R.K.; Phutela, U.G. Biomethanation of agricultural residues: Potential, limitations and possible solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Lesteur, M.; Bellon-Maurel, B.; Gonzalez, C.; Latrille, E.; Roger, J.M.; Junqua, G.; Steyera, J.P. Alternative methods for determining anaerobic biodegradability: A review. Process Biochem. 2010, 45, 431–440. [Google Scholar] [CrossRef]
- Murphy, J.; Braun, R.; Weiland, P.; Wellinger, A. Biogas from Crop Digestion. IEA Bioenergy: Cork, Ireland, 2011. [Google Scholar]
- Raposo, F.; De la Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Kulichkova, G.I.; Ivanova, T.S.; Koettner, M.; Volodko, O.I.; Spivak, S.I.; Tsygankov, S.P.; Blume, Y.B. Plant feedstocks and their biogas production potentials. Open Agric. J. 2020, 14, 219–234. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Nizami, A.-S.; Murphy, J.D. What type of digester configurations should be employed to produce biomethane from grass silage? Renew. Sustain. Energy Rev. 2010, 14, 1558–1568. [Google Scholar] [CrossRef]
- Pererva, Y.; Miller, C.D.; Sims, R.C. Existing empirical kinetic models in biochemical methane potential (BMP) testing, their selection and numerical solution. Water 2020, 12, 1831. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Ibelli-Bianco, C. Predictive regression models for biochemical methane potential tests of biomass samples: Pitfalls and challenges of laboratory measurements. Renew. Sustain. Energy Rev. 2020, 127, 109890. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Frunzo, L.; Liotta, F.; Panico, A.; Pirozzi, F. Bio-methane potential tests to measure the biogas production from the digestion and co-digestion of complex organic substrates. Open Environ. Eng. J. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Weinrich, S.; Schäfer, F.; Bochmann, G.; Liebetrau, J. Value of Batch Tests for Biogas Potential Analysis. In Method Comparison and Challenges of Substrate and Efficiency Evaluation of Biogas Plants; IEA Bioenergy Task 37; University College Cork: Cork, Ireland, 2018; ISBN 978-1-910154-49-6. [Google Scholar]
- Mittweg, G.; Oechsner, H.; Hahn, V.; Lemmer, A.; Reinhardt-Hanisch, A. Repeatability of a laboratory batch method to determine the specific biogas and methane yields. Eng. Life Sci. 2012, 12, 270–278. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and limitations of biochemical methane potential (BMP) tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Amodeo, C.; Hafner, S.D.; Teixeira Franco, R.; Benbelkacem, H.; Moretti, P.; Bayard, R.; Buffière, P. How different are manometric, gravimetric, and automated volumetric BMP results? Water 2020, 12, 1839. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Holliger, C.; Astals, S.; Fruteau de Laclos, H.; Hafner, S.D.; Koch, K.; Weinrich, S. Towards a standardization of biomethane potential tests: A commentary. Water Sci. Technol. 2021, 83, 247–250. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Zhang, Y.; Kusch-Brandt, S.; Heaven, S.; Banks, C.J. Effect of pasteurisation on methane yield from food waste and other substrates in anaerobic digestion. Processes 2020, 8, 1351. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; Mc Carty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Jerger, D.E.; Chynoweth, D.P.; Isaacson, H.R. Anaerobic digestion of sorghum biomass. Biomass 1987, 14, 99–113. [Google Scholar] [CrossRef]
- Zauner, E.; Küntzel, U. Methane production from ensiled plant material. Biomass 1986, 10, 207–223. [Google Scholar] [CrossRef]
- Petersson, A.; Thomsen, M.H.; Hauggaard-Nielsen, H.; Thomsen, A.-B. Potential bioethanol and biogas production using lignocellulosic biomass from winter rye, oilseed rape and faba bean. Biomass Bioenergy 2007, 31, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.L.; Schmidt, J.E.; Angelidaki, I.; Marca, E.; la Cour Jansen, J.; Mosbæk, H.; Christensen, T.H. Method for determination of methane potentials of solid organic waste. Waste Manag. 2004, 24, 393–400. [Google Scholar] [CrossRef]
- Kandel, T.P.; Sutaryo, S.; Møller, H.B.; Jørgensen, U.; Lærke, P.E. Chemical composition and methane yield of reed canary grass as influenced by harvesting time and harvest frequency. Bioresour. Technol. 2013, 130, 659–666. [Google Scholar] [CrossRef]
- Kandel, T.P.; Ward, A.J.; Elsgaard, L.; Møller, H.B.; Lærke, P.E. Methane yield from anaerobic digestion of festulolium and tall fescue cultivated on a fen peatland under different harvest managements. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 670–677. [Google Scholar] [CrossRef]
- Feng, L.; Kristensen, E.F.; Moset, V.; Ward, A.J.; Møller, H.B. Ensiling of tall fescue for biogas production: Effect of storage time, additives and mechanical pretreatment. Energy Sustain. Dev. 2018, 47, 143–148. [Google Scholar] [CrossRef]
- Wahid, R.; Feng, L.; Cong, W.-F.; Ward, A.J.; Møller, H.B.; Eriksen, J. Anaerobic mono-digestion of lucerne, grass and forbs—Influence of species and cutting frequency. Biomass Bioenergy 2018, 109, 199–208. [Google Scholar] [CrossRef]
- Wahid, R.; Nielsen, S.F.; Hernandez, V.M.; Ward, A.J.; Gislum, R.; Jørgensen, U.; Møller, H.B. Methane production potential from Miscanthus sp.: Effect of harvesting time, genotypes and plant fractions. Biosyst. Eng. 2015, 133, 71–80. [Google Scholar] [CrossRef]
- Jurado, E.; Gavala, H.N.; Skiadas, I.V. Enhancement of methane yield from wheat straw, miscanthus and willow using aqueous ammonia soaking. Environ. Technol. 2013, 34, 2069–2075. [Google Scholar] [CrossRef]
- Kakuk, B.; Bagi, Z.; Rákhely, G.; Maróti, G.; Dudits, D.; Kovács, K.L. Methane production from green and woody biomass using short rotation willow genotypes for bioenergy generation. Bioresour. Technol. 2021, 333, 125223. [Google Scholar] [CrossRef]
- German Standard Methods for the Examination of Water, Waste Water and Sludge; Sludge and Sediments (Group S); DIN 38414-8; Determination of the Amenability to Anaerobic Digestion (S 8); Deutsches Institut Fuer Normung e.V.: Berlin, Germany, 1985.
- Fermentation of Organic Materials. Characterisation of the Substrates, Sampling, Collection of Material Data, Fermentation Tests; VDI 4630; VDI-Handbuch Energietechnik, Issue 2006; VDI: Duesseldorf, Germany, 2006.
- Fermentation of Organic Materials. Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; VDI 4630 (updated 2016); VDI-Handbuch Energietechnik, Issue 2016; VDI: Duesseldorf, Germany, 2016.
- Linke, B.; Heiermann, M.; Grundmann, P.; Hertwig, P. Grundlagen, Verfahren und Potenzial der Biogasgewinnung im Land Brandenburg. In Biogas in der Landwirtschaft—Leitfaden für Landwirte und Investoren im Land Brandenburg, 2nd ed.; Heiermann, M., Plöchl, M., Eds.; Ministerium für Landwirtschaft Umweltschutz und Raumordnung des Landes Brandenburg: Potsdam, Germany, 2003; pp. 10–23. [Google Scholar]
- Gallegos, D.; Wedwitschka, H.; Moeller, L.; Zehnsdorf, A.; Stinner, W. Effect of particle size reduction and ensiling fermentation on biogas formation and silage quality of wheat straw. Bioresour. Technol. 2017, 245, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef]
- Machmüller, A.; Hrbek, R.; Kryvoruchko, V.; Amon, T. Biogas Production from Energy Crops produced on Sustainable Crop Rotations. In Proceedings of the 15th European Biomass Conference, Berlin, Germany, 7–11 May 2007. [Google Scholar]
- Bauer, A.; Leonhartsberger, C.; Bösch, P.; Amon, B.; Friedl, A.; Amon, T. Analysis of methane yields from energy crops and agricultural by-products and estimation of energy potential from sustainable crop rotation systems in EU-27. Clean Technol. Environ. Policy 2010, 12, 153–161. [Google Scholar] [CrossRef]
- Heidarzadeh Vazifehkhoran, A.; Triolo, J.M.; Larsen, S.U.; Stefanek, K.; Sommer, S.G. Assessment of the variability of biogas production from sugar beet silage as affected by movement and loss of the produced alcohols and organic acids. Energies 2016, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Lemaigre, S.; Ruf, T.; Delfosse, P.; Emmerling, C. Miscanthus as biogas feedstock: Influence of harvest time and stand age on the biochemical methane potential (BMP) of two different growing seasons. Biomass Convers. Biorefinery 2018, 8, 245–254. [Google Scholar] [CrossRef]
- Helffrich, D.; Oechsner, H. Hohenheim biogas yield test—Comparing various laboratory methods on biomass fermentation. Landtechnik 2003, 58, 148–149. [Google Scholar]
- Haag, N.L.; Nägele, H.J.; Reiss, K.; Biertümpfel, A.; Oechsner, H. Methane formation potential of cup plant (Silphium perfoliatum). Biomass Bioenergy 2015, 75, 126–133. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Ruf, T.; Emmerling, C. Site-adapted production of bioenergy feedstocks on poorly drained cropland through the cultivation of perennial crops. A feasibility study on biomass yield and biochemical methane potential. Biomass Bioenergy 2018, 119, 429–435. [Google Scholar] [CrossRef]
- Hafner, S.D.; de Laclos, H.F.; Koch, K.; Holliger, C. Improving inter-laboratory reproducibility in measurement of biochemical methane potential (BMP). Water 2020, 12, 1752. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; Da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from fresh spring and summer grass: Effect of the harvesting period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Lemaigre, S.; Delfosse, P.; von Francken-Welz, H.; Emmerling, C. Biochemical methane potential (BMP) of six perennial energy crops cultivated at three different locations in W-Germany. Biomass Convers. Biorefin. 2018, 8, 873–888. [Google Scholar] [CrossRef]
- Pouech, P.; Fruteau, H.; Bewa, H. Agricultural crops for biogas production on anaerobic digestion plants. In Biomass for Energy and Industry, Proceeding of the International Conference, Würzburg, Germany, 8–11 June 1998; C.A.R.M.E.N.: Straubing, Germany, 1998; pp. 163–165. [Google Scholar]
- Lehtomäki, A.; Björnsson, L. Two-stage anaerobic digestion of energy crops: Methane production, nitrogen mineralisation and heavy metal mobilisation. Environ. Technol. 2006, 27, 209–218. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Huttunena, S.; Rintala, J. Laboratory investigations on co-digestion of energy crops and crop residues with cow manure for methane production: Effect of crop to manure ratio. Resour. Conserv. Recycl. 2007, 51, 591–609. [Google Scholar] [CrossRef]
- Seppälä, M.; Paavola, T.; Lehtomäki, A.; Rintala, J. Biogas production from boreal herbaceous grasses—Specific methane yield and methane yield per hectare. Bioresour. Technol. 2009, 100, 2952–2958. [Google Scholar] [CrossRef]
- Kaparaju, P.; Luostarinen, S.; Kalmari, E.; Kalmari, J.; Rintala, J. Co-digestion of energy crops and industrial confectionery by-products with cow manure: Batch-scale and farm-scale evaluation. Water Sci. Technol. 2002, 45, 275–280. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Viinikainen, T.; Rintala, J. Screening boreal energy crops and crop residues for methane biofuel production. Biomass Bioenergy 2008, 32, 541–550. [Google Scholar] [CrossRef]
- Parawira, W.; Murto, M.; Zvauya, R.; Mattiasson, B. Anaerobic batch digestion of solid potato waste alone and in combination with sugar beet leaves. Renew. Energy 2004, 29, 1811–1823. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Department of Biological and Environmental Science, University of Jyväskylä, Jyväskylä, Finland. Personal Communication, November 2011.
- Garcia, N.H.; Mattioli, A.; Gil, A.; Frison, N.; Battista, F.; Bolzonella, D. Evaluation of the methane potential of different agricultural and food processing substrates for improved biogas production in rural areas. Renew. Sustain. Energy Rev. 2019, 112, 1–10. [Google Scholar] [CrossRef]
- Cornell, M.; Heaven, S.; Banks, C. Impact of the addition of maize on the anaerobic digestion of cattle slurry. In Proceedings of the 5th IWA International Symposium on Anaerobic Digestion of Solid Wastes and Energy Crops, Hammamet, Tunisia, 25–28 May 2008. [Google Scholar]
- Rincón, B.; Banks, C.J.; Heaven, S. Biochemical methane potential of winter wheat (Triticum aestivum L.): Influence of growth stage and storage practice. Bioresour. Technol. 2010, 101, 8179–8184. [Google Scholar] [CrossRef] [Green Version]
- Rincón, B.; Heaven, S.; Salter, A.M.; Banks, C.J. Anaerobic digestion of spring and winter wheat: Comparison of net energy yields. J. Environ. Sci. Health Part A 2016, 51, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.; Zhang, Y.; Heaven, S.; Banks, C. Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes. Bioresour. Technol. 2009, 100, 6339–6346. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Li, C.; Liu, J.; Yi, Z.; Han, Y. Changes in composition, cellulose degradability and biochemical methane potential of Miscanthus species during the growing season. Bioresour. Technol. 2017, 235, 389–395. [Google Scholar] [CrossRef]
- Virkajärvi, P.; Hyrkäs, M.; Kykkänen, S.; Pyykkönen, V.; Laakso, J.; Kekkonen, P.; Ervasti, S.; Luostarinen, S. Effect of digestate application, N fertilisation and forage species on biomass and biogas production. Sustainable meat and milk production from grasslands 2018. In Proceedings of the 27th General Meeting of the European Grassland Federation, Cork, Ireland, 17–21 June 2018; pp. 688–690. [Google Scholar]
- Nges, I.A.; Li, C.; Wang, B.; Xiao, L.; Yi, Z.; Liu, J. Physio-chemical pretreatments for improved methane potential of Miscanthus lutarioriparius. Fuel 2016, 166, 29–35. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Nges, I.A.; Liu, J. Enhanced biomethane production from miscanthus lutarioriparius using steam explosion pretreatment. Fuel 2016, 179, 267–273. [Google Scholar] [CrossRef]
- Thomas, H.L.; Arnoult, S.; Brancourt-Hulmel, M.; Carrère, H. Methane production variability according to miscanthus genotype and alkaline pretreatments at high solid content. BioEnergy Res. 2019, 12, 325–337. [Google Scholar] [CrossRef]
- Pohl, M.; Mumme, J.; Heeg, K.; Nettmann, E. Thermo-and mesophilic anaerobic digestion of wheat straw by the upflow anaerobic solid-state (UASS) process. Bioresour. Technol. 2012, 124, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Heeg, K.; Pohl, M.; Sontag, M.; Mumme, J.; Klocke, M.; Nettmann, E. Microbial communities involved in biogas production from wheat straw as the sole substrate within a two-phase solid-state anaerobic digestion. Syst. Appl. Microbiol. 2014, 37, 590–600. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Potential of bioethanol production waste for methane recovery. Energy 2019, 173, 133–139. [Google Scholar] [CrossRef]
- Ohlsson, J.A.; Harman-Ware, A.E.; Sandgren, M.; Schnürer, A. Biomass recalcitrance in willow under two biological conversion paradigms: Enzymatic hydrolysis and anaerobic digestion. BioEnergy Res. 2020, 13, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Linke, B.; Schelle, H. Solid state anaerobic digestion of organic wastes. In Proceedings of the Conference Agricultural Engineering into the Third Millenium, AgEng2000, Coventry, UK, 2–7 July 2000. [Google Scholar]
- Heiermann, M.; Plöchl, M.; Linke, B.; Schelle, H. Biogas production in agriculture by co-fermentation of energy crops. Asp. Appl. Biol. 2001, 65, 65–70. [Google Scholar]
- Heiermann, M.; Plöchl, M.; Linke, B.; Schelle, H. Preliminary evaluation of some cereals as energy crops for biogas production. In Proceedings of the World Renewable Energy Congress VII (WREC), Cologne, Germany, 29 June–5 July 2002. [Google Scholar]
- Heiermann, M.; Plöchl, M. Crops—A big potential for biogas production. In Proceedings of the World Renewable Energy Congress VIII, Denver, CO, USA, 28 August–3 September 2004. [Google Scholar]
- Badger, D.M.; Bogue, M.J.; Stewart, D.J. Biogas production from crops and organic wastes 1: Results of batch digestions. N. Z. J. Sci. 1979, 22, 11–20. [Google Scholar]
- Zubr, J. Methanogenic fermentation of fresh and ensiled plant materials. Biomass 1986, 11, 159–171. [Google Scholar] [CrossRef]
- Svensson, L.M.; Christensson, K.; Björnsson, L. Biogas production from crop residues on a farm-scale level: Is it economically feasible under conditions in Sweden. Bioprocess Biosyst. Eng. 2005, 28, 139–148. [Google Scholar] [CrossRef]
- Nizami, A.-S.; Murphy, J.D. Optimizing the operation of a two-phase anaerobic digestion system digesting grass silage. Environ. Sci. Technol. 2011, 45, 7561–7569. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Banks, C.J.; Siegert, I.; Heaven, S.; Borja, R. Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests. Process Biochem. 2006, 41, 1444–1450. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, C.; Zhang, J.; Zhang, R.; Xue, C.; Liu, G.; Chen, C. Study on biomethane production and biodegradability of different leafy vegetables in anaerobic digestion. AMB Express 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilvikiene, V.; Kadziuliene, Z.; Dabkevicius, Z.; Venslauskas, K.; Navickas, K. Feasibility of tall fescue, cocksfoot and reed canary grass for anaerobic digestion: Analysis of productivity and energy potential. Ind. Crops Prod. 2016, 84, 87–96. [Google Scholar] [CrossRef]
- Kaiser, F.; Diepolder, M.; Eder, J.; Hartmann, S.; Prestele, H.; Gerlach, R.; Ziehfreund, G.; Gronauer, A. Ertragspotenziale verschiedener nachwachsender Rohstoffe in landwirtschaftlichen Biogasanlagen. In Biogas in Bayern; Bayerische Landesanstalt für Landwirtschaft: Freising, Germany, 2004; pp. 43–56. [Google Scholar]
- Mähnert, P.; Heiermann, M.; Linke, B. Batch- and semi-continuous biogas production from different grass species. Agric. Eng. Int. CIGR Ej. 2005, 7, 1–11. [Google Scholar]
- Herrmann, C.; Heiermann, M.; Idler, C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 2011, 102, 5153–5161. [Google Scholar] [CrossRef]
- Herrmann, C.; Heiermann, M.; Idler, C.; Prochnow, A. Particle size reduction during harvesting of crop feedstock for biogas production I: Effects on ensiling process and methane yields. Bioenergy Res. 2012, 5, 926–936. [Google Scholar] [CrossRef]
- Herrmann, C.; Idle, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Kreuger, E.; Nges, I.A.; Björnsson, L. Ensiling of crops for biogas production: Effects on methane yield and total solids determination. Biotechnol. Biofuels 2011, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Gissén, C.; Prade, T.; Kreuger, E.; Nges, I.A.; Rosenqvist, H.; Svensson, S.E.; Lantz, M.; Mattsson, J.E.; Börjesson, P.; Björnsson, L. Comparing energy crops for biogas production—Yields, energy input and costs in cultivation using digestate and mineral fertilisation. Biomass Bioenergy 2014, 64, 199–210. [Google Scholar] [CrossRef]
- Bruni, E.; Jensen, A.P.; Pedersen, E.S.; Angelidaki, I. Anaerobic digestion of maize focusing on variety, harvest time and pre-treatment. Appl. Energy 2010, 87, 2212–2217. [Google Scholar] [CrossRef]
- Kreuger, E.; Prade, T.; Escobar, F.; Svensson, S.-E.; Englund, J.-E.; Björnsson, L. Anaerobic digestion of industrial hemp—Effect of harvest time on methane energy yield per hectare. Biomass Bioenergy 2011, 35, 893–900. [Google Scholar] [CrossRef]
- Prade, T.; Svensson, S.E.; Hörndahl, T.; Kreuger, E. Impact of harvest date and cutting length of grass ley and whole-crop cereals on methane yield and economic viability as feedstock for biogas vehicle fuel production. Bioenergy Res. 2019, 12, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Allen, E.; Wall, D.M.; Herrmann, C.; Murphy, J.D. A detailed assessment of resource of biomethane from first, second and third generation substrates. Renew. Energy 2016, 87, 656–665. [Google Scholar] [CrossRef]
- Nges, I.A.; Wang, B.; Cui, Z.; Liu, J. Digestate liquor recycle in minimal nutrients-supplemented anaerobic digestion of wheat straw. Biochem. Eng. J. 2015, 94, 106–114. [Google Scholar] [CrossRef]
- Kolbl, S.; Paloczi, A.; Panjan, J.; Stres, B. Addressing case specific biogas plant tasks: Industry oriented methane yields derived from 5 L Automatic Methane Potential Test Systems in batch or semi-continuous tests using realistic inocula, substrate particle sizes and organic loading. Bioresour. Technol. 2014, 153, 180–188. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, K.; Zhang, Y.; Zheng, Z.; Cai, Y.; Wen, B.; Cui, Z.; Wang, X. Improving the methane yield of maize straw: Focus on the effects of pretreatment with fungi and their secreted enzymes combined with sodium hydroxide. Bioresour. Technol. 2018, 250, 204–213. [Google Scholar] [CrossRef]
- Stewart, D.J.; Bogue, M.J.; Badger, D.M. Biogas production from crops and organic wastes 2: Results from continuous digestion tests. N. Z. J. Sci. 1984, 27, 285–294. [Google Scholar]
- Scherer, P.A.; Dobler, S.; Rohardt, S.; Loock, R.; Büttner, B.; Nöldeke, P.; Brettschuh, A. Continuous biogas production from fodder beet silage as sole substrate. Water Sci. Technol. 2003, 48, 229–233. [Google Scholar] [CrossRef]
- Nges, I.A.; Björnsson, L. High methane yields and stable operation during anaerobic digestion of nutrient-supplemented energy crop mixtures. Biomass Bioenergy 2012, 47, 62–70. [Google Scholar] [CrossRef]
- Zhu, H.; Stadnyk, A.; Béland, M.; Seto, P. Co-production of hydrogen and methane from potato waste using a two-stage anaerobic digestion process. Bioresour. Technol. 2008, 99, 5078–5084. [Google Scholar] [CrossRef] [PubMed]
- Kakuk, B.; Kovács, K.L.; Szuhaj, M.; Rákhely, G.; Bagi, Z. Adaptation of continuous biogas reactors operating under wet fermentation conditions to dry conditions with corn stover as substrate. Anaerobe 2017, 46, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veluchamy, C.; Gilroyed, B.H.; Kalamdhad, A.S. Process performance and biogas production optimizing of mesophilic plug flow anaerobic digestion of corn silage. Fuel 2019, 253, 1097–1103. [Google Scholar] [CrossRef]
- Rincón, B.; Heaven, S.; Banks, C.J.; Zhang, Y. Anaerobic digestion of whole-crop winter wheat silage for renewable energy production. Energy Fuels 2012, 26, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Pohl, M.; Heeg, K.; Mumme, J. Anaerobic digestion of wheat straw—Performance of continuous solid-state digestion. Bioresour. Technol. 2013, 146, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Symons, G.E.; Buswell, A.M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Buswell, A.M.; Hatfield, W.D. Anaerobic Fermentation—Bulletin 32; State of Illinois Department of Registration and Education: Urbana, IL, USA, 1936. [Google Scholar]
- Czepuck, K.; Oechsner, H.; Schumacher, B.; Lemmer, A. Hohenheim biogas yield test. Landtechnik 2006, 61, 82–83. [Google Scholar]
- Linke, B.; Baganz, K.; Schlauderer, R. Nutzung von Feldfrüchten zur Biogasgewinnung. Agaratechnische Forsch. 1999, 5, 81–90. [Google Scholar]
- DLG (Ed.) Futterwerttabellen—Wiederkäuer; DLG-Verlag: Frankfurt, Germany, 1997. [Google Scholar]
- LfL (Bayerische Landesanstalt für Landwirtschaft). Biogasausbeuten Verschiedener Substrate (Database). Available online: https://www.lfl.bayern.de/iba/energie/049711/ (accessed on 6 June 2021).
- Rodrigues, R.P.; Rodrigues, D.P.; Klepacz-Smolka, A.; Martins, R.C.; Quina, M.J. Comparative analysis of methods and models for predicting biochemical methane potential of various organic substrates. Sci. Total Environ. 2019, 649, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Koettner, M. The role of biogas in German farm business development. In Proceedings of the Bioexell European Biogas Conference—Biogas in Society, Enniskillen, UK, 21–23 October 2004. [Google Scholar]
- KTBL (Ed.) Betriebsplanung Landwirtschaft 2008/09; KTBL: Darmstadt, Germany, 2009. [Google Scholar]
- Karpenstein-Machan, M. Energiepflanzenbau für Biogasanlagenbetreiber; DLG Verlag: Frankfurt, Germany, 2005. [Google Scholar]
- KTBL (Ed.) Wirtschaftlichkeitsrechner Biogas. Available online: https://daten.ktbl.de/biogas/ (accessed on 6 June 2021).
- Weiland, P. Production and energetic use of biogas from energy crops and wastes in Germany. Appl. Biochem. Biotechnol. 2003, 109, 263–274. [Google Scholar] [CrossRef]
- Hafner, S.D.; Astals, S. Systematic error in manometric measurement of biochemical methane potential: Sources and solutions. Waste Manag. 2019, 91, 147–155. [Google Scholar] [CrossRef]
- Müller, W.-R.; Frommert, I.; Jörg, R. Standardized methods for anaerobic biodegradability testing. Rev. Environ. Sci. Biotechnol. 2004, 3, 141–158. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Snieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S.; Graban, L.; Lajszner, W. Short rotation coppices, grasses and other herbaceous crops: Biomass properties versus 26 genotypes and harvest time. Ind. Crops Prod. 2018, 119, 22–32. [Google Scholar] [CrossRef]
- White, P.M.; Viator, R.P.; Webber, C.L. Temporal and varietal variation in sugarcane post-harvest residue biomass yields and chemical composition. Ind. Crops Prod. 2020, 154, 112616. [Google Scholar] [CrossRef]
- Butkute, B.; Lemežienė, N.; Kanapeckas, J.; Navickas, K.; Dabkevičius, Z.; Venslauskas, K. Cocksfoot, tall fescue and reed canary grass: Dry matter yield, chemical composition and biomass convertibility to methane. Biomass Bioenergy 2014, 66, 1–11. [Google Scholar] [CrossRef]
- Ierna, A.; Sortino, O.; Mauromicale, G. Biomass, seed and energy yield of Cynara cardunculus L. as affected by environment and season. Agronomy 2020, 10, 1548. [Google Scholar] [CrossRef]
- Bochmann, G.; Montgomery, L.F.R. Storage and pre-treatment of substrates for biogas production. In The Biogas Handbook; Woodhead Publishing Series in Energy; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Oxford, UK, 2013; pp. 85–103. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.; Lidén, G. Semi-continuous co-digestion of solid slaughterhouse waste, manure, and fruit and vegetable waste. Renew. Energy 2008, 33, 726–734. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Bio-methanization of energy crops through mono-digestion for continuous production of renewable biogas. Renew. Energy 2009, 34, 2940–2945. [Google Scholar] [CrossRef]
- Mähnert, P.; Linke, B. Kinetic study of biogas production from energy crops and animal waste slurry: Effect of organic loading rate and reactor size. Environ. Technol. 2009, 30, 93–99. [Google Scholar] [CrossRef]
- Pizarro-Loaiza, C.A.; Torres-Lozada, P.; Illa, J.; Palatsi, J.; Bonmatí, A. Effect of harvesting age and size reduction in the performance of anaerobic digestion of Pennisetum grass. Processes 2020, 8, 1414. [Google Scholar] [CrossRef]
- Brauer-Siebrecht, W.; Jacobs, A.; Christen, O.; Götze, P.; Koch, H.-J.; Rücknagel, J.; Märländer, B. Silage maize and sugar beet for biogas production in rotations and continuous cultivation: Dry matter and estimated methane yield. Agronomy 2016, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Wannasek, L.; Ortner, M.; Kaul, H.-P.; Amon, B.; Amon, T. Double-cropping systems based on rye, maize and sorghum: Impact of variety and harvesting time on biomass and biogas yield. Eur. J. Agron. 2019, 110, 125934. [Google Scholar] [CrossRef]
- Graß, R.; Malec, S.; Wachendorf, M. Biomass performance and competition effects in an established temperate agroforestry system of willow and grassland—Results of the 2nd rotation. Agronomy 2020, 10, 1819. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kusch-Brandt, S.; Salter, A.M.; Heaven, S. Estimating the Methane Potential of Energy Crops: An Overview on Types of Data Sources and Their Limitations. Processes 2021, 9, 1565. https://doi.org/10.3390/pr9091565
Zhang Y, Kusch-Brandt S, Salter AM, Heaven S. Estimating the Methane Potential of Energy Crops: An Overview on Types of Data Sources and Their Limitations. Processes. 2021; 9(9):1565. https://doi.org/10.3390/pr9091565
Chicago/Turabian StyleZhang, Yue, Sigrid Kusch-Brandt, Andrew M. Salter, and Sonia Heaven. 2021. "Estimating the Methane Potential of Energy Crops: An Overview on Types of Data Sources and Their Limitations" Processes 9, no. 9: 1565. https://doi.org/10.3390/pr9091565
APA StyleZhang, Y., Kusch-Brandt, S., Salter, A. M., & Heaven, S. (2021). Estimating the Methane Potential of Energy Crops: An Overview on Types of Data Sources and Their Limitations. Processes, 9(9), 1565. https://doi.org/10.3390/pr9091565








