Abstract
Cancer is a major cause of death worldwide, as exemplified by millions of cancer diagnoses every year. The use of chemotherapy in treating cancer has many disadvantages which include recurrence of cancer, associated with drug resistance, and severe side effects that are harmful to the patients. A better source of anticancer drugs can come from nature. Strobilanthes crispus (S. crispus) is a herbal medicinal plant that is indigenous in Madagascar and the Malay Archipelago. The plant possesses high vitamin and mineral content as well as phytochemicals—like phenols, catechins, tannins, and flavonoids—that are known to have therapeutic effects. Numerous preclinical studies have reported very versatile pharmacological effects of this plant, such as anticancer, antimicrobial, antioxidant, anti-angiogenesis, anti-diabetes, anti-ulcerogenic, and wound healing. Herein, this paper reviews the anticancer properties of S. crispus, providing information for future research and further exploration.
1. Introduction
Cancer refers to uncontrolled cellular growth which is mainly caused by genetic and environmental factors [1]. Hanahan et al. enumerated six hallmarks of cancer as resistant to necrosis or apoptosis, persistent signals of cell proliferation, escape from growth-suppressing factors, uncontrolled cellular replication, sustained blood supply, and lastly capable of metastasizing [2]. Thus, it is very challenging to remove the cancerous cells thoroughly from the cancer patients. Not surprisingly, cancer is the second leading cause of deaths worldwide and the cancer burden is still continuously growing [3]. Common types of cancer include lung cancer, breast cancer, colorectal cancer, prostate cancer, stomach cancer, and liver cancer [4].
Various therapies have been developed for cancer treatments. For example, chemotherapy, radiation therapy, hormonal therapy, stem cell transplantation, and surgery [1,5]. However, these treatments usually come with severe side effects, such as severe pain, fatigue, becoming debilitated, anemia, leukopenia, cardiotoxicity, and even opportunistic infections [1,5,6,7]. Therefore, it is important to seek for alternative treatment strategy that would bring a similar anticancer effect but with less severe side effects.
Natural plants have been traditionally used or consumed by humans as a method in aiding the recovery from illnesses [8]. Phytotherapy, also known as herbal medicine or herbalism, is the use of plants’ extracts for either treatment or health-promoting purposes [8,9,10]. To date, various plant extracts have been discovered to confer medical benefit (as shown in Table 1). Various solvents have been used in the plant extraction processes. The choice of solvent depends on which compound is desired in the extract. Furthermore, the solvent chosen should not affect the test models. Toxicity of the solvent should be carefully considered before selecting the solvent. Usually, the solvent will be removed from the extract in the extraction process for use.

Table 1.
Examples of plants that possess medical properties 1.
Strobilanthes crispus, which is a shrub belonging to the family of Acanthaceae, is naturally found in Madagascar as well as the Malay Archipelago [36,37]. It is also commonly known in local dialects as “pecah beling”, “pecah kaca”, “keji biling”, “bayam karang”, “pokok ngokilo”, “lidah jin”, or “jin batu” [37,38]. This plant normally grows alongside the river or in uninhabited fields [38]. The leaves of the plant are oblong-lanceolate in shape, rather obtuse and shallowly crenate-crispate [39]. The upper surface of the leaves is rough, darker green in color, and covered by short hair [37,39]. As for the flowers of S. crispus, they are yellow in color and have the characteristics of short, dense, and panicled spikes [39,40].
S. crispus is conventionally known as a folklore medical plant in Indonesia and also Malaysia. This plant is consumed by the people either freshly or by boiling in water like tea. The tea tastes slightly bitter due to the high content of cystoliths of calcium carbonate and the alkaline infusion gotten from this plant [37,38,41]. They customarily ingest this plant as a remedy for various diseases or medical conditions—such as diabetes, calculi (kidney stones), hypertension, and constipation—as well as to enhance immunity in order to prevent the development of cancer [37,41,42].
Many studies have reported that S. crispus contains numerous phytochemicals (as shown in Table 2) and the identified compounds have been proven scientifically to possess various favorable medical properties, such as anti-oxidant [43,44,45], anti-microbial [46,47], anti-diabetic [48,49], anti-ulcerogenic [50], as well as wound healing [51,52]. Most importantly, according to a number of studies carried out, the results showed that this plant also exhibited anti-cancer features. This is particularly significant since cancer is still a tremendous burden and it has caused millions of death worldwide. Thus, this review article aims to collect and compile scientifically proven information of S. crispus in order to provide an overall review of this plant relating to its anti-cancer properties against various types of cancers. A section on its toxicological studies is also included.

Table 2.
Notable phytochemicals isolated from S. crispus.
2. Anticancer Properties of Strobilanthes crispus against Various Cancers
2.1. Breast Cancer
2.1.1. In Vitro Studies
Breast cancer is the presence of malignant breast nodule, mass or abscess. The lump or mass is usually painless, hard and irregular in shape. However, it can also be tender, soft, rounded, or painful. Muslim et al. evaluated the cytotoxic and anti-angiogenesis effects of S. crispus on MCF-7 and T-47D cells by using the aqueous and methanolic extracts of its leaves. Water is a suitable solvent for the extraction of polar compounds while methanol has been identified as an effective solvent that resulted in a high extraction yield. Cytotoxicity test showed that IC50 of the aqueous extract was 120.7 µg/mL for MCF-7 cells whereas IC50 values of the methanolic extract were 160.16 µg/mL and 121.53 µg/mL for MCF-7 and T-47D cells respectively. In addition, both extracts showed positive anti-angiogenesis effects based on ex vivo rat aortic ring assay. The aqueous extract was reported to have stronger anti-angiogenesis effect where it showed 16.67 ± 8.11% of inhibition while the methanolic extract inhibited 6.25 ± 3.6% of angiogenesis. The concentration of both extracts was at 100 µg/mL. The anti-angiogenesis effect exhibited by the plant was likely to be contributed by the presence of the antioxidant phytosterol compounds such as α-sitosterol (7.08%), phytol (3.78%), campesterol (2.63%), and stigmasterol (7.89%) [44].
Yaacob et al. did a research on evaluating the anticancer activity of a sub-fraction of dichloromethane extract (remarked as SC/D-F1, SC/D-F2, SC/D-F3, …, SC/D-F15) of S. crispus on MCF-7 and MDA-MB-231 breast cancer cell lines. Dichloromethane is not miscible with water. It is able to dissolve a wide range of organic compounds and useful for lipid extraction. In this study, 15 dichloromethane sub-fractions were obtained by using thin layer chromatography. LDH Cytotoxicity Detection Kit was used to measure the cytotoxicity. Among these fractions, SC/D-F9 showed the most cytotoxicity in which it had the lowest EC50 values of 8.5 µg/mL and 10.0 µg/mL, respectively on MCF-7 and MDA-MB-231 cells, compared to the others. A comparison between this particular sub-fraction and tamoxifen (a chemotherapeutic drug) was also made regarding the ability to induce cellular death. The results showed that at the EC50 value of 8.5 µg/mL, SC/D-F9 induced 44% MCF-7 cell death in 24 h and 57% MCF-7 cell death in 48 h. Tamoxifen only induced substantial death (similar to that of SC/D-F9 in 48 h) at high concentration (15 µM). As for the MDA-MB-231 cells, SC/D-F9 induced 80% cell death within 48 h at the EC50 value of 10.0 µg/mL, corresponding to the effect of tamoxifen at the concentration of 15 µM on the particular cell line. Since MCF-7 and MDA-MB-231 are estrogen receptor (ER)-dependent and ER-independent cell lines, respectively, the results suggested that the tumoricidal effects of SC/D-F9 is independent of the expression of estrogen receptor on the cells. In addition, the authors stated that SC/D-F9 caused cell death by inducing apoptosis through a signaling pathway involving caspase 3 and/or 7, which was proven by using fluorescence staining. Most importantly, the sub-fraction, SC/D-F9 was non-cytotoxic to normal breast epithelial cell line (MCF10A) with treatment up to 72 h while tamoxifen caused the cell death significantly. Hence, it can be inferred that SC/D-F9 has the more potent effect than the commonly used drug, tamoxifen in terms of causing the cancer cell death [52]. It was quoted that the cytotoxicity was due to the high content of gallic acid (phenol, 29.0 ± 0.867 mg/g) and catechin (flavonoid, 59.0 ± 0.333 mg/g).
Chong et al. did an investigation on the ethanol extract of S. crispus leaves on MCF-7 and MDA-MB-231 cells. Ethanol has been known as a good solvent for polyphenol extraction. The results showed that the ethanol extract was not effective against MDA-MB-231 cells (IC50 > 100 μg/mL). On the other hand, the extract was able to exert anti-proliferative and cytotoxic effects on the MCF-7 cells detected by using MTT and BrdU assays at the IC50 concentration of 30 µg/mL. It was found that 12% of the cells were arrested at sub-G1 phase after extract treatment at 24 h and increased to 35% at 48 h followed by 47% at 72 h. The induction of apoptosis by S. crispus was identified using TUNEL assay which showed that 30% of cells were TUNEL positive after 48-h exposure and 50% were TUNEL positive after 72-h exposure to treatment. Furthermore, the ethanol extract of S. crispus also caused a significant increase (p < 0.05) in the concentration of cytochrome c after 24 to 36 h of exposure. There was also an increase in the level of initiator caspase 9 after 6- and 36-h exposure although it was not significantly different (p > 0.05) as compared to the control cells. Level of active caspase 3/7 after 48- and 72- exposures were also significantly elevated (p < 0.05). The level of cyclin dependent kinase (CDK) 2 and cyclin dependent kinase 4 were also significantly increased (p < 0.05) after treatment for 24 h. In addition to that, the expression of tumor suppressor p53 gene was upregulated significantly (p < 0.05) and the level of XIAP (an apoptosis inhibitor) was downregulated significantly (p < 0.05) after 48 h of exposure. The author suggested that S. crispus extract induced DNA fragmentation and thus apoptosis through mitochondrial activated apoptosis pathway involving CDK2, CDK4, and p53. The author also proposed that stigmasterol may be the factor inducing p-53 mediated apoptosis in MCF-7 cells [56].
Rahmat et al. examined the anti-proliferative effects of the extracts from S. crispus leaves (catechin, ethanol, methanol, chloroform, hexane, and ethyl acetate) as well as two bioactive compounds (β-sitosterol and stigmasterol) isolated from the leaves of this plant on MCF-7 and MDA-MB-231 cells [57]. The solvents used in the extraction are with different polarities, and hence would be able to extract different compounds from the plant. The cytotoxicity test revealed that all the extracts required a large dose (IC50 > 100 µg/mL) to exert the anti-proliferative effect on MCF-7 cells with the exception of methanol extract. The methanol extract exhibited the anti-proliferative effect on MDA-MB-231 cells with IC50 value of 27.2 µg/mL. The findings also showed that β-sitosterol had a better effect on MCF-7 cells (IC50 = 71.2 µM) compared to MDA-MB-231 cells (IC50 > 247.5 µM). As for stigmasterol, it had similar anti-proliferative effect on both MCF-7 and MDA-MB-231 cells with IC50 values of 156.0 and 185.9 µM, respectively.
Baraya et al. studied the anti-migration, anti-invasion and anti-metastasis effects of S. crispus leaves in MDA-MB-231 breast cancer cells by using a standardized sub-fraction called F3 [58]. F3 contained a variety of constituents, including β-sitosterol, stigmasterol, campesterol, lutein, pheophytin a, 131-hydroxy-132-oxo-pheophytin a, and 132- hydroxy-pheophytin a. The results showed that F3 exerted anti-proliferative effect with the IC50 value of 84.27 µg/mL after 24 h of exposure and 74.41 µg/mL after 48 h of exposure. In terms of anti-migration effect, F3, with the concentration of 50 µg/mL and higher, remarkably (p = 0.018) inhibited the migration of the cancerous cell in a dose- and time-dependent manner. F3 also significantly (p = 0.04) inhibited the invasion in a dose-dependent manner when the cells were treated with above 50 µg/mL of this extract. The anti-metastatic effect of F3 was evident in in vivo experiment with the observed downregulation of the six metastatic markers. MMP-9, MUC-1, N-cadherin, twist, VEGF, and vimentin were significantly (p < 0.05) reduced in F3-treated tumor-bearing mice compared to the untreated group. On the other hand, F3 also caused significant upregulation (p < 0.002) of E-cadherin expression, a negative diagnostic biomarker of breast cancer, as compared to untreated group.
In another study, the effects of a bioactive sub-fraction of S. crispus (SCS) leaves and its combination with tamoxifen in terms of cell cycle modulation on MCF-7 and MDA-MB-231 cells were analyzed by using flow cytometry [59]. With the concentration of 8.5 µg/mL or 10.0 µg/mL, SCS astonishingly increased (p < 0.05) the percentage of MCF-7 cells in G1 phase (73.4%) and G2/M phase (9.4%) while reducing the percentage of cells in S phase (17.2%) compared to the corresponding phases in untreated cells (58.7%, 5.4%, and 35.9%, respectively). On the other hand, tamoxifen (5 µM) caused the MCF-7 cells to be arrested in G1 phase (72.8%) and reduced the percentage of the cells in S phase (22.3%) compared to untreated cells. As for the combination of SCS with tamoxifen (8.5 µg/mL or 10.0 µg/mL of SCS combined with 5 µM of tamoxifen), the percentage of cells arrested in G1 phase was 70.6% and the percentage in S phase was reduced to 23.1%. As for MDA-MB-231 cells, SCS caused remarkable (p < 0.05) accumulation of cells in G1 phase (74.0%) while reducing the percentage of cells in S phase (19.4%) compared to the corresponding phases of untreated controls (51.3% and 34.8%, respectively). It had no effect on the percentage of MDA-MB-231 cells in G2/M phase. For the same cells treated with tamoxifen, the cell population in G1 phase was increased to 63.5% while the percentage of cells in S phase was decreased to 26.4%. The effects of SCS combining with tamoxifen was also noticeable in increasing the percentage of the cells in G1 phase (70.2%) as well as in decreasing the cell population in S phase (18.9%). The research group also investigated the expression of cell cycle regulatory proteins. The results revealed that in MCF-7 cells, SCS induced an increase in cyclin D1, p21 and p53 proteins, and a decrease in cyclin E after 24-h treatment while it did not induce any significant change in level of cyclins A and B. For 48-h treatment, there was an unexpected decrease in cyclin D1 expression. Similar observations were obtained when using tamoxifen alone or combination with SCS. As for MDA-MB-231 cells, SCS and the combination with tamoxifen caused a reduction in cyclin D1 expression. Cyclin E expression was also observed to be decreased but cyclin B expression was slightly increased after 48 h of combination treatment. Taken all together, it was revealed that the growth inhibitory effects of S. crispus on both MCF-7 and MDA-MB-231 cells are analogous to tamoxifen and this plant has synergistic effects with tamoxifen in treating breast cancer.
Another study done by Gordani et al. investigated the anti-proliferative effects of S. crispus on MCF-7 cells using different extracts (methanol, hexane, chloroform, ethyl acetate, and aqueous extracts) from the leaves and the stem of the plant [60]. Results of MTT assay indicated that the aqueous extract of the leaves and the ethyl acetate extract of the stem had the strongest anti-proliferative activity with the IC50 values of 23 µg/mL and 38 µg/mL, respectively. Besides that, the methanol and chloroform extracts of the leaves as well as the chloroform extract of the stem also showed moderate inhibition with the IC50 values of 74, 80, and 86 µg/mL, respectively.
Koh et al. also evaluated the anticancer mechanisms of S. crispus hexane extract on MDA-MB-231 cells [61]. In this study, leaf chloroform (LC), leaf ethyl acetate (LEA), leaf hexane (LH), leaf methanol (LM), stem chloroform (SC), stem ethyl acetate (SEA), stem hexane (SH), and stem methanol (SM) extracts were used. Figure 1 shows the steps of plant extraction. Results of MTT assay showed that SH showed strong inhibition against the cancer cells with the IC50 value of 42.50 µg/mL while LH showed mild anti-proliferative activity with the IC50 value of 192.50 µg/mL. However, the other extracts showed no cytotoxic effects on the cancer cells. The IC50 value of SH (42.50 µg/mL) was lower than that of 5-flurouracil (5-FU). Selectivity index (SI) was determined in which both SH and LH had no good selectivity (0.26 and 0.44 respectively) towards the cancer cells over normal cells. However, 5-FU even had a worse selectivity (0.16) than the two extracts. Since SH had the lowest IC50 value, this extract was further studied to investigate its effects on cell cycle modulation. SH slightly increased the cell population in Sub-G and S phases (5.04% and 8.57%, respectively) while reducing the percentage of G0/G1 phase (57.86%) compared to control groups (0.56%, 0.94%, and 70.25%, respectively). The percentage of the cells treated with SH in G2/M phase was similar to that of the control groups. However, the differences of cell cycle phases between the control and treated groups were not statistically significant. Besides the effects on cell cycle arrest, SH delayed the cell doubling time (p > 0.05) in which it caused a 2.5-fold delay in cell proliferation (control group: 25.22 ± 8.40 h; SH-treated group: 62.63 ± 32.33 h) when treated at the IC50 value. However, the cell doubling time was significantly (p < 0.05) delayed to a longer time (84.87 ± 40.55 h) when the cells were treated with double IC50 value. The effects of SH on caspase-8 activation, which is involved in apoptotic pathway, were also studied and the results showed that 1.05-fold increase was induced although it was not statistically significant. In short, the findings suggested that stem hexane extract showed the most potent activity on anticancer property through cell cycle arrest at Sub-G and S phases which eventually leads to apoptosis through caspase-8 activation. The effects were said to be contributed by the presence of stigmasterol and β-sitosterol which are more effectively extracted in hexane.
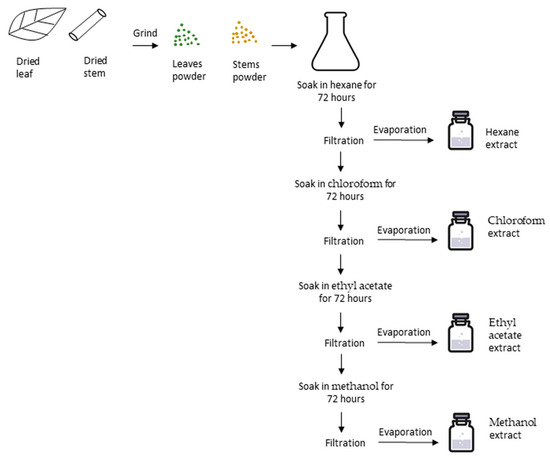
Figure 1.
Steps of plant extraction from Strobilanthus crispus leaves and stems. Dried leaves and stems were separated, crushed into fine pieces and then ground into powder form. The extraction was performed by mixing 500 mL of the first solvent into 100 g of powdered leaves or stems, and then macerating in the dark at room temperature for 72 h. The suspensions were filtered and then evaporated to obtain the first crude extract. The residues were then macerated in the second solvent and the processes were repeated to obtain the second extract. Eventually, the extracts obtained were as follows: leaf hexane (LH), leaf chloroform (LC), leaf ethyl acetate (LEA), leaf methanol (LM), stem hexane (SH), stem chloroform (SC), stem ethyl acetate (SEA), and stem methanol (SM).
Endrini et al. evaluated the cytotoxic effect of γ-sitosterol isolated from the leaves of S. crispus on MCF-7 cells by using MTT assay [62]. This bioactive compound had positive effect with IC50 value of 28.8 µg/mL. Another study done by Fadzelly et al. investigated the anti-proliferative effect on MCF-7 and metastatic MDA-MB-231 cells by using unfermented and fermented tea made from young and old S. crispus leaves [63]. The results showed that the hot water extract of both unfermented and fermented tea from old leaves demonstrated cytotoxic effects on MCF-7 cells with IC50 values of 80.5 µg/mL and 72.5 µg/mL, respectively. However, in MDA-MB-231 cells, no extracts showed considerable effects (IC50 > 100 µg/mL). This study suggested that consumption of either unfermented or fermented S. crispus tea made from old leaves could serve as an additional nutraceutical treatment in breast cancer patients.
In an investigation of the cytotoxic properties of essential oil from S. crispus, the results of MTT assay indicated that the essential oil from the plant had no effect on both MCF-7 and MDA-MB-231 cells [64]. Ng et al. also studied the methanolic leaf extract of the plant against T-47D cells and the results showed that the extract was effective against the cells with IC50 value of 99.83 ± 4.75 µg/mL [65].
2.1.2. In Vivo Studies
Yaacob et al. studied the anti-tumor effects of dichloromethane fraction (F3) of S. crispus on N-methyl-N-nitrosourea (NMU)-induced rat mammary tumor models [66]. In this study, 15 inbred female Sprague Dawley rats (36 days of age) were obtained and 10 rats were injected with NMU intraperitoneally in three weekly doses of 50 mg/kg body weight starting from 43 days of age. The results showed that 75% of the F3-treated rats were observed with complete tumor regression and the remaining (25%) rats were observed with partial tumor regression compared to the untreated control (p < 0.05). The significant reduction of tumor volume (p < 0.01) was found in the beginning of the fourth week of treatment. In addition, no recurrence of tumor and no complications were observed in the animals throughout the study. Nevertheless, the average tumor weight at necropsy was not significantly different from the untreated group. Also, significant incidental weight loss in the F3-treated rats was observed but the values were similar to the untreated group. In conclusion, administration of the dichloromethane fraction, F3, was found to effectively alleviate the overall tumor burden in NMU-induced rat mammary model compared to untreated group.
Immunomodulatory effects of the dichloromethane fraction (F3) of S. crispus were evaluated by Yankuzo et al. In terms of cellular immune parameters, the results showed that administration of F3 significantly increased (p < 0.05) the number of infiltrating CD4+ and CD8+ immune cells as well as the expression of CIITA and MHC-II on the mammary cancer cells compared to the untreated group. In addition to that, the serum level of chemokine ligand 2 (CCL2) was found to be significantly decreased (p < 0.05) after eight weeks of treatment, while the level of interferon gamma (IFN-γ) was increased the most among the 34 cytokines examined, although it was not significant (p = 0.083) [67]. The author suggested that a rise in the number of IFN-γ activated more CD4+ and CD8+ immune cells, accompanied by upregulation of CIITA expression which in turn promoted the expression of MHC-II molecules that are responsible for the binding of CD4+ T cells, could lead to the destruction of the mammary tumor [68,69,70,71,72].
2.2. Liver Cancer
2.2.1. In Vitro Studies
Liver cancer is cancer that arises from the cells of liver. The most common type of liver cancer is hepatocellular carcinoma. The signs and symptoms of liver cancer include lump in the right upper abdomen, jaundice, abdominal pain, weight loss, and fatigue. A previous research has investigated the anti-proliferative effects of the catechin, ethanol, methanol, chloroform, hexane, and ethyl acetate extracts of S. crispus leaves as well as two isolated bioactive compounds, β-sitosterol and stigmasterol, on HepG-2 cancer cells [57]. The results of MTT assay showed that methanolic and chloroform extracts exerted the best effects with the IC50 values of 29.3 µg/mL and 28 µg/mL, respectively, while the other extracts required large doses (IC50 > 100 µg/mL). As for the two bioactive compounds, β-sitosterol, with the IC50 value of 53.0 µM, showed better effect than stigmasterol which had IC50 value of 182.0 µM.
Another study has examined the cytotoxic effects of different solvent extracts (hexane, chloroform, ethyl acetate, methanol, and water) of the leaves and the stems of S. crispus in HepG-2 cells [61]. Among the leaf extracts, chloroform and ethyl acetate extracts showed mediocre effects with similar IC50 values (175.70 ± 35.40 µg/mL, 176.70 ± 15.30 µg/mL, respectively) while no noticeable effects were exhibited by the other extracts. Among the stem extracts, hexane extract showed the best cytotoxic effect (IC50 = 38.80 ± 8.50 µg/mL), followed by chloroform extract (IC50 = 173.30 ± 5.80 µg/mL) while the others showed no significant cytotoxic effect. Besides that, the selectivity indexes (SI) of the chloroform leaf extract (1.05) and chloroform stem extract (>1.15) were higher than those of the other extracts. Stem hexane extract, which had the most potent cytotoxic effect, was further investigated for its effect on cell cycle progression and cell doubling time. Stem hexane extract at IC50 dose (38.80 ± 8.50 µg/mL) had no significant effect in cell cycle progression and cell doubling time. However, significant increase (p < 0.001) in the cell doubling time (control group: 17.86 ± 0.96 h; 2 × IC50 treated-group: 89.18 h) was observed when the treatment concentration was double the IC50 value. As for the cell cycle modulation, stem hexane extract-treated cells also had a significant increase (p < 0.05) in G0/G1 phase and significant decrease (p < 0.05) in sub-G, S, and G2/M phases. Caspase-8 activity was also significantly elevated (p < 0.01). Thus, the author concluded that stem hexane extract demonstrated the highest potential in anti-cancer effect through arresting the cells at G0/G1 phase and eventually induced caspase 8-mediated apoptosis. The anticancer property might be due to the presence of stigmasterol and β-sitosterol which were extracted more effectively in hexane solvent.
Endrini et al. examined the anticancer effect on HepG-2 cells using γ-sitosterol and the result showed that it exerted positive effect with IC50 value of 21.8 µg/mL [62]. This compound also inhibited the expression of c-myc gene. In another study, the effect of chloroform leaf extract on apoptotic pathway was investigated by using TUNEL assay which employed fluorescein [73]. Measures of 20 and 30 µg/mL of the chloroform extracts were used to treat the cells. Condensation of nuclei, fragmentation of DNA and apoptotic bodies were observed in the cells treated with 20 µg/mL extract and these observations were more pronounced in the cells treated with 30 µg/mL extract, suggesting that apoptosis was induced by the chloroform leaf extracts as its mechanism of action.
Hussin et al. explored the anticancer mechanisms on DNA damage, apoptosis, and gene expression in HepG-2 cells by using S. crispus juice at different concentrations (0.001%, 0.01%, 0.1%, 1%, and 10%) [74]. The MTT assay results showed that the cytotoxicity effect was dose- and time-dependent. The cell cycle was arrested at sub-G1 phase and apoptotic death was increased in the cells treated with juice at 0.1% and more. The number of cell death had 5-, 7-, and 10-fold changes at the concentrations of 0.1%, 0.4%, and 1.0% as compared to untreated cells. This was evident by the significant (p < 0.01) increase of cell population in sub-G1 phase from 3% to 25% and decreased of cell population in G2/M phase from 33% to 7%. The extent of DNA damage increased significantly in relation to the doses above 0.1% (p < 0.05). Treatment with 0.1%, 0.4% and 1% concentrations increased the c-myc gene expression but decreased both c-fos and c-erbB2 gene expressions, as compared to the controls. It was concluded that S. crispus juice exerted its cytotoxic property by inducing apoptosis in the cells through modulation of c-myc, c-fos, and c-erbB2 genes.
Tan et al. tested the photocytotoxic effect of the methanolic leaf extract of S. crispus on HepG-2 cancer cells using photodynamic therapy (PDT) [75]. The cells were incubated with the extract for 2 h prior to illumination with light at 660 nm for 10 min. There was no significant cell death after 2-h incubation with the methanolic extract. Interestingly, after light activation, treatment with methanolic extracts (3.125, 6.25, 12.5, 25, 50, and 100 µg/mL) exhibited positive anti-proliferative effect with the IC50 value of 8.51 ± 0.70 µg/mL. Under microscopic observation, the extract-treated cells showed features of cell shrinkage, membrane blebbing and cell debris. The author proposed that the anticancer effects of the extract might be contributed by the presence of flavonoids, phenolic acids, saponins, and tannins.
Another study done by Ng et al. tested the cytotoxicity effect of the methanolic leaf extract on Hep-G2 cells [65]. The findings showed that the extract had poor anticancer activity against the cancer cells with IC50 value of 111.52 ± 2.56 µg/mL. Similarly, Muslim et al. found that both methanolic and aqueous extracts of the leaves required high concentration (IC50 > 200 µg/mL) to exert the cytotoxic effect on Hep-G2 cells [44]. Rahmat et al. studied the cytotoxic effects of the essential oil extracted from S. crispus leaves against Hep-G2 cells and the result showed that the essential oil had no effect against the cells [64].
2.2.2. In Vivo Studies
Hanachi et al. investigated the effect of S. crispus leaf extract (SC) on the lesion scoring and P450 isoenzyme activity in the liver of diethylnitrosamine/acetylaminofluorene (DEN/AAF)-induced hepatocarcinoma rats. The results showed that the grade of inflammation and necrosis for the untreated DEN/AAF-induced liver cancer group was the highest, which had scored 2.3. This is followed by the glycyrrhizin-treated group, which had the score of 2.0. SC-treated group had a score of 1.0. The portal of SC-treated group had inflammation, but no necrosis was found. As for the score of lobular necrosis, untreated DEN/AAF-induced liver cancer group, with the score of 2.3, was found significantly different (p < 0.05) as compared to the SC-treated cancer groups and three normal groups. In the SC-treated group with the score of 1.0, inflammation without necrosis was observed and some area was normal without inflammation, which also had significant difference with all other groups (p < 0.05). Hereof, SC exhibited a positive effect on reducing inflammation and necrosis. As for the P450 isoenzyme activity evaluation, SC-treated group was found to have insignificant decrease in level of aniline hydroxylase activity (p < 0.05) which was near normal level. The detoxification activity of glutathione s-transferase (GST) was also studied and the results indicated that the SC-treated group had a significant decrease (p < 0.05) of GST activity in the liver cytosol after 12 weeks. In addition, the findings on the investigation of the stage of fibrosis showed that the score of fibrosis was significantly higher (p < 0.05) in untreated cancer groups than that of normal control groups. On the other hand, the stage of fibrosis was significantly lower (p < 0.05) in the SC-treated cancer groups as compared to the untreated cancer groups. No changes were found in the hepatocytes of normal rats after supplemented with SC. Taken together, these results suggested that 5.0% (w/v) S. crispus leaf extract exhibited anticancer effect by reducing the activity of aniline hydroxylase and glutathione s-transferase (GST) in liver cancer cells [76,77,78].
Suherman et al. also studied the effect of the aqueous extract of S. crispus leaves in the DEN/AAF-induced rats by examining the concentrations of plasma and liver enzymes, which are γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), and glutathione (GSH) [79]. After determining the enzyme level, the results showed that in the DEN/AAF-induced cancer groups (C), there were significant increases (p < 0.05) in the levels of GGT, ALP, and GSH as compared to the normal control group (N). On the other hand, all the SC supplemented-diet and glycyrrhizin-treated (all doses) groups had significantly decreased (p < 0.05) the levels of the three enzymes. In the same study, the effects of SC leaf extract on the activity of two other enzymes, glutathione s-transferase (GST) and uranyl diphosphate glucoronyl transferase (UDPGT) were also examined. According to the findings, the level of cytosolic GST and microsomal UDPGT was significantly high (p < 0.05) in the cancer groups (C) as compared to the normal control group (N). All the SC supplemented-diet and glycyrrhizin-treated groups caused significant reduction (p < 0.05) in the activity of both GST and UDPGT enzymes. Histologically, 5% of S. crispus aqueous extract showed a better effect than 1%, 2.5%, and 7.5% of the extracts, as the 5% extract caused the greatest reduction in the lesion scoring [80]. More importantly, administration of SC extract did not physiologically and histologically affect the liver in normal rats in both studies. Hence, these studies demonstrated that S. crispus leaf extract, at the optimum dose of 5% (w/v), is able to alleviate the severity of liver cancer by lowering the activity of γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP) and glutathione (GSH), glutathione s-transferase (GST), and uranyl diphosphate glucoronyl transferase (UDPGT) [79,80].
2.3. Colon Cancer
2.3.1. In Vitro Studies
Cancer that begins in the colon is called a colon cancer. The common signs of colon cancer include changes in bowel habits such as constipation or diarrhea, blood in the stool, anemia, weight loss, and others. The anti-proliferative effect of S. crispus on Caco-2 cancer cells was determined by using catechin, ethanol, methanol, chloroform, hexane, and ethyl acetate extracts as well as two isolated bioactive compounds, β-sitosterol and stigmasterol, from the leaves [57]. The MTT results indicated that both methanolic and chloroform extracts displayed anti-proliferative effect on Caco-2 cells with IC50 values of 22.3 and 25.1 μg/mL, respectively. The two bioactive compounds, β-sitosterol and stigmasterol, also exhibited anti-proliferative effect on the cancer cells with IC50 values of 20.0 and 132.5 μM respectively. The rest of the extracts required a larger dose (IC50 > 100 μg/mL) to have the anticancer effect.
The anticancer effect of γ-sitosterol isolated from the S. crispus was tested on Caco-2 cells [62]. This compound exerted strong anti-proliferative ability with IC50 value of 8.3 μg/mL. They also showed that the compound inhibited the c-myc gene expression, leading to activation of apoptosis.
In another study, the anti-proliferative effect of the ethanol extract of the leaves against HT-29 cells was determined by using MTT and BrdU assays [56]. The findings revealed that the ethanol extract was effective in causing the cancer cell death with IC50 value of 52 ± 6.3 μg/mL; however, there was no significant difference (p > 0.05) as compared to positive control (doxorubicin, IC50 = 52.2 ± 2.9 μg/mL).
Ismail et al. examined the anti-proliferative effect of different S. crispus extracts (hexane, dichloromethane, ethyl acetate, and methanol) from the leaves and flowers on HT-29 cells [81]. The results showed that the ethyl acetate and methanolic extracts of the leaves demonstrated anticancer effect on the cells with IC50 values of 70.2 ± 1.4 and 59.0 ± 0.8 μg/mL, respectively, while no anticancer effect was observed in the hexane and dichloromethane extracts. As for the flower extracts, the dichloromethane and ethyl acetate extracts had anti-proliferative effect on the cells with IC50 values of 90.3 ± 1.1 and 42.0 ± 1.8 μg/mL, respectively, while the hexane and methanolic extracts had no effect.
Ng et al. studied the anticancer property of methanolic extract of S. crispus leaves on HCT-116 cells [65]. The extract showed minimal anti-proliferative effect (IC50 > 200 μg/mL) on the cells. Similar results were reported from the study done by Muslim et al. [44].
2.3.2. In Vivo Studies
Al-Henhena et al. investigated the chemopreventive effect of ethanol extract of the leaves on the azoxymethane (AOM)-induced colonic aberrant crypt foci (ACF) colorectal cancer rat model, and the extract was also tested for its total phenolic and flavonoid content [82]. The result showed that the extract decreased the ACF numbers in the rat colon. Treatment with S. crispus extract significantly (p < 0.01) reduced approximately 71–74% of the number of ACF and remarkably (p < 0.05) attenuated the distribution of ACF segment which was in the middle and distal parts of the colon, as compared to AOM group which was in the middle and proximal parts. This suggests that the extract restored the anatomical integrity by enhancing the cellular defense mechanism. In addition to that, the weights of body and colon increased significantly (p < 0.05) in both 250 and 500 mg/kg extract-treated groups compared to AOM group. The author suggested that the increase in the weights might have relation to the enhanced stability of the colonic wall. The total phenolic content (737.67 ± 0.024 mg, expressed as gallic acid equivalent in mg/g) and the total flavonoid content (262.86 ± 0.005 mg, expressed as quercetin equivalent in mg/g) were also said to possibly contribute to the therapeutic effects.
2.4. Prostate Cancer
In Vitro Studies
The prostate is a small gland of walnut shape in the pelvis of men. Prostate cancer is a form of cancer that develops in this gland. In its early stages, prostate cancer often has no symptoms. Prostate cancer that is more advanced may cause signs and symptoms such as trouble urinating, decreased force in the stream of urine, blood in the urine, blood in the semen, bone pain, losing weight, and erectile dysfunction. A previous study evaluated the anticancer activities of different sub-fractions of dichloromethane extract of S. crispus on PC-3 and DU-145 prostate cancer cell lines [52]. A total of 15 sub-fractions were produced after application of thin layer chromatography (TLC) and their cytotoxicity against the cancer cells was determined using LDH Cytotoxicity Detection Kit. SC/D-F9 fraction showed the most potent cytotoxic effects with EC50 values of 7.4 and 7.2 µg/mL on PC-3 and DU-145 cells, respectively. This sub-fraction was also compared to commonly used chemotherapeutic drugs, paclitaxel, docetaxel and doxorubicin. At the constant EC50 dose of 7.4 µg/mL, SC/D-F9 caused 90% death in PC-3 cells while paclitaxel (50 nM), docetaxel (20 nM) and doxorubicin (100 nM) only caused less than 30% cell death. In DU-145 cells, SCD-F9 induced more than 50% cell death while docetaxel (20 nM) only caused 40% of cell death while doxorubicin and paclitaxel only caused less than 10% cell death. The author suggested that the high content of gallic acid (phenol, 29.0 ± 0.867 mg/g) and catechin (flavonoid, 59.0 ± 0.333 mg/g) found in the particular fraction may contribute to its cytotoxic property.
2.5. Cervical Cancer
In Vitro Studies
Cervical cancer happens when cells change in cervix. Most cases of cervical cancer are caused by infection with human papillomavirus. Cervical cancer may not have signs and symptoms at early stage. Advanced cervical cancer may cause vaginal bleeding or discharge. The anti-proliferative property of the ethanol extract of S. crispus leaves against HeLa cervical cancer cells was investigated by Chong et al. [56]. The result revealed that the extract insignificantly (p > 0.05) inhibited the proliferation of the cancer cells with IC50 of 78 ± 1.5 μg/mL. Chong et al. also studied the anti-proliferative effect of the hexane and aqueous extracts of the leaves and stem of the plant against HeLa cervical cancer cells [83]. Using the MTT assay, the percentage viabilities of the extracts at different concentrations (12.5, 25, 50, 100, and 200 µg/mL) were determined. The results showed that hexane stem extract exerted positive anti-proliferative effects with IC50 value of 160 ± 10.01 µg/mL while other extracts showed little or no anti-proliferative effect. Cells showed characteristics of apoptosis, which include cellular membrane blebbing, chromatin condensation and cellular shrinkage, after 72 h of hexane stem extract treatment. Flow cytometry was also done to examine the cell cycle arrest and the findings indicated that there was an increase (0.25% to 6.10%) in sub-G1 phase and slight increase (3.06% to 5.08%) in S phase accompanied by decreases in G0/G1 phase and in G2/M phase. The authors suggested that the enhanced apoptosis was mediated via the significant elevation (p < 0.05) in caspase-3/7 activity as well as slight increase in caspase-9.
2.6. Nasopharyngeal Cancer
In Vitro Studies
Nasopharyngeal cancer is occurring in the nasopharynx. Some common symptoms of nasopharyngeal cancer include neck lump, hearing loss, stuffy nose, and nosebleeds. Five different solvent extracts (hexane, chloroform, ethyl acetate, methanol, and aqueous) of the leaves and stems of S. crispus were tested on CNE-1 cells [84]. The MTT assay results indicated that among the leaf extracts, the ethyl acetate extract showed the strongest anti-proliferative effect on the cells (IC50 value of 119.00 ± 48.10 µg/mL), followed by hexane extract (IC50 = 123.50 ± 37.50 µg/mL) and chloroform extract (IC50 = 161.70 ± 20.20 µg/mL). For the stem extracts, hexane extract showed the most potent anti-proliferative effect with IC50 value of 49.40 ± 8.00 µg/mL, followed by chloroform extract (IC50 = 148.30 ± 23.20 µg/mL) and then ethyl acetate extract (IC50 = 163.50 ± 16.30 µg/mL). Methanolic and aqueous extracts of both leaves and stems had no significant effect on the cells. In terms of selectivity index (SI), as compared to 5-fluorouracil (3.15), the SI order of the different extracts was as follows: ethyl acetate leaves (1.40) > chloroform stem (1.35) > chloroform leaves (1.14) > ethyl acetate stem (1.06) > hexane leaves (0.68) > hexane stem (0.22). Overall, the authors showed low selectivity towards the cancer cells. In terms of cell doubling time, the leaf chloroform extract caused a significant ~2-fold increase (p < 0.05), compared to untreated control cells. This increase was also seen in both leaf and stem hexane extracts but it was not significant. CNE-1 cells treated with stem ethyl acetate extract was found to increase the cell doubling time by 82.50%. Cell cycle analysis was also done by using flow cytometry to determine the apoptogenic effect of the extracts. Leaf ethyl acetate, leaf chloroform, stem hexane and stem chloroform extracts significantly increased (p < 0.05) the sub-G phase from 0.69% to 22.08%, 12.85%, 4.07%, and 57.67%, respectively. Stem chloroform extract also remarkably (p < 0.05) reduced the G0/G1 population by 67.55%. Significant reduction in the cell population in G2/M phase was seen in cells treated with leaf hexane, leaf chloroform and stem chloroform extracts. Microscopic observation revealed that cells treated with various S. crispus leaf and stem extracts caused notable morphological changes such as presence of floating dead cells and apoptotic bodies as well as reduction of number of viable cells. The effect of S. crispus extracts on caspase activation was also studied and the results showed that all extracts decreased the apoptotic executioner caspase 3/7 activity whereas there was no effect on apoptotic caspase 8/9 activity (production of apoptotic bodies), suggesting that the caspase-mediated apoptosis induced by S. crispus was not the sole process responsible for the anticancer mechanism.
2.7. Lung Cancer
In Vitro Studies
Lung cancer is the cancer that arises from the lining of the bronchi, or other areas of the respiratory system, including the trachea, bronchioles, and alveoli. Lung cancers are generally divided into two types: non-small cell lung cancer and small cell lung cancer. The early symptoms of lung cancer may be a slight cough or shortness of breath, and as the cancer develops, these symptoms may become more severe. A cytotoxicity study of the methanolic leaf extract of S. crispus was performed on NCI-H23 lung cell line [44]. The results showed that the methanolic leaf extract was not effective against the cells (IC50 > 200 µg/mL). The findings were similar as the one done by Ng et al. [65].
3. Toxicology Studies
3.1. In Vitro Studies
There are numerous studies done by examining the cytotoxic effects of various S. crispus extracts against normal cell lines [56,58,63]. Normal breast epithelial cell line (MCF10A) and Chang liver (normal) cell line were used in some studies and the results showed that S. crispus extracts were not cytotoxic against normal cells.
3.2. In Vivo Studies
Al-Henhena et al. evaluated the cytotoxic effect of S. crispus ethanol leaf extract in Sprague–Dawley rats treated with vehicle (10% Tween-20) or treated with 2500 mg/kg ethanol extract in vehicle [82]. The findings showed that the dose at 2500 mg/kg was not toxic to the rats due to absence of mortality nor signs of toxicity. The serum parameters such as glucose, albumin, total protein, urea, creatinine, and enzymes (ALT, AST, ALP) were not significantly different between the control and treated groups. Hence, it was concluded that such high dose of S. crispus was safe as there were no adverse side effects observed.
Similarly, another in vivo toxicological study was done by Norfarizan-Hanoon et al. in Sprague–Dawley rats by orally feeding them the juice of S. crispus leaves [85]. The values of body weight, relative liver and kidney weights, aspartate aminotransferase (AST) activity, alanine aminotransferase (ALT) activity, alkaline phosphatase (ALP) activity, serum albumin level and creatinine level were tested and recorded at day 0 and day 14. The findings showed that there were no significant differences (p > 0.05) in all the parameters except the ALT activity in female groups fed with 700, 2100, 3500, and 4900 mg/kg BW of S. crispus juice, in which the level was lower than before, in comparison to the female untreated control groups. The reduction in ALT activity suggested that the hepatocytes were in healthy state without inflammation. In addition to that, the morphology and biochemical profile of both the liver and kidney had no changes after S. crispus juice treatment. Also, no signs of toxicity, adverse pharmacological effects or abnormal behavioral changes were observed throughout the 14-day study. Overall, the juice of S. crispus leaves caused no toxic effect to the normal Sprague–Dawley rats and was safe without causing adverse effects to the liver and kidney functions even at the maximum dose of 4900 mg/kg BW.
Lim et al. also studied the oral toxicity effect of S. crispus ethanol leave extracts in 20 female Sprague–Dawley rats. Regarding the findings, no signs of toxicity, lethality, and abnormal behavioral changes were observed in the rats throughout the 14-day experiment. Between the control group and three treatment groups (orally fed with 150, 300, and 600 mg/kg of ethanol leave extract), no significant differences (p > 0.05) were observed in terms of body weight, food intake, water intake, serum biochemical parameters, and relative weight of organs. Taken together, it was concluded that ethanol extracts of S. crispus leaves were not toxic to the rats and their liver and kidney functions. In addition, no-observed-adverse-effect-level (NOAEL) of S. crispus ethanol leaf extract was determined as the highest dose of 600 mg/kg per day. Moreover, acceptable daily intake (ADI) was also calculated to be 6 mg/kg per day. In conclusion, continuous 14-day oral administration of S. crispus ethanol leaf extract at 150, 300, and 600 mg/kg was safe to the female rats without affecting the normal liver and kidney functions as well as causing no sign of toxicity to them [86].
4. Conclusions
Strobilanthes crispus has been increasingly investigated for its potential as an anticancer therapy over a few decades. The plant’s extracts have shown potent anticancer effects against a wide range of cancers—including breast cancer, liver cancer, and colon cancer—through numerous in vitro and in vivo studies. However, its effect against other types of cancer such as cervical, prostate, nasopharyngeal, and lung cancer is still not conclusive due to the scarcity of those related studies. Another issue to be addressed is the limited number of toxicological studies; clinical trials are yet to be conducted to determine the safety and efficacy of the extracts towards human body. Future studies should be focused on exploring the use of other bioactive compounds in S. crispus, as well as evaluating the bioavailability, potency, and possible side effects of the extracts through clinical trials so that the pharmacological potential of the plant could be fully utilized.
Author Contributions
Conceptualization, M.G.N. and R.Y.K.; Methodology, M.G.N. and R.Y.K.; Validation, C.H.N., K.Y.N., S.M.C., A.P.K.L. and R.Y.K.; Writing—original draft preparation, M.G.N.; Writing—review and editing, C.H.N., K.Y.N. and R.Y.K.; Supervision, R.Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Higher Education, Malaysia (grant number FRGS/1/2019/SKK08/MUSM/02/2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors would like to thank Biomedical Science Program, International Medical University, Malaysia for the support to this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbas, Z.; Rehman, S. An Overview of Cancer Treatment Modalities. In Neoplasm; InTech: London, UK, 2018. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/health-topics/cancer (accessed on 17 July 2020).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–2491. [Google Scholar] [CrossRef]
- Lasley, I. What Cancer Treatments May Be Used. In 21st Century Cancer Treatment; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2010; pp. 16–55. [Google Scholar]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/cancer/survivors/patients/side-effects-of-treatment.htm (accessed on 17 July 2020).
- National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects (accessed on 17 July 2020).
- Vickers, A. Herbal medicine. West. J. Med. 2001, 175, 125–128. [Google Scholar] [CrossRef]
- Falzon, C.C.; Balabanova, A. Phytotherapy. Prim. Care Clin. Off. Pract. 2017, 44, 217–227. [Google Scholar] [CrossRef]
- Ghosh, D. Seed to Patient in Clinically Proven Natural Medicines**Partly adapted from Zangara and Ghosh (2014), with permission from CCR Press. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 925–931. [Google Scholar]
- Zhan, W.; Liu, Y.; Li, D.; Liu, Y. Advancing insights on the anti-obesity biochemical mechanism of (−)-epigallocatechin gallate (EGCG) by inhibiting α-amylase activity. RSC Adv. 2016, 6, 96918–96927. [Google Scholar] [CrossRef]
- Lee, S.; Al Razqan, G.S.; Kwon, D.H. Antibacterial activity of epigallocatechin-3-gallate (EGCG) and its synergism with β-lactam antibiotics sensitizing carbapenem-associated multidrug resistant clinical isolates of Acinetobacter baumannii. Phytomedicine 2017, 24, 49–55. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Liu, Z.-H.; Wan, X.-C.; Li, D.-X.; Tai, L.-L. The anti-hyperuricemic effect of epigallocatechin-3-gallate (EGCG) on hyperuricemic mice. Biomed. Pharmacother. 2018, 97, 168–173. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Braicu, C.; Irimie, A.I.; Zanoaga, O.; Gherman, C.; Berindan-Neagoe, I.; Campian, R.S.; Pileczki, V. Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis and autophagy in oral cancer SSC-4 cells. Onco. Targets. Ther. 2015, 461, 461–470. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Wang, X.; Zeng, S.; Zhang, X.; Zhao, J.; Zhang, X.; Chen, X.; Yang, W.; Yang, Y.; Dong, Z.; et al. The natural polyphenol curcumin induces apoptosis by suppressing STAT3 signaling in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 303. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; You, M.; Xu, Y.; Li, F.; Zhang, D.; Li, X.; Hou, Y. Inhibition of curcumin on myeloid-derived suppressor cells is requisite for controlling lung cancer. Int. Immunopharmacol. 2016, 39, 265–272. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B. Applications of the Phytomedicine Echinacea purpurea (Purple Coneflower) in Infectious Diseases. J. Biomed. Biotechnol. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.M.; Anderson, M.; Schoop, S.R.; Hudson, J.B. Bactericidal and anti-inflammatory properties of a standardized Echinacea extract (Echinaforce®): Dual actions against respiratory bacteria. Phytomedicine 2010, 17, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, W. Gambogic Acid is a Novel Anti-cancer Agent that Inhibits Cell Proliferation, Angiogenesis and Metastasis. Anticancer Agents Med. Chem. 2012, 12, 994–1000. [Google Scholar] [CrossRef]
- Cascão, R.; Vidal, B.; Raquel, H.; Neves-Costa, A.; Figueiredo, N.; Gupta, V.; Fonseca, J.E.; Ferreira Moita, L. Potent Anti-Inflammatory and Antiproliferative Effects of Gambogic Acid in a Rat Model of Antigen-Induced Arthritis. Mediators Inflamm. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Liu, L.; Qi, X.-J.; Zhong, Z.-K.; Zhang, E.-N. Nanomedicine-based combination of gambogic acid and retinoic acid chlorochalcone for enhanced anticancer efficacy in osteosarcoma. Biomed. Pharmacother. 2016, 83, 79–84. [Google Scholar] [CrossRef]
- Lee, D.C.; Yang, C.L.; Chik, S.C.; Li, J.C.; Rong, J.; Chan, G.C.; Lau, A.S. Bioactivity-guided identification and cell signaling technology to delineate the immunomodulatory effects of Panax ginseng on human promonocytic U937 cells. J. Transl. Med. 2009, 7, 34. [Google Scholar] [CrossRef]
- Li, X.-T.; Chen, R.; Jin, L.-M.; Chen, H.-Y. Regulation on Energy Metabolism and Protection on Mitochondria of Panax Ginseng Polysaccharide. Am. J. Chin. Med. 2009, 37, 1139–1152. [Google Scholar] [CrossRef]
- Attele, A.S.; Zhou, Y.-P.; Xie, J.-T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.-S. Antidiabetic Effects of Panax ginseng Berry Extract and the Identification of an Effective Component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef]
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological Activities and Chemistry of Saponins from Panax ginseng C. A. Meyer. Phytochem. Rev. 2005, 4, 159–175. [Google Scholar] [CrossRef]
- Xu, S.; Little, P.J.; Lan, T.; Huang, Y.; Le, K.; Wu, X.; Shen, X.; Huang, H.; Cai, Y.; Tang, F.; et al. Tanshinone II-A attenuates and stabilizes atherosclerotic plaques in Apolipoprotein-E knockout mice fed a high cholesterol diet. Arch. Biochem. Biophys. 2011, 515, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, Y.; Lu, Y.; Li, L.; Abdolmaleky, H.; Blackburn, G.L.; Zhou, J.-R. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Cancer 2011, 129, 1042–1052. [Google Scholar] [CrossRef]
- Yin, X.; Yin, Y.; Cao, F.-L.; Chen, Y.-F.; Peng, Y.; Hou, W.-G.; Sun, S.-K.; Luo, Z.-J. Tanshinone IIA Attenuates the Inflammatory Response and Apoptosis after Traumatic Injury of the Spinal Cord in Adult Rats. PLoS ONE 2012, 7, e38381. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, S.; Liu, P. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, X.; Shen, M.; Ru, D.; Gao, P.; Zhang, X.; Huang, C.; Sun, Y.; Li, H.; Duan, Y. Co-Delivery of Triptolide and Curcumin for Ovarian Cancer Targeting Therapy via mPEG-DPPE/CaP Nanoparticle. J. Biomed. Nanotechnol. 2018, 14, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, G.; Jiang, Z.; Yang, J.; Sun, L.; Zhang, L. Triptolide inhibits human breast cancer MCF-7 cell growth via downregulation of the ERα-mediated signaling pathway. Acta Pharmacol. Sin. 2015, 36, 606–613. [Google Scholar] [CrossRef]
- Bai, S.; Hu, Z.; Yang, Y.; Yin, Y.; Li, W.; Wu, L.; Fang, M. Anti-Inflammatory and Neuroprotective Effects of Triptolide via the NF-κB Signaling Pathway in a Rat MCAO Model. Anat. Rec. 2016, 299, 256–266. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef]
- Burkill, I.H.; Birtwistle, W. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture Malaysia: Kuala Lumpur, Malaysia, 2002; ISBN 9839863347. [Google Scholar]
- Sunarto, P. Penerbit Direktorat Jenderal Pengawasan Obat dan Makanan, 1st ed.; Materia Medika Indonesia: Jakarta, Indonesia, 1977. [Google Scholar]
- Noraida, A. Penyembuhan Semula Jadi Dengan Herba; PTS Millennia Sdn. Bhd.: Selangor, Malaysia, 2005; ISBN 9833372295. [Google Scholar]
- Stone, B.C. Flora of Java (Spermatophytes Only). Volume I: Gymnospermae, Families 1-7; Angiospermae, Families 8-110. C. A. Backer, R.C. Bakhuizen van den Brink, Jr. Flora of Java (Spermatophytes Only). Volume II: Angiospermae, Families 8-110. C. A. Backer, R.C.B.Q. Rev. Biol. 1969, 44, 424–425. [Google Scholar] [CrossRef]
- Nurraihana, H.; Norfarizan-Hanoon, N.A. Phytochemistry, pharmacology and toxicology properties of Strobilanthes crispus. Int. Food Res. J. 2013, 20, 2045–2056. [Google Scholar]
- Perry, L.M.; Metzger, J. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1980; ISBN 0262160765 9780262160766. [Google Scholar]
- Samuel, A.J.S.J.; Kalusalingam, A.; Chellappan, D.K.; Gopinath, R.; Radhamani, S.; Husain, H.A.; Muruganandham, V.; Promwichit, P. Ethnomedical survey of plants used by the Orang Asli in Kampung Bawong, Perak, West Malaysia. J. Ethnobiol. Ethnomed. 2010, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Manickam, E.; Danial, A.M.; Rahmat, A.; Yahaya, A. Chemical composition and antioxidant activity of Strobilanthes crispus leaf extract. J. Nutr. Biochem. 2000, 11, 536–542. [Google Scholar] [CrossRef]
- Muslim, N.S.; Ng, K.W.; Itam, A.; Nassa, Z.D.; Ismail, Z.; Abdul Maji, A.M.S. Evaluation of Cytotoxic, Anti-angiogenic and Antioxidant Properties of Standardized Extracts of Strobilanthes crispus Leaves. Int. J. Pharmacol. 2010, 6, 591–599. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Najim, N.; Zain, M.M.; Hamdan, S. Antioxidant, Total Phenolic Content and Cytotoxicity Evaluation of Selected Malaysian Plants. Molecules 2011, 16, 3433–3443. [Google Scholar] [CrossRef] [PubMed]
- Muskhazli, M.; Dirnahayu, M.; Nor Azwady, A.A.; Nurhafiza, Y.; Nor Dalilah, E.; Che Ku Nurshaira, C.K.N. Antibacterial Activity of Methanolic Crude Extracts from Selected Plant Against Bacillus cereus. Pertanika J. Trop. Agric. Sci. 2009, 32, 175–183. [Google Scholar]
- Hamad Abou Muamar, A.F. Isolation, Identification And Evaluation Of Antibacterial Activity Of The Semi-Purified Compound From Strobilanthes Crispus (L. Bremek). Master’s Thesis, Universiti Putra Malaysia, Seri Kembangan, Selangor, Malaysia, 1999. [Google Scholar]
- Norfarizan, N.A.; Asmah, R.; Rokiah, M.Y.; Fauziah, O.; Faridah, H. Antihyperglycemic, Hypolipidemic and Antioxidant Enzymes Effect of Strobilanthes crispus Juice in Normal and Streptozotocin-Induced Diabetic Male and Female Rats. Int. J. Pharmacol. 2009, 5, 200–207. [Google Scholar] [CrossRef]
- Mahmood, A.A.; Fard, A.A.; Harita, H.; Zahra, A.A.; Salmah, I. Evaluation of gastroprotective effects of Strobianthes crispus leaf extract on ethanol-induced gastric mucosal injury in rats. Sci. Res. Essays 2011, 6, 2306–2314. [Google Scholar] [CrossRef]
- Al-Henhena, N.; Mahmood, A.A.; Al-magrami, A.; Nor Syuhada, A.B.; Zahra, A.A.; Summaya, M.D.; Suzi, M.S.; Salmah, I. Histological study of wound healing potential by ethanol leaf extract of Strobilanthes crispus in rats. J. Med. Plants Res. 2011, 5, 3660–3666. [Google Scholar]
- Norfarizan, N.A.; Asmah, R.; Rokiah, M.Y.; Fauziah, O.; Faridah, H. Effects of Strobilanthes crispus Juice on Wound Healing and Antioxidant Enzymes in Normal and Streptozotocin-Induced Diabetic Rats. J. Biol. Sci. 2009, 9, 662–668. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Hamzah, N.; Nik Mohamed Kamal, N.N.; Zainal Abidin, S.A.; Lai, C.S.; Navaratnam, V.; Norazmi, M.N. Anticancer activity of a sub-fraction of dichloromethane extract of Strobilanthes crispus on human breast and prostate cancer cells in vitro. BMC Complement. Altern. Med. 2010, 10, 42. [Google Scholar] [CrossRef]
- Liza, M.S.; Abdul Rahman, R.; Mandana, B.; Jinap, S.; Rahmat, A.; Zaidul, I.S.M.; Hamid, A. Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah Kaca). Food Bioprod. Process. 2010, 88, 319–326. [Google Scholar] [CrossRef]
- Soediro, L.; Pellecuer, J.; Andary, C.; Privat, G. Strobilanthes crispus (L) B1 I: Pemeriksaan senyawaan turunan asam kafeat verbascosid. Acta Pharm. Indones. 1983, 8, 1–10. [Google Scholar]
- Cheong, B.E.; Zakaria, N.A.; Cheng, A.Y.F.; Teoh, P.L. GC-MS Analysis of Strobilanthes crispus Plants and Callus. Trans. Sci. Technol. 2016, 3, 155–161. [Google Scholar]
- Chong, H.Z.; Rahmat, A.; Yeap, S.K.; Md Akim, A.; Alitheen, N.B.; Othman, F.; Gwendoline-Ee, C.L. In vitro cytotoxicity of Strobilanthes crispus ethanol extract on hormone dependent human breast adenocarcinoma MCF-7 cell. BMC Complement. Altern. Med. 2012, 12, 35. [Google Scholar] [CrossRef]
- Rahmat, A.; Edrini, S.; Akim, A.M.; Ismail, P.; Yap, T.Y.H.; Fadzelly, M.A.B. Anticarcinogenic Properties of Strobilanthes crispus Extracts and its Compounds in vitro. Int. J. Cancer Res. 2005, 2, 47–49. [Google Scholar] [CrossRef]
- Baraya, Y.S.; Wong, K.K.; Yaacob, N.S. Strobilanthes crispus inhibits migration, invasion and metastasis in breast cancer. J. Ethnopharmacol. 2019, 233, 13–21. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Kamal, N.N.N.M.; Wong, K.K.; Norazmi, M.N. Cell Cycle Modulation of MCF-7 and MDA-MB-231 by a Sub-Fraction of Strobilanthes crispus and its Combination with Tamoxifen. Asian Pacific J. Cancer Prev. 2016, 16, 8135–8140. [Google Scholar] [CrossRef] [PubMed]
- Gordani, N.; Cheong, B.E.; Teoh, P.L. Antiproliferative Effect of Strobilanthes crispus on MCF-7 Cell Line. Trans. Sci. Technol. 2017, 4, 414–419. [Google Scholar]
- Koh, R.Y.; Lim, F.P.; Ling, L.S.Y.; Ng, C.P.L.; Liew, S.F.; Yew, M.Y.; Tiong, Y.L.; Ling, A.P.K.; Chye, S.M.; Ng, K.Y. Anticancer mechanisms of Strobilanthes crispa Blume hexane extract on liver and breast cancer cell lines. Oncol. Lett. 2017, 14, 4957–4964. [Google Scholar] [CrossRef]
- Endrini, S.; Rahmat, A.; Ismail, P.; Taufiq-Yap, Y.H. Cytotoxic effect of γ-sitosterol from Kejibeling (Strobilanthes crispus) and its mechanism of action towards c-myc gene expression and apoptotic pathway. Med. J. Indones. 2015, 23, 203–208. [Google Scholar] [CrossRef]
- Fadzelly, M.A.B.; Teh, A.H.; Rahmat, A.; Othman, F.; Hashim, N.; Fakurazi, S. Antiproliferative Properties and Antioxidant Activity of Various Types of Strobilanthes crispus Tea. Int. J. Cancer Res. 2006, 2, 152–158. [Google Scholar] [CrossRef]
- Rahmat, A.; Edrini, S.; Ismail, P.; Yap, T.Y.H.; Fadzelly, M.A.B. Chemical Constituents, Antioxidant Activity and Cytotoxic Effects of Essential Oil from Strobilanthes crispus and Lawsonia inermis. J. Biol. Sci. 2006, 6, 1005–1010. [Google Scholar] [CrossRef]
- Ng, K.-W.; Salhimi, S.; Majid, A.; Chan, K.-L. Anti-Angiogenic and Cytotoxicity Studies of Some Medicinal Plants. Planta Med. 2010, 76, 935–940. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Yankuzo, H.M.; Devaraj, S.; Wong, J.K.M.; Lai, C.-S. Anti-Tumor Action, Clinical Biochemistry Profile and Phytochemical Constituents of a Pharmacologically Active Fraction of S. crispus in NMU-Induced Rat Mammary Tumour Model. PLoS ONE 2015, 10, e0126426. [Google Scholar] [CrossRef]
- Yankuzo, H.M.; Baraya, Y.S.; Mustapha, Z.; Wong, K.K.; Yaacob, N.S. Immunomodulatory effects of a bioactive fraction of Strobilanthes crispus in NMU-induced rat mammary tumor model. J. Ethnopharmacol. 2018, 213, 31–37. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. Tumor-Infiltrating CD8 + Lymphocytes Predict Clinical Outcome in Breast Cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.-J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Holling, T.M.; Schooten, E.; van Den Elsen, P.J. Function and regulation of MHC class II molecules in T-lymphocytes: Of mice and men. Hum. Immunol. 2004, 65, 282–290. [Google Scholar] [CrossRef]
- Shi, B.; Vinyals, A.; Alia, P.; Broceno, C.; Chen, F.; Adrover, M.; Gelpi, C.; Price, J.; Fabra, A. Differential expression of MHC class II molecules in highly metastatic breast cancer cells is mediated by the regulation of the CIITA transcriptionImplication of CIITA in tumor and metastasis development. Int. J. Biochem. Cell Biol. 2006, 38, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Endrini, S.; Suherman, S.; Rahmat, A.; Ismail, P.; Taufiq-Yap, Y.; Othman, F. Effects of strobilanthes crispus extract on the apoptotic pathway of human liver carcinoma cell lines. Yars. Med. J. 2019, 15. [Google Scholar] [CrossRef]
- Hussin, F.; Eshkoor, S.A.; Rahmat, A.; Othman, F.; Akim, A.; Eshak, Z. Strobilanthes crispus Juice Concentrations and Anticancer Effects on DNA Damage, Apoptosis and Gene Expression in Hepatocellular Carcinoma Cells. Asian Pacific J. Cancer Prev. 2015, 16, 6047–6053. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-A.; Lim, S.; Law, C.; Yue, C.; Poh, T.; Saad, W.; Ismail, S.; Yusoff, K.; Loke, C. Antioxidative and Photocytotoxic Effects of Standardized Clinacanthus nutans and Strobilanthes crispus Extracts toward HepG2 Liver Cells. Pharmacogn. Mag. 2019, 15, 613. [Google Scholar] [CrossRef]
- Hanachi, P.; Fauziah, O.; Asmah, R. Lesion scoring and P450 Isoenzyme activity in liver of hepatocarcinogenesis rats treated with Strobilanthes Crispus. Int. J. Cancer Manag. 2008, 1, 11–15. [Google Scholar]
- Fauziah, O.; Hanachi, P.; Yogespiriya, S.; Asmah, R. Reducing Effect of Strobilanthes crispus Leaf Extract in Hepatocarcinogenesis Rats. Int. J. Cancer Res. 2005, 1, 109–112. [Google Scholar] [CrossRef]
- Yogespiriya, S.; Hanachi, P.; Patimah, I.; Asmah, R.; Fauziah, O. Histological Study During Hepatocarciniogenesis in Rats Treated With Strobilanthes crispus Extract. J. Biol. Sci. 2005, 5, 153–157. [Google Scholar] [CrossRef][Green Version]
- Suherman, J.; Asmah, R.; Fauziah, O.; Patimah, I.; Nor Haslinda, A. Effect of Strobilanthes crispus on Tumour Marker Enzymes and Glutathione During Chemical Hepatocarcinogenesis in the Rat. Pakistan J. Biol. Sci. 2004, 7, 947–951. [Google Scholar] [CrossRef][Green Version]
- Suherman, J.; Asmah, R.; Fauziah, O.; Patimah, I.; Siti Muskinah, H.M. Effect of Strobilanthes crispus on the Histology and Tumour Marker Enzymes in Rat Liver During Hepatocarcinogenesis. J. Med. Sci. 2005, 5, 130–135. [Google Scholar] [CrossRef]
- Ismail, M.; Bagalkotkar, G.; Iqbal, S.; Adamu, H.A. Anticancer Properties and Phenolic Contents of Sequentially Prepared Extracts from Different Parts of Selected Medicinal Plants Indigenous to Malaysia. Molecules 2012, 17, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Al-Henhena, N.; Khalifa, S.A.M.; Ying, R.P.Y.; Hassandarvish, P.; Rouhollahi, E.; Al-Wajeeh, N.S.; Ali, H.M.; Abdulla, M.A.; El-Seedi, H.R. Chemopreventive effects of Strobilanthes crispus leaf extract on azoxymethane-induced aberrant crypt foci in rat colon. Sci. Rep. 2015, 5, 13312. [Google Scholar] [CrossRef]
- Chong, Y.H.; Koh, R.Y.; Ling, A.P.K.; Chye, S.M.; Yew, M.Y. Strobilanthes crispus Extract Induces Apoptosis Through Enhanced Caspases Activities in Cervical Cancer Cells. In Proceedings of the International Conference on Biological, Environment and Food Engineering (BEFE-2014), Bali, Indonesia, 4–5 August 2014; International Institute of Chemical, Biological & Environmental Engineering: Barcelona, Spain, 2014. [Google Scholar]
- Koh, R.Y.; Sim, Y.C.; Toh, H.J.; Liam, L.K.; Lynn Ong, R.S.; Yew, M.Y.; Tiong, Y.L.; Ling, A.P.K.; Chye, S.M.; Ng, K.Y. Cytotoxic and apoptogenic effects of Strobilanthes crispa Blume extracts on nasopharyngeal cancer cells. Mol. Med. Rep. 2015, 12, 6293–6299. [Google Scholar] [CrossRef]
- Norfarizan-Hanoon, N.A.; Asmah, R.; Fauziah, O.; Rokiah, M.Y.; Faridah, H. Absence of Toxicity of Strobilanthes crispa Juice in Acute Oral Toxicity Study in Sprague Dawley Rats. Sains Malays. 2012, 41, 403–409. [Google Scholar]
- Lim, K.T.; Lim, V.; Chin, J.H. Subacute oral toxicity study of ethanolic leaves extracts of Strobilanthes crispus in rats. Asian Pac. J. Trop. Biomed. 2012, 2, 948–952. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
