Towards the Circular Economy of Rare Earth Elements: Lanthanum Leaching from Spent FCC Catalyst by Acids
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
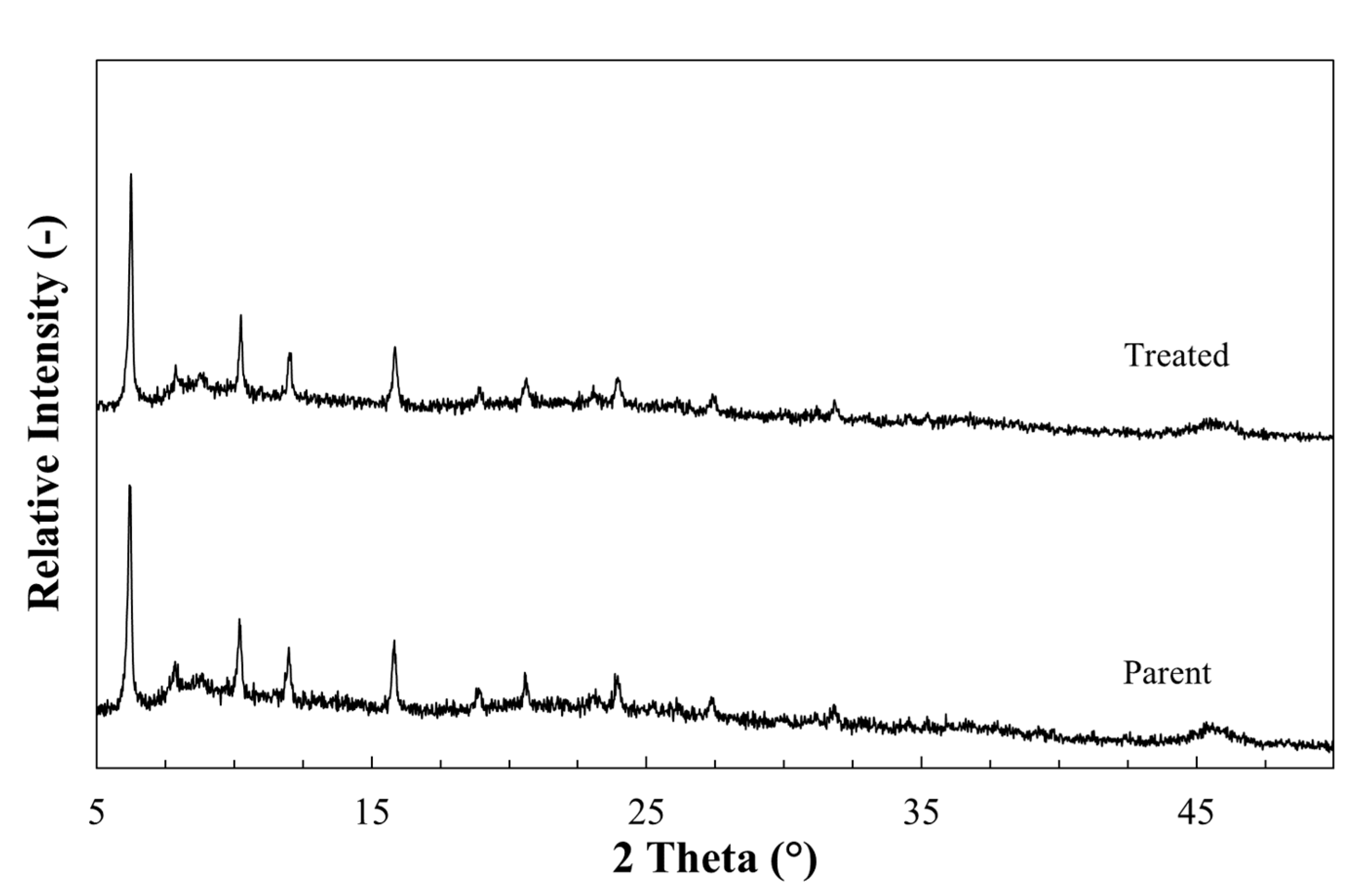
3.1. Characterisation of as Received Sample
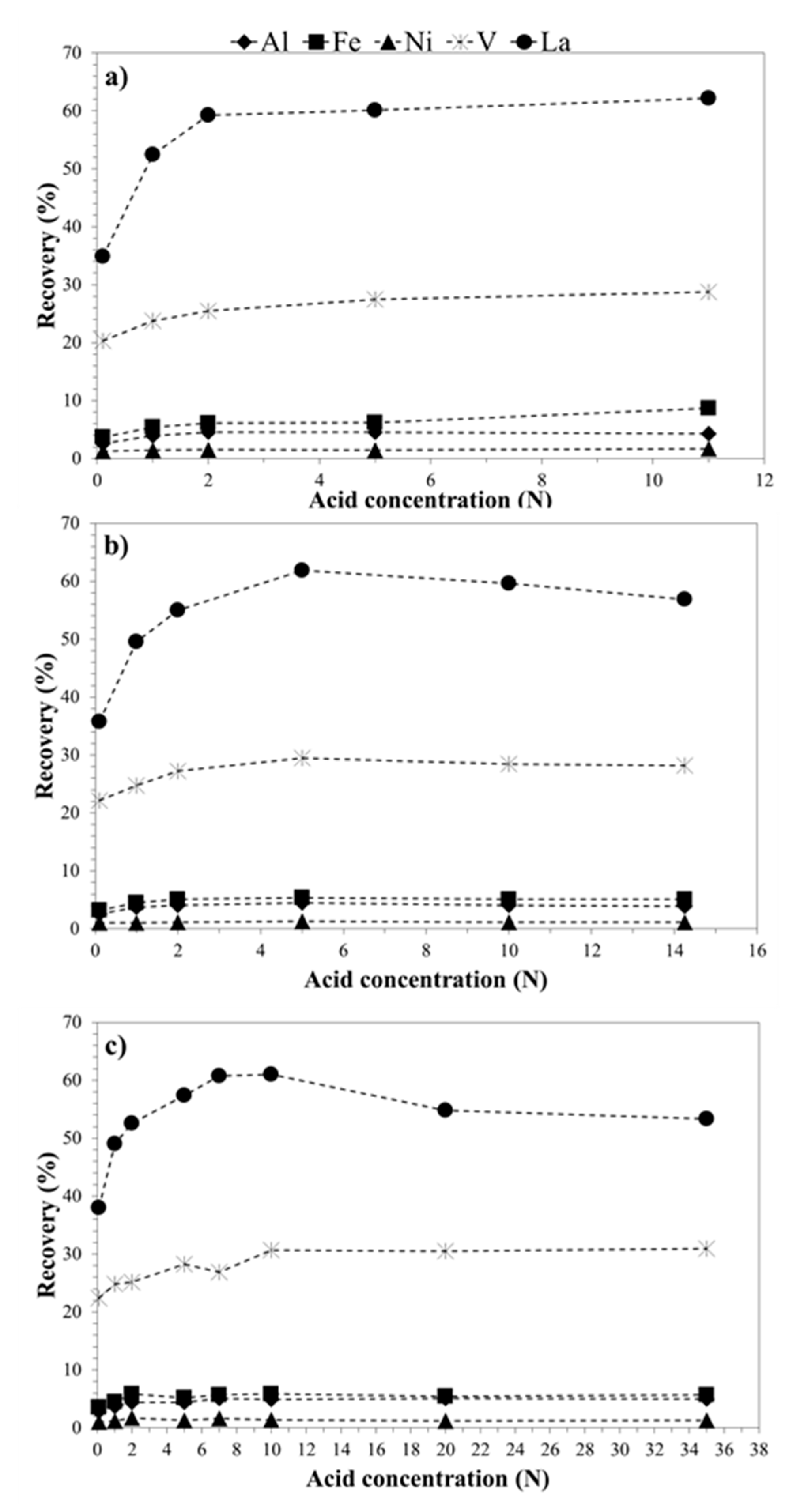
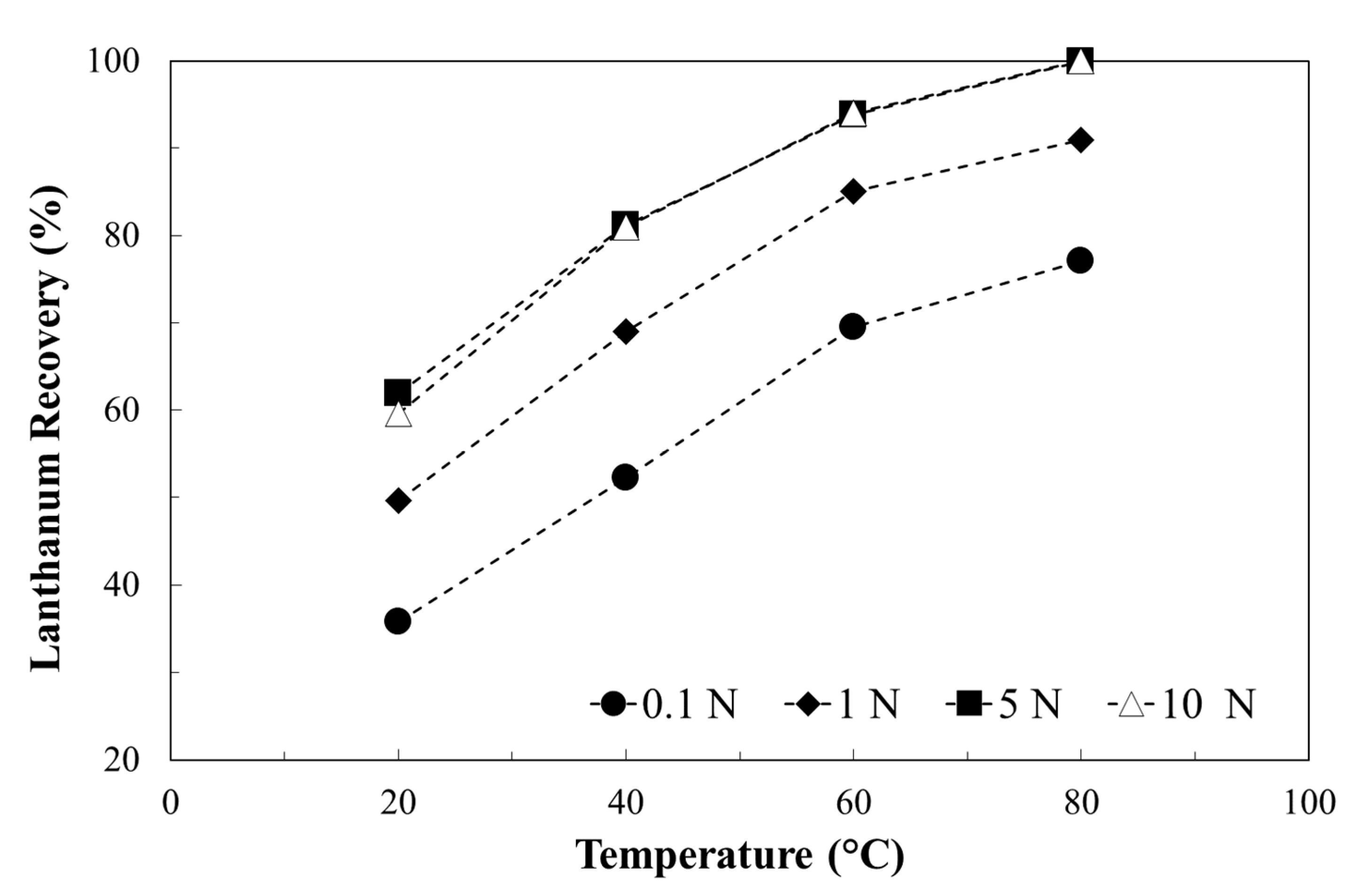
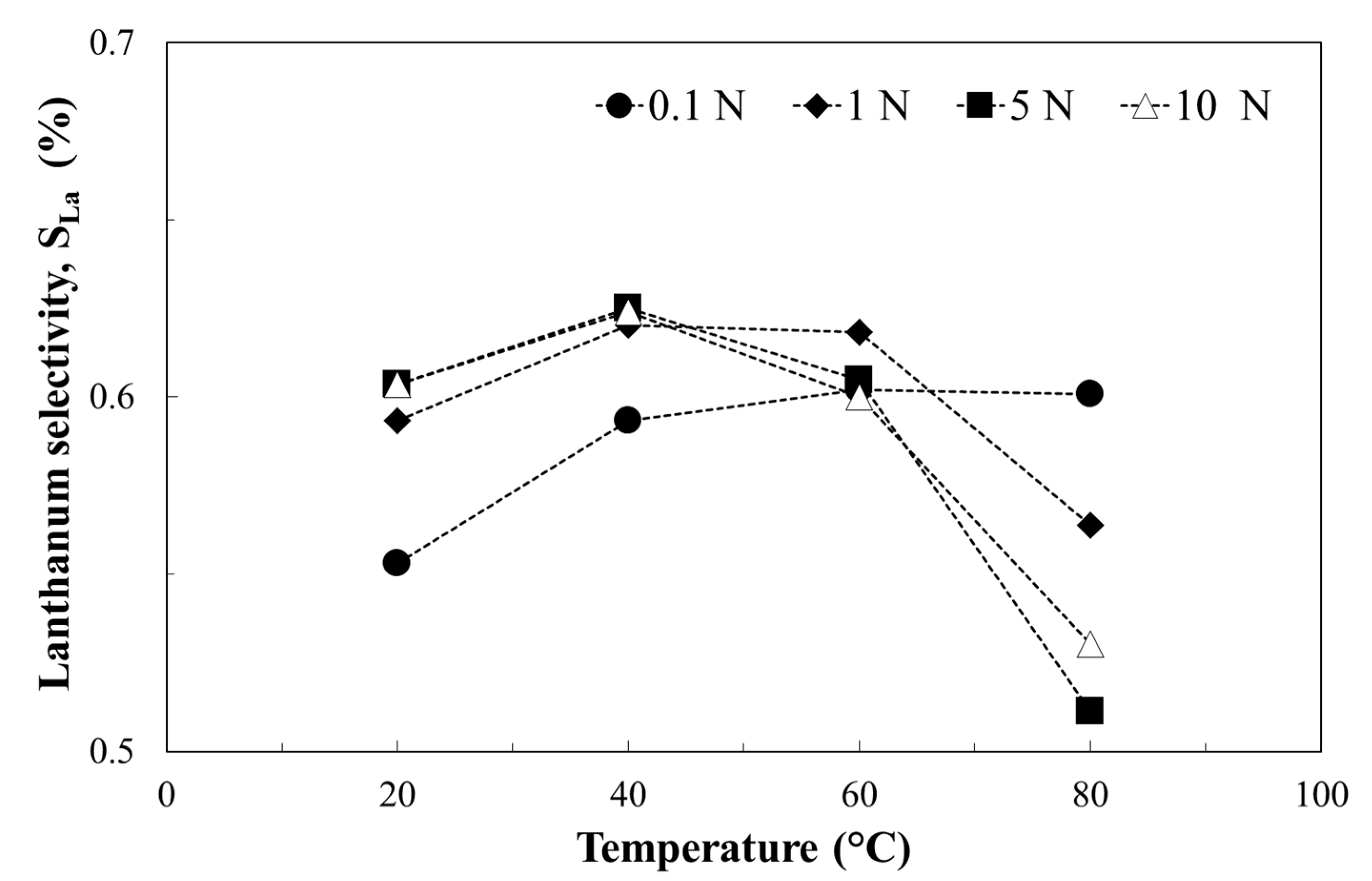
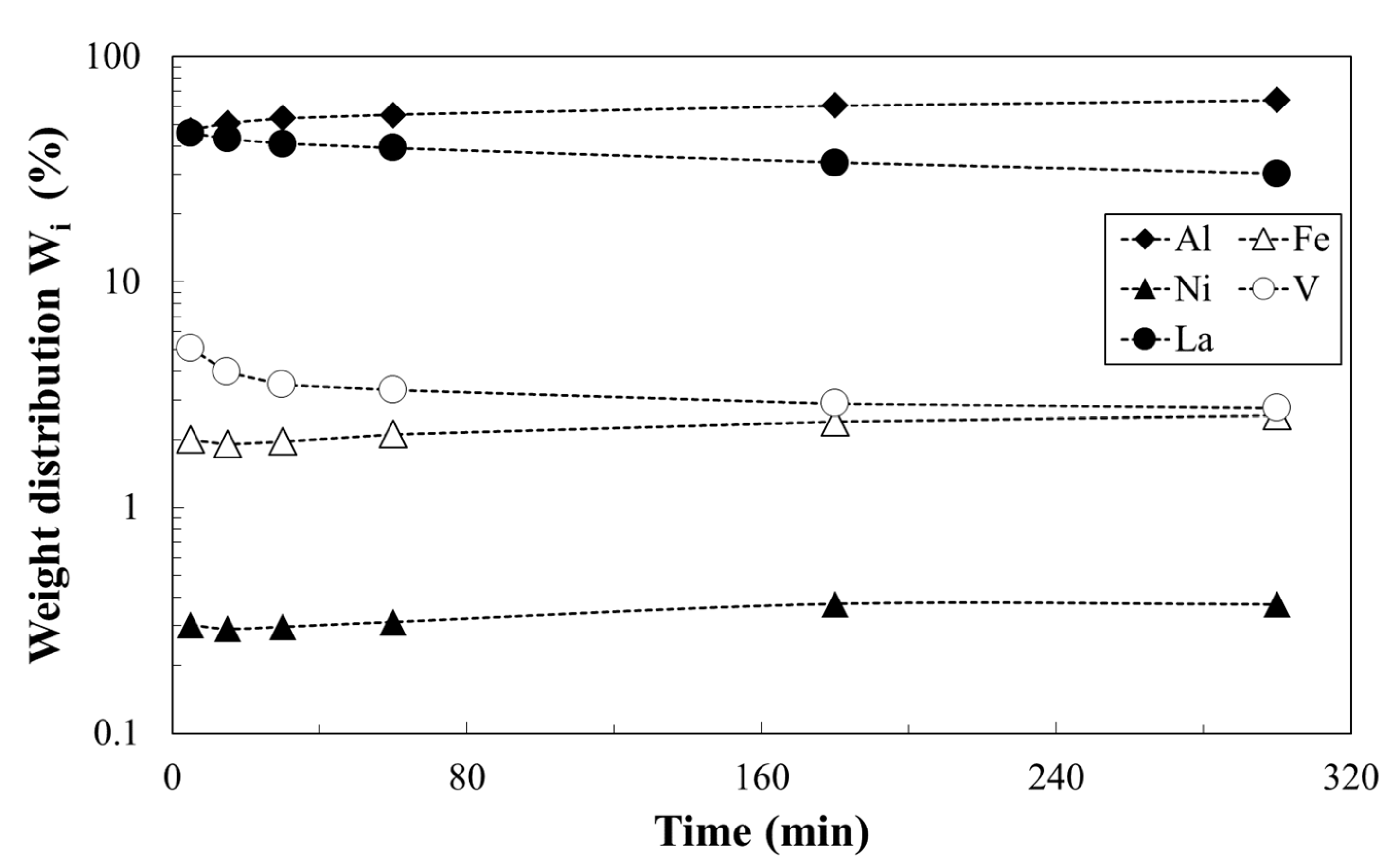
3.2. Metals Leaching by Acids: The Effect of Operation Condition on Lanthanum Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- BP Statistical Review of World Energy. 2019. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 8 July 2020).
- Blasi, A.; Sposato, C.; Devincenzis, G.; Garzone, P.; Morgana, M. Definition of the Process to Separate Light Rare Earths by Working with (2-Ethylexyl)-Mono(2-Ethylexyl)ester Phosphonic Acid (P507) in a Mixer Settler Battery. In Proceedings of the TMS Annual Meeting, San Diego, CA, USA, 16–20 February 2014; Volume 197. [Google Scholar]
- Macheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Resour. Conserv. Recycl. 2019, 142, 101. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Ogden, M.D.; Moon, E.M.; Soldenhoff, K.L. REE behavior and sorption on weak acid resins from buffered media. J. Ind. Eng. Chem. 2018, 59, 440. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Fernaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Sposato, C.; Blasi, A.; Romanelli, A.; Braccio, G.; Morgana, M. Behavior of sec-octylphenoxy acetic acid (CA-12) in yttrium recovery from high concentrated heavy rare earths mixture. In Rare Metal Technology; Springer: Cham, Switzerland, 2017; pp. 225–233. [Google Scholar]
- Blasi, A.; Sposato, C.; Romanelli, A.; Braccio, G.; Morgana, M. Study of a synergistic solvent extracting system to separate yttrium and heavy rare earths: A deep investigation on system behavior. In Rare Metal Technology; Springer: Cham, Switzerland, 2017; pp. 277–284. [Google Scholar]
- Sposato, C.; Blasi, A.; Devincenzis, G.; Garzone, P.; Morgana, M. Comparison among different extractants, AS (2-ethylhexyl)-mono (2-ethylhexyl) ester phosphonic acid (p507), secondary-octyl phenoxy acetic acid (CA-12) and BIS(2, 4, 4-trimethylpentyl)phosphinic acid (CYANEX272), in the separation of heavy rare earths via hydrometallurgical processes. In Proceedings of the TMS Annual Meeting, San Diego, CA, USA, 16–20 February 2014; Volume 201. [Google Scholar]
- Molino, A.; Giordano, G.; Motola, V.; Fiorenza, G.; Nanna, F.; Braccio, G. Electrciity production by biomass steam gasification using a high efficiency technology and low environmental impact. Fuel 2013, 103, 179–192. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Perspectives for the recovery of rare earths from end-of-life fluorescent lamps. J. Rare Earths 2014, 32, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Yurramendi, L.; Gijsemans, L.; Forte, F.; Aldana, J.L.; del Rio, C.; Binnemans, K. Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 2019, 187, 38–44. [Google Scholar] [CrossRef]
- Huckenbeck, T.; Otto, R.; Haucke, E.; Osram, A.G. Verfahren zur Rückgewinnung Seltener Erden aus Leuchtstofflampen Sowie Zugehörige Leuchtstoffe und Lichtquellen. WO2012143240 A2, 26 October 2012. [Google Scholar]
- Sun, T.; Kennedy, M.W.; Yurramendi, L.; Aldana, J.L.; Del Rio, C.; Arnout, S.; Trannel, G.; Aune, R.E. Pyrometallurgical Treatment of Apatite Concentrate with the Objective of Rare Earth Element Extraction: Part I. J. Sustain. Metall. 2017, 3, 829–845. [Google Scholar] [CrossRef] [Green Version]
- Prusty, S.; Pradhan, S.; Mishra, S. Ionic liquid as an emerging alternative for the separation and recovery of Nd, Sm and Eu using solvent extraction technique—A review. Sustain. Chem. Pharm. 2021, 21, 100434. [Google Scholar]
- Hidayah, N.N.; Abidin, S.Z. The evolution of mineral processing in extraction of rare earth elements using liquid-liquid extraction: A review. Miner. Eng. 2018, 121, 146–157. [Google Scholar] [CrossRef]
- Hammache, Z.; Bensaadi, S.; Berbar, Y.; Audebrand, N.; Szymczyk, A.; Amara, M. Recovery of rare earth elements from electronic waste by diffusion dialysis. Sep. Purif. Technol. 2021, 254, 117641. [Google Scholar] [CrossRef]
- Arrachart, G.; Couturier, J.; Dourdain, S.; Levard, C.; Pellet-Rostaining, S. Recovery of rare earth (REEs) using ionic solvents. Processes 2021, 9, 1202. [Google Scholar] [CrossRef]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of valuable elements from red mud with focus on using liquid media—A review. Processes 2021, 6, 38. [Google Scholar]
- Wang, K.; Adidharma, H.; Radosz, M.; Wan, P.; Xu, X.; Russell, C.K.; Tian, H.; Fan, M.; Yu, J. Recovery of rare earth elements with ionic liquids. Green Chem. 2017, 19, 4469–4493. [Google Scholar] [CrossRef]
- de Oliveira, R.P.; Benvenuti, J.; Espinosa, D.C.R. A review of the current progress in recycling technologies for gallium and rare earth elements from light-emitting diodes. Renew. Sustain. Energy Rev. 2021, 145, 111090. [Google Scholar] [CrossRef]
- Becci, A.; Beolchini, F.; Amato, A. Sustainable strategies for the exploitation of End-of-Life permanent magnets. Processes 2021, 9, 857. [Google Scholar] [CrossRef]
- Lorenz, T.; Bertau, M. Recycling of Rare Earth Elements. Phys. Sci. Rev. 2017, 2, 20160067. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Klocke, D.J.; Buchanan, J.S. Effects of high-level additions of ZSM-5 to a fluid catalytic cracking (FCC) RE-USY catalyst. Appl. Catal. Gen. 1995, 131, 121. [Google Scholar] [CrossRef]
- Degnan, T.F.; Chitnis, G.K.; Schipper, P.H. History of ZSM-5 fluid catalytic cracking additive development at Mobil. Microporous Mesoporous Mater. 2000, 35, 245. [Google Scholar] [CrossRef]
- Ibarra, A.; Hita, I.; Azkoiti, M.J.; Arandes, J.M.; Bilbao, J. Catalytic cracking of raw bio-oil under FCC unit conditions over different zeolite-based catalysts. J. Ind. Eng. Chem. 2019, 75, 372. [Google Scholar] [CrossRef]
- Harding, R.H.; Peters, A.W.; Nee, J.R.D. New developments in FCC catalyst technology. Appl. Catal. Gen. 2001, 221, 389. [Google Scholar] [CrossRef]
- Rawlence, D.J.; Gosling, K. FCC catalyst performance evaluation. Appl. Catal. 1988, 43, 213. [Google Scholar] [CrossRef]
- Catizzone, E.; Cirelli, Z.; Aloise, A.; Lanzafame, P.; Migliori, M.; Giordano, G. Methanol conversion over ZSM-12, ZSM-22 and EU-1 zeolites: From DME to hydrocarbons production. Catal. Today 2018, 304, 39. [Google Scholar] [CrossRef]
- Palčić, A.; Catizzone, E. Application of nanosized zeolites in methanol conversion processes: A short review. Curr. Opin. Green Sustain. Chem. 2021, 27, 100393. [Google Scholar] [CrossRef]
- Catizzone, E.; van Daele, S.; Bianco, M.; Di Michele, A.; Aloise, A.; Migliori, M.; Valtchev, V.; Giordano, G. Catalytic application of ferrierite nanocrystals in vapour-phase dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2019, 243, 273. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922. [Google Scholar] [CrossRef]
- Escobar, A.S.; Pereira, M.M.; Pimenta, R.D.M.; Lau, L.Y.; Cerqueira, H.S. Interaction between Ni and V with USHY and rare earth HY zeolite during hydrothermal deactivation. Appl. Catal. Gen. 2005, 286, 196. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Gong, X.; Guo, Y.; Wang, Y.; Lu, G. Current status and perspectives of rare earth catalytic materials and catalysis. J. Catal. 2014, 35, 1238. [Google Scholar]
- Xiaoning, W.; Zhen, Z.; Chunming, X.; Aijun, D.; Li, Z.; Guiyuan, J. Effects of light rare earth on acidity and catalytic performance of HZSM-5 zeolite for catalytic cracking of butane to light olefins. J. Rare Earth 2007, 25, 321. [Google Scholar] [CrossRef]
- Falabella, E.; Trigueiro, F.E.; Zotin, F.M.Z. The role of rare earth elements in zeolites and cracking catalysts. Catal. Today 2013, 115, 218–219. [Google Scholar]
- De la Puente, G.; Falabella, E.; Zotin, F.M.Z.; Camorim, V.L.D.; Sedran, U. Influence of different rare earth ions on hydrogen transfer over Y zeolite. Appl. Catal. Gen. 2000, 197, 41. [Google Scholar] [CrossRef]
- Sterte, J.; Otterstedt, J.E. Catalytic cracking of heavy oil: Use of alumina—montmorillonites both as catalysts and as matrices for rare earth exchanged zeolite Y molecular sieve. Appl. Catal. 1988, 38, 131. [Google Scholar] [CrossRef]
- Scherzer, J. Designing FCC catalysts with high-silica Y zeolites. Appl. Catal. 1991, 75, 1. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17. [Google Scholar] [CrossRef] [Green Version]
- Shengqiang, Z.; Xiuyang, H.; Dahui, W. Review on comprehensive recovery of valuable metals from spent electrode materials of nickel-hydrogen batteries. Rare Metal Mater. Eng. 2015, 44, 73. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ferella, F.; De Michelis, I.; Vegliò, F. Treatment of fluid catalytic cracking spent catalysts to recover lanthanum and cerium: Comparison between selective precipitation and solvent extraction. J. Ind. Eng. Chem. 2015, 24, 92. [Google Scholar] [CrossRef]
- Lanzafame, P.; Papanikolaou, G.; Perathoner, S.; Centi, G.; Migliori, M.; Catizzone, E.; Giordano, G. Reassembly mechanism in Fe-Silicalite during NH4OH post-treatment and relation with acidity and catalytic reactivity. Appl. Catal. Gen. 2019, 508, 186–196. [Google Scholar] [CrossRef]
- Woltermann, G.M.; Magee, J.S.; Griffith, S.D. Chapter 4 Commercial Preparation and Characterization of FCC Catalysts. Stud. Surf. Sci. Catal. 1993, 76, 105–144. [Google Scholar]
- Gonzalez, M.R.; Pereyra, A.M.; Torres Sánchez, R.M.; Basaldella, E.I. Chromium removal by zeolite-rich materials obtained from an exhausted FCC catalyst: Influence of chromium incorporation on the sorbent structure. J. Colloid Interf. Sci. 2013, 408, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Gupta, P.; Ray, S.S. Structure and composition of hard coke deposited on industrial fluid catalytic cracking catalysts by solid state 13C nuclear magnetic resonance. Appl. Catal. Gen. 2013, 466, 123–130. [Google Scholar] [CrossRef]
- Migliori, M.; Catizzone, E.; Aloise, A.; Bonura, G.; Gómez-Hortigüela, L.; Frusteri, L.; Cannilla, C.; Frusteri, F.; Giordano, G. New insights about coke deposition in methanol-to-DME reaction over MOR-, MFI-and FER-type zeolites. J. Ind. Eng Chem. 2018, 68, 196. [Google Scholar] [CrossRef]
- Tonnetto, G.; Atias, J.; De Lasa, H. FCC catalysts with different zeolite crystallite sizes: Acidity, structural properties and reactivity. Appl. Catal. Gen. 2004, 270, 9–25. [Google Scholar] [CrossRef]
- Escobar, A.S.; Pinto, F.V.; Cerqueira, H.S.; Pereira, M.M. Role of nickel and vanadium over USY and RE-USY coke formation. Appl. Catal. A 2006, 315, 68–73. [Google Scholar] [CrossRef]
- Roncolatto, R.E.; Cardoso, M.J.B.; Cerqueira, H.S.; Lam, Y.L.; Schmal, M. XPS study of spent FCC catalyst regenerated under different conditions. Ind. Eng. Chem. Res. 2007, 46, 1148–1152. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J.; Roper, D.H. Separation Process Principles, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Giudici, R.; Kouwenhoven, H.W.; Prins, R. Comparison of nitric and oxalic acid in the dealumination of mordenite. Appl. Catal. Gen. 2000, 203, 101–110. [Google Scholar] [CrossRef]
- Mintova, S.; Valtchev, V.; Onfroy, T.; Marichal, C.; Knözinger, H.; Bein, T. Variation of the Si/Al ratio in nanosized zeolite Beta crystals. Microporous Mesoporous Mater. 2006, 90, 237–245. [Google Scholar] [CrossRef]
- Gao, X.; Owens, W.T. Process for Metal Recovery from Catalyst Waste. U.S. Patent 2012/0156116 A1, 21 June 2012. [Google Scholar]
- Disegna, W.L.; Foster, R.L. Catalyst Demetallization. U.S. Patent 3252918, 24 May 1966. [Google Scholar]

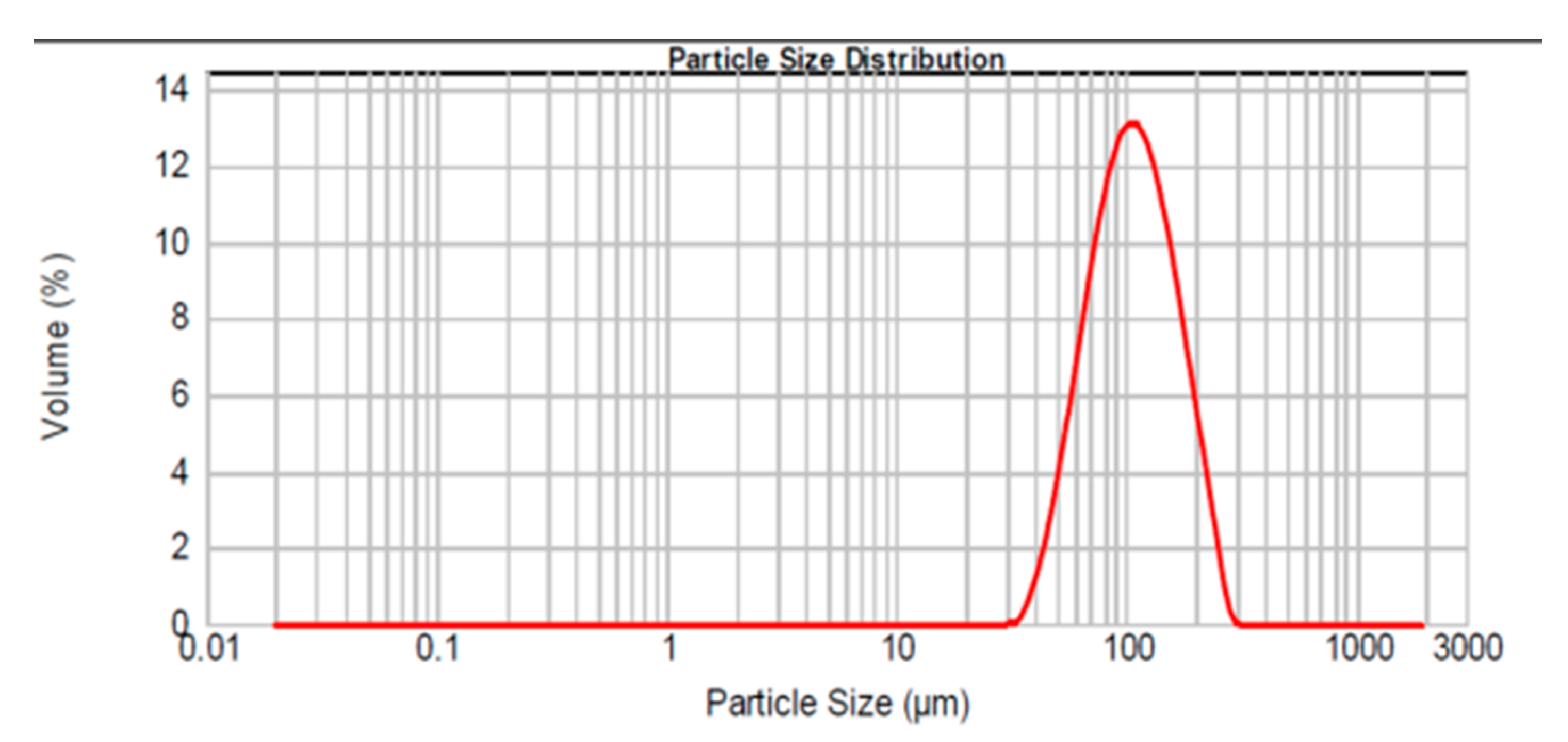
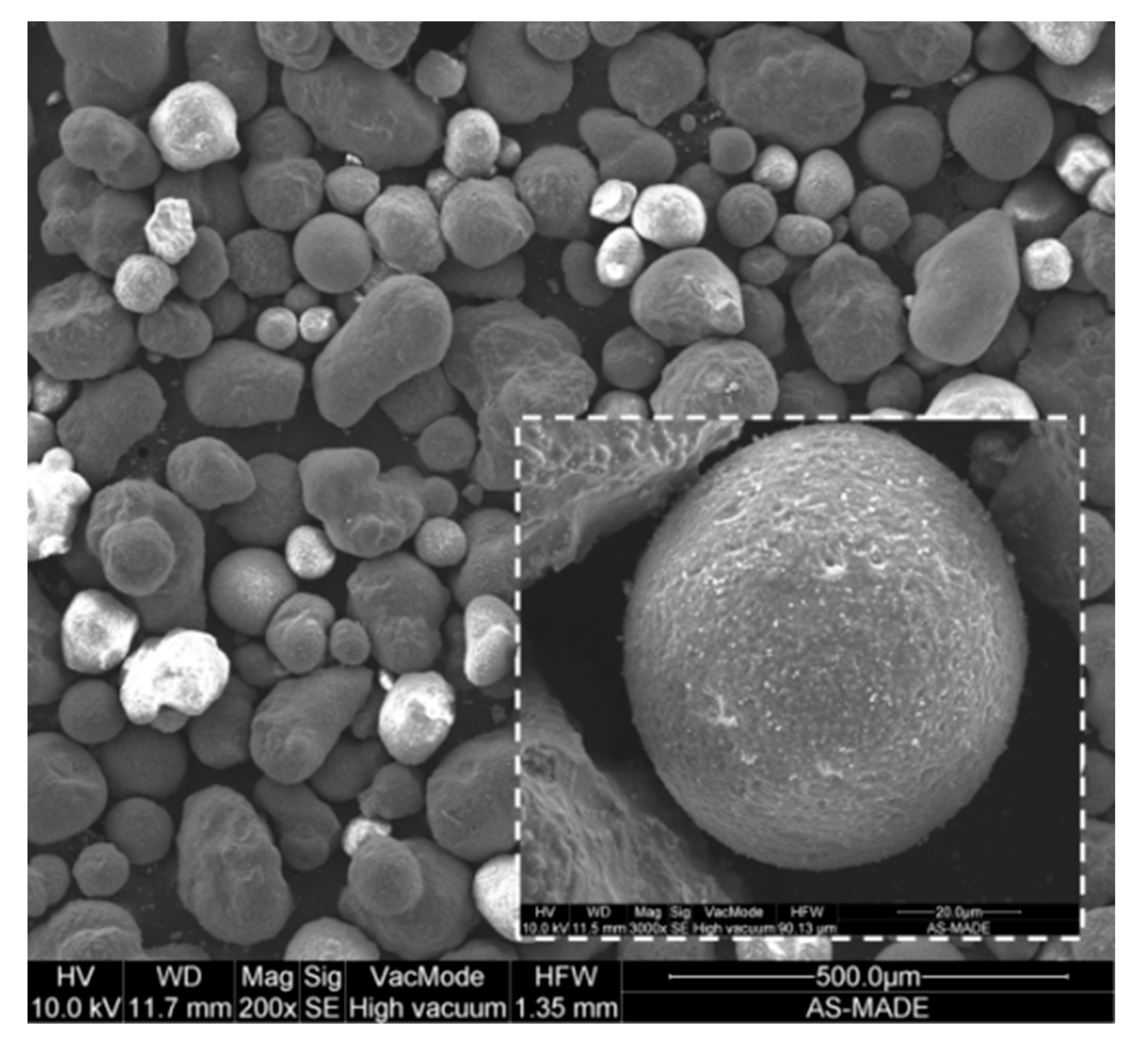
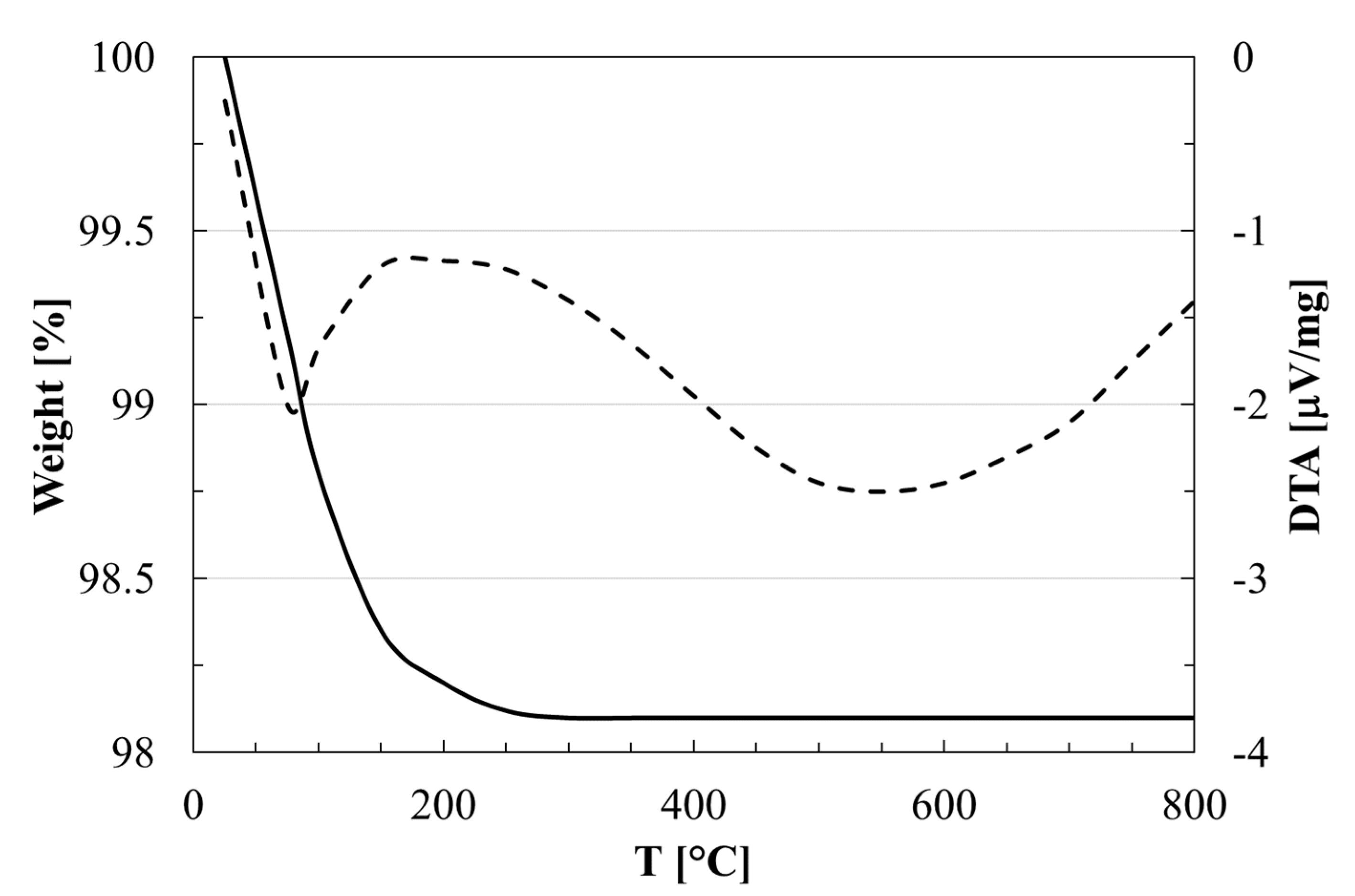



| Average particle size (µm) a | 104 |
| Specific area (m2/g) b | 107 |
| Total pore volume (cm3/g) c | 0.128 |
| Micropore volume (cm3/g) d | 0.039 |
| Mesopore volume (cm3/g) | 0.089 |
| Chemical composition (wt%) e | |
| Al | 22.70 |
| La | 1.49 |
| Fe | 0.74 |
| Ni | 0.49 |
| V | 0.35 |
| Co | 0.0028 |
| Cr | 0.0055 |
| Si, O, H, Na | rest of 100% |
| Temperature (°C) | [HNO3] (N) | Al (%) | Fe (%) | Ni (%) | V (%) |
|---|---|---|---|---|---|
| 20 °C | 0.1 | 2.6 | 3.2 | 1.0 | 22.1 |
| 1 | 3.7 | 4.5 | 1.1 | 24.7 | |
| 2 | 4.0 | 5.1 | 1.1 | 27.2 | |
| 5 | 4.5 | 5.4 | 1.3 | 29.5 | |
| 10 | 4.0 | 5.1 | 1.2 | 28.4 | |
| 40 °C | 0.1 | 4.6 | 5.4 | 1.6 | 24.2 |
| 1 | 5.8 | 6.6 | 1.8 | 28.1 | |
| 2 | 6.1 | 7.0 | 1.9 | 29.1 | |
| 5 | 6.5 | 7.9 | 1.9 | 31.8 | |
| 10 | 6.5 | 7.9 | 2.0 | 32.3 | |
| 60 °C | 0.1 | 7.6 | 7.8 | 2.3 | 28.3 |
| 1 | 9.3 | 10.4 | 2.7 | 30.1 | |
| 2 | 9.6 | 11.2 | 2.7 | 32.4 | |
| 5 | 10.9 | 13.3 | 3.1 | 33.8 | |
| 10 | 10.8 | 13.3 | 3.1 | 35.5 | |
| 80 °C | 0.1 | 10.5 | 9.9 | 2.9 | 27.9 |
| 1 | 15.1 | 16.5 | 4.3 | 34.5 | |
| 2 | 18.0 | 19.6 | 5.0 | 37.3 | |
| 5 | 21.7 | 24.4 | 6.3 | 43.0 | |
| 10 | 19.6 | 24.4 | 6.2 | 41.6 |
| La wt% | Al wt% | Fe wt% | V wt% | Ni wt% |
|---|---|---|---|---|
| 96.3 | 3.33 | 0.13 | 0.22 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sposato, C.; Catizzone, E.; Blasi, A.; Forte, M.; Romanelli, A.; Morgana, M.; Braccio, G.; Giordano, G.; Migliori, M. Towards the Circular Economy of Rare Earth Elements: Lanthanum Leaching from Spent FCC Catalyst by Acids. Processes 2021, 9, 1369. https://doi.org/10.3390/pr9081369
Sposato C, Catizzone E, Blasi A, Forte M, Romanelli A, Morgana M, Braccio G, Giordano G, Migliori M. Towards the Circular Economy of Rare Earth Elements: Lanthanum Leaching from Spent FCC Catalyst by Acids. Processes. 2021; 9(8):1369. https://doi.org/10.3390/pr9081369
Chicago/Turabian StyleSposato, Corradino, Enrico Catizzone, Alessandro Blasi, Marilena Forte, Assunta Romanelli, Massimo Morgana, Giacobbe Braccio, Girolamo Giordano, and Massimo Migliori. 2021. "Towards the Circular Economy of Rare Earth Elements: Lanthanum Leaching from Spent FCC Catalyst by Acids" Processes 9, no. 8: 1369. https://doi.org/10.3390/pr9081369
APA StyleSposato, C., Catizzone, E., Blasi, A., Forte, M., Romanelli, A., Morgana, M., Braccio, G., Giordano, G., & Migliori, M. (2021). Towards the Circular Economy of Rare Earth Elements: Lanthanum Leaching from Spent FCC Catalyst by Acids. Processes, 9(8), 1369. https://doi.org/10.3390/pr9081369








