Abstract
The m.8993T>G mutation of the mitochondrial MT-ATP6 gene is associated with NARP syndrome (neuropathy, ataxia and retinitis pigmentosa). The equivalent point mutation introduced in yeast Saccharomyces cerevisiae mitochondrial DNA considerably reduced the activity of ATP synthase and of cytochrome-c-oxidase, preventing yeast growth on oxidative substrates. The overexpression of the mitochondrial oxodicarboxylate carrier (Odc1p) was able to rescue the growth on the oxidative substrate by increasing the substrate-level phosphorylation of ADP coupled to the conversion of α-ketoglutarate (AKG) into succinate with an increase in Complex IV activity. Previous studies showed that equivalent point mutations in ATP synthase behave similarly and can be rescued by Odc1p overexpression and/or the uncoupling of OXPHOS from ATP synthesis. In order to better understand the mechanism of the ATP synthase mutation bypass, we developed a core model of mitochondrial metabolism based on AKG as a respiratory substrate. We describe the different possible metabolite outputs and the ATP/O ratio values as a function of ATP synthase inhibition.
1. Introduction
Mitochondria support aerobic respiration and produce most of the cellular ATP by oxidative phosphorylation, i.e., the coupling of a series of redox reactions and the electron transport chain (ETC) with ATP synthase through a transmembraneous proton gradient. The ETC is mainly fed by the NADH and succinate generated in the TCA cycle and a few other possible dehydrogenases.
ATP synthase organizes into a matrix domain (F1 inside mitochondria) where ATP is synthesized and a membrane-embedded domain (Fo) moves protons across the membrane [1,2,3,4]. Dozens of point mutations in the mitochondrial MT-ATP6 gene (coded by the ATP6 gene in yeast mtDNA) have been identified as leading to deleterious neuromuscular disorders [5]. This gene codes for the subunit A in ATP synthase. It is in contact with the c-ring in the Fo membrane domain and has been proposed to optimize the transmembrane conduction of protons [6,7]. The m.8993T>G mutation of the mitochondrial MT-ATP6 gene affecting the mitochondrial energy transduction has been particularly studied [8]. This mutation has been associated in humans with numerous cases of neuropathy, ataxia and retinitis pigmentosa (NARP) and maternally-inherited Leigh syndrome.
The equivalent mutations introduced in the yeast Saccharomyces cerevisiae (NARP mutant) [9] dramatically slow down its respiratory growth. It was recently shown that this mutation prevents proton release in the mitochondrial matrix, strongly decreasing ATP synthesis to 10% of the wild type (WT) value [6]. Concomitantly with the ATP synthase defect, a decrease in the Complex IV amount was observed (the retrograde signaling pathway linking ATP synthase and Complex IV biogenesis [8,10,11]).
An additional molecule of ATP (in yeast) or GTP (in the liver) is produced in the TCA cycle by Succinyl-CoA synthetase, also named succinate thiokinase. It is called substrate-level phosphorylation (SLP) because it involves the transfer of a phosphate from a donor molecule (here, the succinyl-Pi) to ADP to form ATP. Following the pioneering work of Schwimmer et al. [10], a rescue of the NARP mutation in yeast was mediated through the mitochondrial carrier Odc1p overexpression [8]. Odc1p is a mitochondrial carrier that exchanges 2-oxoglutarate (AKG) against succinate, malate or citrate (and several other metabolites not a priori involved in the rescuing process) [12]. Odc1p overexpression presumably increases AKG uptake in mitochondria, promoting a subsequent increase in ATP synthesis by SLP (SLP-ATP) [10]. In both cases, the rescue is accompanied by an increase in the Complex IV amount without a full restoration of the WT value. A model of the interdependence between ATP synthase and the Complex IV amount via the increase in the mitochondrial membrane potential has been proposed in [8], in a relationship with mitochondrial membrane hyperpolarization (see also [13]).
An Odc1p carrier was identified and characterized by Palmieri et al. [12]. It operates by a strict counter-exchange mechanism. An Odc1p carrier can principally uptake 2-oxoglutarate against itself (112.5), succinate (19), malate (56) and citrate (28.6). The numbers in brackets indicate in mmol/min/g protein the rate of 2-oxoglutarate uptake in proteoliposomes preloaded with the corresponding metabolite (Table 2 in [12]). The diversity of the output catalyzed by Odc1p offers different metabolic solutions to enhance SLP-ATP. In order to have a better understanding of the rescue mechanism by Odc1p, we developed in this paper a metabolic model of yeast mitochondria that characterizes the different possible metabolic pathways bypassing oxidative phosphorylation ATP deficiency in isolated yeast mitochondria using α-ketoglutarate (AKG) as a respiratory substrate. We show that several metabolite outputs following an AKG input are possible and depend upon the activity of ATP synthase. We also show that the simple property of Odc1p as a transporter is not sufficient to explain the ATP synthase rescue. We propose a few new hypotheses to explore. Finally, we discuss the therapeutic application of this study in humans.
2. Materials and Methods
The model of mitochondrial metabolism was written with Copasi [14] and is detailed in Supplementary Material S1. It involves a relevant representation of the coupling between the respiratory chain and ATP synthesis through a chemiosmotic proton gradient ΔµH+ considered here as a pseudo-substrate called PMF (proton motive force). For those reactions that depend only on the ΔpH or on the Δψ component of the ΔµH+, we take, following Bohnensack [15], ΔpH = 0.2 PMF and Δψ = 0.8 PMF. In our model, the PMF, as postulated by Mitchell’s chemiosmotic theory [16], is the result at a steady state of the opposite activities of the respiratory chain on the one hand and of ATP synthase on the other hand, even though a few recent results indicate a more complex mechanism [17]. Copasi also allows the calculation of EFMs (elementary flux modes, i.e., the minimal set of fluxes at a steady state) [18,19] but with integer stoichiometric coefficients. This explains why a few reactions had high stoichiometric coefficients and why a few coefficients of the reactions in the EFMs were high (Supplementary Material S2). The kinetic parameters of the rate equations are listed in Supplementary Material S1.
The model includes the different exchanges catalyzed by Odc1p under the form of three reactions for the AKG input:
T2: AKGc + MALm = AKGm + MALc.
T22: AKGc + SUCCm = AKGm + SUCCc.
T23: AKGc + CITm = AKGm + CITc.
In addition, the dicarboxylate carrier (DIC) catalyzes the exchanges:
T31: MALm + Pic = MALc + Pim.
T33: SUCCm + Pic = SUCCc + Pim.
3. Results
The aim of the model was to simulate the experiments on isolated yeast mitochondria with 10 mM AKG as a respiratory substrate and to characterize all possible metabolic pathways inside mitochondria and metabolite outputs including ATP synthesis. We also studied the oxygen consumption flux and calculated the ATP/O ratio for which experimental results already exist [20].
In a first part, we will describe the elementary flux modes (EFMs), i.e., the minimal pathways at a steady state inside a metabolic network [18]. In this context, minimal means that the removal of a step prevents the establishment of a steady state. They either connect the input with output metabolites or are cycles at a steady state. Any set of fluxes at a steady state in a metabolic network can be written as a non-negative linear combination of EFMs [18,21].
3.1. Determination of EFMs with 2-Oxoglutarate (AKG) as a Respiratory Substrate
The EFMs are described in Supplementary Material S2. The direction of the carriers in the model were such that only AKG was allowed as a respiratory substrate. In these conditions, we obtained 15 possible EFMs. Among these 15 EFMs, 8 involved the input of only AKG, ADP, Pi and O and the output of ATP (represented in Figure 1 and in Supplementary Material S2). The outputs were succinate only (EFMs 3 and 11; Figure 1a,d, respectively), malate only (EFMs 4 and 12; Figure 1b,e, respectively), malate + citrate (EFMs 6 and 13; Figure 1c,f, respectively), succinate + citrate (EFM 14; Figure 1g) or succinate + malate + citrate (EFM 15; Figure 1i). EFMs 11, 12 and 13 involved a membrane leak (L), which consumed the PMF without ATP synthesis (ASYNT = 0) with a rather low ATP/O of 0.5 or 1. There was a final EFM without oxygen consumption, EFM 5 (represented in Figure 1h). This EFM involved the reversion of ATP synthase (-ASYNT). Even though oxygen consumption was experimentally observed, this EFM could not be excluded because it could participate in a linear combination of EFMs with a net consumption of oxygen. All in all, the ATP/O values ranged from 0.5 to 6. Two EFMs (EFM 3 and EFM 6) displayed an ATP/O of 2.15 close to the published value of 2.3 [10,20]. However, because the actual fluxes in a metabolic network can be seen as a linear combination of EFMs, the experimental value of 2.3 could result from a combination of different EFMs with different ATP/O values. Thus, any of the 9 EFMs could be implicated either alone (EFMs 3 or 6, Figure 1a or Figure 1c) or in a combination.
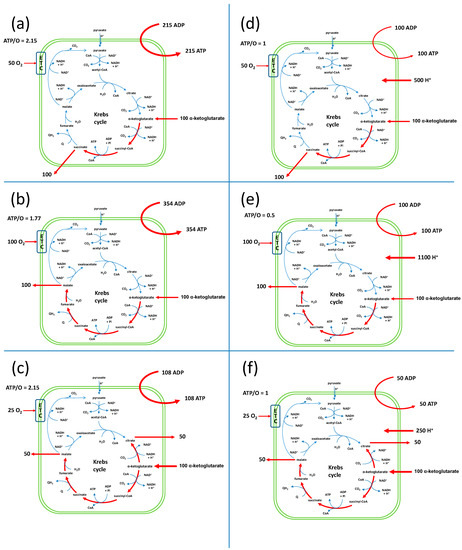
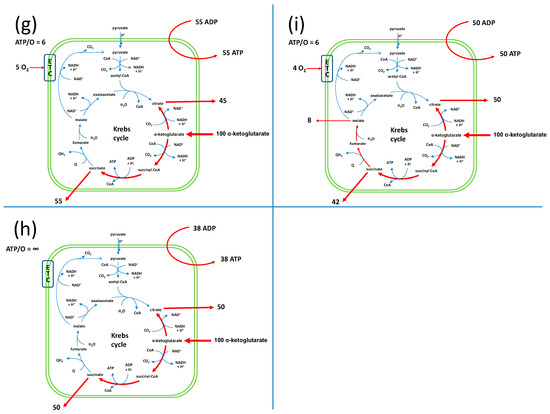
Figure 1.
Schematic representation of the EFMs that explain the set of fluxes in isolated yeast mitochondria respiring on AKG. All values are given for an input of 100 AKG per time unit (in arbitrary units) in order to facilitate the comparison of the release fluxes. The EFMs are detailed in Supplementary Material S2.
To obtain a further insight into the actual metabolic fluxes and the pathway taken by AKG inside the mitochondria, we developed a kinetic model using Copasi [14] involving the kinetic properties known for each step, as described in Supplementary Material S1.
3.2. Dynamics of Oxygen Consumption and ATP Generation and Determination of the Ratio ATP/O as a Function of ATP Synthase Activity
Three properties of the model must be emphasized.
- (1)
- As the thermodynamic span between the pair NADH/NAD and QH2/Q was much greater than the span between succinate/fumarate and QH2/Q, NADH was oxidized before QH2 (Figure 2b) and the accumulation of QH2 blocked the respiratory complex II (RCII or SDH).
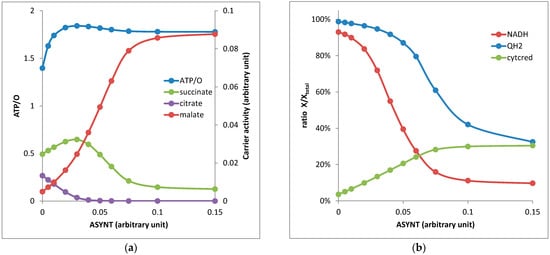 Figure 2. Energy metabolism of isolated yeast mitochondria respiring on AKG as a function of ATP synthase activity: (a) ATP/O ratio (left scale) and output fluxes through the Odc1 carrier against the AKG input (in arbitrary units, right scale) and (b) NADH/NADtotal and QH2/Qtotal and reduced cyt c/cyt ctotal ratios. The parameters of the kinetic model are listed in Supplementary Material S1.
Figure 2. Energy metabolism of isolated yeast mitochondria respiring on AKG as a function of ATP synthase activity: (a) ATP/O ratio (left scale) and output fluxes through the Odc1 carrier against the AKG input (in arbitrary units, right scale) and (b) NADH/NADtotal and QH2/Qtotal and reduced cyt c/cyt ctotal ratios. The parameters of the kinetic model are listed in Supplementary Material S1. - (2)
- When the ratio NADH/NAD was high (low ATP synthase activity), the reversion of IDH3 associated with NADH reoxidation was possible.
- (3)
- When both NADH/NAD and QH2/Q ratios were low (high activity of the respiratory chain and ATP synthase), RCII was active and succinate was easily transformed in fumarate competing with the output of succinate against AKG through Odc1p. The AKG/succinate exchange thus decreased and was largely replaced by the AKG/malate exchange (Figure 2a; high ASYNT activity).
Let us now describe the behavior of mitochondrial metabolism with AKG as a respiratory substrate as a function of the ATP synthase activity (corresponding with different degrees of activity or a different amount of the ATP synthase inhibitor, oligomycin) (Figure 2).
When ASYNT = 0, the respiratory chain activity was low due to the high PMF value leading to high NADH/NAD and QH2/Q ratios. The RCII activity was very low so that succinate went against AKG. The high NADH level reversed the IDH3 activity, leading to a citrate output against AKG. There was a very low malate output in these conditions. An ATP/O = 1.3 was obtained, which fitted well with the 1.1 value experimentally observed [20].
An intermediate ASYNT value of 0.04 consumed the PMF, which decreased and liberated the respiratory chain that consumed the NADH (Figure 2b). The NADH decrease prevented IDH3 reversion and the production of citrate (Figure 2a). The slight decrease in QH2 (10%) allowed an increase in malate production but the succinate output remained high. An ATP/O = 1.9 was observed in these conditions.
At a higher ASYNT activity above 0.1 (WT), the NADH and QH2 concentrations became low and liberated the RCII activity, which consumed a greater part of succinate that was then transformed into fumarate and malate excreted against AKG. The ATP/O was equal to 1.8, somewhat lower than the observed value of 2.3. We will discuss this point later.
3.3. Decomposition of the Steady States in EFMs
As any set of reactions at a steady state can be expressed as a non-negative combination of the EFMs of the metabolic network, we expressed the different steady states of Figure 2 as a function of the EFMs represented in Figure 1. It appeared that only six EFMs were sufficient to describe all the set of fluxes, EFMa, EFMb, EFMd, RFMg, EFMh and EFMi (EFMa indicates EFM represented in Figure 1a, etc.). At a low ATP synthase activity, the set of fluxes was mainly represented by EFMd (output of succinate), EFMg and EFMh (output of succinate and citrate) and EFMi (output of succinate, malate and citrate). As the ATP synthase activity increased, EFMd, g, h and i were progressively replaced by EFMs a and b and then mainly by b (93%) at a high ATP synthase activity. These changes were in accordance with the metabolite outputs and the variation of the ATP/O ratio described in Figure 2a.
3.4. Overexpression of Odc1p
Curiously, in our model, the effect of increasing the activity of the Odc1p carrier (× 10) was rather weak (Table 1, No Odc1 Leak). This could be explained by several factors. First, the growth of the yeast strain NARP + Odc1p was much less than the growth of the wild type (Figure 1 in [8]), suggesting that the increase in ATP synthesis with Odc1p overexpression was far from reaching the WT value; second, it was shown that in the yeast NARP mutant, the activity of Complex IV, the last step of respiratory chain, decreased by 80% so that the 0.030 value of ATP synthesis in NARP (Table 1) was probably overestimated. The effect of Odc1p overexpression not only increased the expression of the carrier but also increased the level of Complex IV (40% of the WT activity, Table 1 in [8]). This was attributed to the retrograde signaling pathway linking ATP synthase and Complex IV biogenesis [8,10,11].

Table 1.
ATP synthesis, respiration and ATP/O in a wild type (WT), NARP mutation and NARP + 10 times Odc1p overexpression calculated with a Copasi model in arbitrary units.
Another factor could play an important role in the rescue of the yeast NARP mutant by Odc1p overexpression: one of the strong constraints of SLP-ATP production is the mandatory reoxidation of NADH produced by α-ketoglutarate dehydrogenase, the reaction preceding the thiokinase reaction that produces ATP in the TCA cycle. Several ways exist in mitochondria to reoxidize NADH [22]. One occurring at a low activity of ATP synthase is the reversion of isocitrate dehydrogenase in the presence of a high concentration of NADH leading to the citrate output as shown in Figure 2. The other classical one is the activation of the respiratory chain due to the PMF consumption by ATP synthase. Another way to consume the PMF and NADH, consequently activating the TCA cycle and SLP, is an increase in the membrane leak. In yeast, this leak is naturally high leading to a high state 4 and a mild uncoupling between respiration and ATP synthesis (Table 1 in [8]). The introduction in our model of a leak accompanying the Odc1 overexpression further increased the ATP synthesis rate (Table 2).

Table 2.
ATP synthesis, respiration and ATP/O in a wild type (WT), NARP mutation and NARP + 10 times Odc1p overexpression with a leak accompanying the Odc1p overexpression. Calculations are performed using the Copasi model in arbitrary units.
4. Discussion
The introduction of human mitochondrial mutations in yeast is an ingenious strategy to explore curative procedures of mitochondrial diseases. This is how the overexpression of the Odc1p carrier was discovered in yeast [10] with the possible implication of substrate-level phosphorylation in the TCA cycle to supply cellular ATP needs.
In order to understand the bases of the rescue of ATP synthase mutants by Odc1p and the metabolic constraints in this metabolic bypass, we developed a theoretical model of mitochondrial metabolism with alpha-ketoglutarate (AKG) as a respiratory substrate (Supplementary Material S1).
4.1. EFMs
We first described the elementary flux modes (EFMs) of the metabolic network. EFMs are the minimal pathways in a metabolic network at a steady state. Any set of metabolic fluxes of a metabolic network at a steady state can be expressed as the sum of EFMs weighted with non-negative coefficients summing to one.
The description of the EFMs of the yeast mitochondrial metabolism showed that, in principle, any TCA segment can be taken from AKG leading to any TCA intermediate output (Figure 1) with ATP/O ratios ranging from 0.5 to 6 except for the EFM of Figure 1h, for which there was no oxygen consumption. The already reported ATP/O ratio of 2.3 [20] was close to the one of 2.15 measured in the EFMs of Figure 1a,c, which corresponded in our system to an exit of succinate on the one hand or malate and citrate on the other hand. However, because the metabolic fluxes at a steady state could be the combination of several EFMs with different ATP/O values, a value of 2.3 could be achieved in this combination.
4.2. Kinetic Model and Decomposition of EFMs
To gain an insight into this matter, we introduced in our model the rate equations of the different steps (Supplementary Material S1) and we studied the steady state of the metabolic network at different ATP synthase activity values (Figure 2). We observed a rewiring of mitochondrial metabolism when the amount of ATP synthase was decreased with an ATP/O passing from a value of 1.8–1.9 to a value of 1.3 close to the corresponding experimental value of 1.1 reported in [20] in the presence of oligomycin, an inhibitor of ATP synthase. However, one may wonder the reason(s) of the greater difference between the theoretical value of 1.8 and the experimental one of 2.3 at a high ATP synthase activity. As we noticed earlier, two EFMs presented an ATP/O of 2.15, close to the experimental value. One presented an output of citrate that was not observed in our kinetic model at a high ATP synthase activity. The other supposed that all AKG entry occurred in exchange with the succinate output. As discussed above, the succinate output necessitates that SDH (complex II) is inactive, which in our case was obtained at a high QH2 concentration. Another solution came from the participation of EFMs with ATP/O = 6. Once more these EFMs involved a citrate output that necessitated a high NADH concentration. This was not the case at a high ATP synthase concentration in our kinetic model in which the release of metabolites changed from mainly malate and succinate at a high ATP synthase activity to succinate plus malate and ultimately a null ATP synthase activity to mainly a succinate release (plus a weaker citrate and malate release). The decomposition of the steady states into six EFMs (Figure 3) confirmed this result with essentially EFMb (corresponding with Figure 1b and ATP/O = 1.77) and, to a lesser extent, EFMa (ATP/O = 2.15) at a high ATP synthase activity.
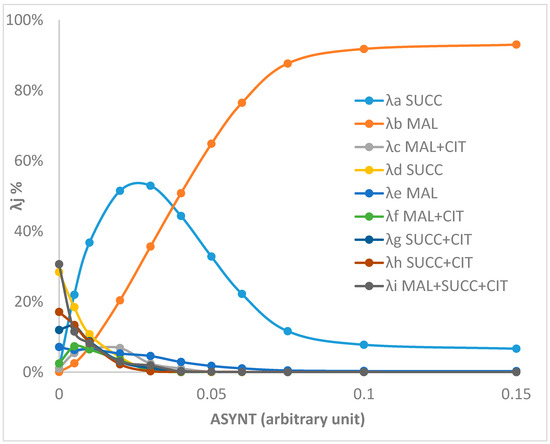
Figure 3.
Decomposition of the fluxes as a function of the EFMs of the network involving AKG, ADP and Pi input (see details in Supplementary Material S2). The λj values refer to EFMj represented in Figure 1. For each ASYNT value, the metabolism at a steady state is equal to Σλj*EFMj (j = a, b,…, i) with Σλj = 1. The metabolite(s) indicated after the λj is/are the metabolite(s) that goes/go out.
Obviously, the conclusions of our model are dependent upon the rate equations of the enzymatic reactions. In order to solve the discrepancy in the ATP/O values, experiments are underway in our laboratory to refine the kinetic parameters of our system by measuring the activities of isolated reactions but also the fluxes of metabolites released from the mitochondria with AKG as a respiratory substrate.
4.3. Odc1p Rescue
It has been shown that CCCP, an uncoupler of the respiratory chain from the ATP synthase activity, can also rescue ATP deficiency in yeast [8]. We can thus hypothesize that part of the Odc1p effect could be to increase the membrane leak favoring SLP-ATP not only by increasing the AKG feeding part of the TCA cycle producing ATP but also NADH consumption by an activation of the respiratory chain. We tested this hypothesis in our model, which showed that increasing the leak in the NARP + Odc1p conditions actually increased SLP-ATP synthesis (Table 2). This effect is comparable with the role of uncoupling proteins in cancer [23,24]. This should also be reconciled with the fact that the overexpression of Odc1 can cure different types of ATP synthase deficiencies [8,10,25], probably by a more general mechanism than a simple AKG transport. Work is in progress in our laboratory to study the possible uncoupling properties of the Odc1 carrier.
4.4. Implications for NARP Syndrome Human Odc1p Counterpart
What is the relevance of this yeast study on human NARP syndrome?
Several reports show that substrate-level phosphorylation is able to rescue ATP synthase deficiency or to favor ATP production in cells fed with glutamine, a precursor of AKG [26,27,28]. However, our theoretical study evidenced the constraints in enhancing SLP through a rewiring of the energy metabolism, namely, the necessity to reoxidize the NADH synthesized by alpha-ketoglutarate dehydrogenase [22,26].
It also evidenced the (perhaps multifactorial) role of the mitochondrial carriers such as Odc1p. Concerning human NARP syndrome, the question is: is there a human homologue of Odc1p? As a matter of fact, Odc1p has been identified by a sequence analysis of the nuclear genome of Saccharomyces cerevisiae [12] and then in humans as an orthologue [29]. As in yeast, the human oxodicarboxylate carrier catalyzed the 2-oxoadipate and 2-oxoglutarate by a counter-exchange mechanism [29]. Nevertheless, contrary to yeast Odc1p, the human carrier does not transport malate and succinate. Thus, the human oxodicarboxylate carrier does not appear to be a good candidate to rescue NARP mutations as in yeast. However, in their work aiming at rescuing human NARP mutations, Sgarbi et al. [27] were able to increase mitochondrial substrate -level phosphorylation and propose a mechanism in which the oxoglutarate carrier exchanged 2-oxoglutarate against malate in a medium enriched in 2-oxoglutarate and aspartate. Thus, the human oxoglutarate carrier (OGC) could play the role of the oxodicarboxylate carrier (Odc1) in yeast. The comparison of the fluxes of metabolites released from the mitochondria of yeast and mammals with AKG as a respiratory substrate and different levels of ATP synthase activity should help in understanding the mitochondrial metabolic pathways taken to rescue ATP synthase deficiencies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9081424/s1, S1: Rate equations of the theoretical model with the kinetic parameters; S2: EFMs of the model (S1) and decomposition of the steady state fluxes on the EFM space (S3); S3: Sbml file of the Copasi model.
Author Contributions
Conceptualization, A.D., J.-P.M. and S.R.; methodology, J.-P.M. and S.R.; software, J.-P.M. and S.R.; validation, A.D., J.-P.M., S.R. and E.Y.; formal analysis, A.D., J.-P.M., S.R. and E.Y.; writing—original draft, A.D., J.-P.M. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by AFM, contract N° 23095 MitoBAD and EMERGENCE GSO.
Acknowledgments
This work benefitted from helpful discussions with Stéphane Duvezin-Caubet, Jean-Paul di Rago, Michel Rigoulet and Déborah Tribouillard-Tanvier.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AKG | α-ketoglutarate/2-oxoglutarate |
| ASYNT-ATP | ATP synthesized by the mitochondrial ATP-synthase |
| PMF | Proton motive force (ΔµH+) |
| SLP | Substrate-level phosphorylation |
| SLP-ATP | ATP synthesized by Succinyl-CoA synthetase (succinate thiokinase) |
References
- Zhou, A.; Rohou, A.; Schep, D.G.; Bason, J.V.; Montgomery, M.G.; Walker, J.E.; Grigorieff, N.; Rubinstein, J.L. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife 2015, 4, e10180. [Google Scholar] [CrossRef]
- Dautant, A.; Velours, J.; Giraud, M.-F. Crystal Structure of the Mg·ADP-inhibited State of the Yeast F1c10-ATP Synthase. J. Biol. Chem. 2010, 285, 29502–29510. [Google Scholar] [CrossRef] [Green Version]
- Spikes, T.E.; Montgomery, M.G.; Walker, J.E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl. Acad. Sci. USA 2020, 117, 23519–23526. [Google Scholar] [CrossRef]
- Guo, H.; Rubinstein, J.L. Cryo-EM of ATP synthases. Curr. Opin. Struct. Biol. 2018, 52, 71–79. [Google Scholar] [CrossRef]
- Dautant, A.; Meier, T.; Hahn, A.; Tribouillard-Tanvier, D.; Di Rago, J.-P.; Kucharczyk, R. ATP Synthase Diseases of Mitochondrial Genetic Origin. Front. Physiol. 2018, 9, 329. [Google Scholar] [CrossRef]
- Su, X.; Dautant, A.; Rak, M.; Godard, F.; Ezkurdia, N.; Bouhier, M.; Bietenhader, M.; Mueller, D.M.; Kucharczyk, R.; di Rago, J.-P.; et al. The pathogenic m.8993 T > G mutation in mitochondrial ATP6 gene prevents proton release from the subunit c-ring rotor of ATP synthase. Hum. Mol. Genet. 2021, 30, 381–392. [Google Scholar] [CrossRef]
- Su, X.; Dautant, A.; Godard, F.; Bouhier, M.; Zoladek, T.; Kucharczyk, R.; Di Rago, J.-P.; Tribouillard-Tanvier, D. Molecular Basis of the Pathogenic Mechanism Induced by the m.9191T>C Mutation in Mitochondrial ATP6 Gene. Int. J. Mol. Sci. 2020, 21, 5083. [Google Scholar] [CrossRef]
- Su, X.; Rak, M.; Tetaud, E.; Godard, F.; Sardin, E.; Bouhier, M.; Gombeau, K.; Caetano-Anollés, D.; Salin, B.; Chen, H.; et al. Deregulating mitochondrial metabolite and ion transport has beneficial effects in yeast and human cellular models for NARP syndrome. Hum. Mol. Genet. 2019, 28, 3792–3804. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.; Tetaud, E.; Duvezin-Caubet, S.; Ezkurdia, N.; Bietenhader, M.; Rytka, J.; di Rago, J.-P. A Yeast Model of the Neurogenic Ataxia Retinitis Pigmentosa (NARP) T8993G Mutation in the Mitochondrial ATP Synthase-6 Gene. J. Biol. Chem. 2007, 282, 34039–34047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwimmer, C.; Lefebvre-Legendre, L.; Rak, M.; Devin, A.; Slonimski, P.P.; di Rago, J.-P.; Rigoulet, M. Increasing Mitochondrial Substrate-level Phosphorylation Can Rescue Respiratory Growth of an ATP Synthase-deficient Yeast. J. Biol. Chem. 2005, 280, 30751–30759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butow, R.A.; Avadhani, N.G. Mitochondrial Signaling: The Retrograde Response. Mol. Cell 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Palmieri, L.; Agrimi, G.; Runswick, M.J.; Fearnley, I.M.; Palmieri, F.; Walker, J. Identification in Saccharomyces cerevisiae of Two Isoforms of a Novel Mitochondrial Transporter for 2-Oxoadipate and 2-Oxoglutarate. J. Biol. Chem. 2001, 276, 1916–1922. [Google Scholar] [CrossRef] [Green Version]
- Lebiedzinska, M.; Karkucińska-Więckowska, A.; Wojtala, A.; Suski, J.M.; Szabadkai, G.; Wilczynski, G.; Wlodarczyk, J.; Diogo, C.V.; Oliveira, P.J.; Tauber, J.; et al. Disrupted ATP synthase activity and mitochondrial hyperpolarisation-dependent oxidative stress is associated with p66Shc phosphorylation in fibroblasts of NARP patients. Int. J. Biochem. Cell Biol. 2013, 45, 141–150. [Google Scholar] [CrossRef]
- Hoops, S.; Sahle, S.; Gauges, R.; Lee, C.; Pahle, J.; Simus, N.; Singhal, M.; Xu, L.; Mendes, P.; Kummer, U. COPASI—A COmplex PAthway SImulator. Bioinformatics 2006, 22, 3067–3074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnensack, R. Control of energy transformation in mitochondria. Analysis by a quantitative model. Biochim. et Biophys. Acta (BBA) Bioenerg. 1981, 634, 203–218. [Google Scholar] [CrossRef]
- Mitchell, P. Vectorial Chemistry and the Molecular Mechanics of Chemiosmotic Coupling: Power Transmission by Proticity. Biochem. Soc. Trans. 1976, 4, 399–430. [Google Scholar] [CrossRef]
- Toth, A.; Meyrat, A.; Stoldt, S.; Santiago, R.; Wenzel, D.; Jakobs, S.; von Ballmoos, C.; Ott, M. Kinetic coupling of the respiratory chain with ATP synthase, but not proton gradients, drives ATP production in cristae membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 2412–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, S.; Hilgetag, C.C. On elementary flux modes in biochemical reaction systems at steady state. J. Biol. Syst. 1994, 2, 165–182. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Sanchez-Valdenebro, I.; Nuño, J.C.; Montero, F.; Schuster, S. METATOOL: For studying metabolic networks. Bioinformatics 1999, 15, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Rigoulet, M.; Velours, J.; Guerin, B. Substrate-level phosphorylation in isolated yeast mitochondria. JBIC J. Biol. Inorg. Chem. 1985, 153, 601–607. [Google Scholar] [CrossRef]
- Schuster, S.; Dandekar, T.; Fell, D.A.; Schuster, S.; Dandekar, T.; Fell, D.A.; Schuster, S.; Dandekar, T.; Fell, D.A.; Schuster, S.; et al. Detection of elementary flux modes in biochemical networks: A promising tool for pathway analysis and metabolic engineering. Trends Biotechnol. 1999, 17, 53–60. [Google Scholar] [CrossRef]
- Chinopoulos, C. Acute sources of mitochondrial NAD+ during respiratory chain dysfunction. Exp. Neurol. 2020, 327, 113218. [Google Scholar] [CrossRef]
- Valle, A.; Oliver, J.O.; Roca, P. Role of Uncoupling Proteins in Cancer. Cancers 2010, 2, 567–591. [Google Scholar] [CrossRef]
- Samudio, I.; Fiegl, M.; Andreeff, M. Mitochondrial Uncoupling and the Warburg Effect: Molecular Basis for the Reprogramming of Cancer Cell Metabolism: Figure 1. Cancer Res. 2009, 69, 2163–2166. [Google Scholar] [CrossRef] [Green Version]
- Tilques, M.D.T.D.; Tribouillard-Tanvier, D.; Tétaud, E.; Testet, E.; di Rago, J.-P.; Lasserre, J.-P. Overexpression of mitochondrial oxodicarboxylate carrier (ODC1) preserves oxidative phosphorylation in a yeast model of the Barth syndrome. Dis. Model. Mech. 2017, 10, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Chinopoulos, C.; Seyfried, T.N. Mitochondrial Substrate-Level Phosphorylation as Energy Source for Glioblastoma: Review and Hypothesis. ASN Neuro 2018, 10, 1759091418818261. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, G.; Casalena, G.A.; Baracca, A.; Lenaz, G.; DiMauro, S.; Solaini, G. Human NARP Mitochondrial Mutation Metabolism Corrected With α-Ketoglutarate/Aspartate. Arch. Neurol. 2009, 66, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Kirk, K.; Shurubor, Y.I.; Zhao, D.; Arreguin, A.; Shahi, I.; Valsecchi, F.; Primiano, G.; Calder, E.L.; Carelli, V.; et al. Rewiring of Glutamine Metabolism Is a Bioenergetic Adaptation of Human Cells with Mitochondrial DNA Mutations. Cell Metab. 2018, 27, 1007–1025.e5. [Google Scholar] [CrossRef] [Green Version]
- Fiermonte, G.; Dolce, V.; Palmieri, L.; Ventura, M.; Runswick, M.J.; Palmieri, F.; Walker, J.E. Identification of the Human Mitochondrial Oxodicarboxylate Carrier. Bacterial Expression, Reconstitution, Functional Characterization, Tissue Distribution, and Chromosomal Location. J. Biol. Chem. 2001, 276, 8225–8230. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).