Abstract
The recently defined and yet rather new topic of healthy aging is attracting more attention worldwide. As the world population is getting older, it is rapidly becoming essential to develop and maintain functional abilities at older age and develop mechanisms to protect the senior population from chronic diseases. One of the most effective components, as well as processes associated with aging, is the recently discovered and Nobel prize-awarded—nitric oxide (NO) (as a signaling molecule), which, followed by later discoveries, showed to have a positive metabolic, immunological, and anti-inflammatory effect. Nitrates are one of the most debated topics of the last decade in the scientific community due to their pathways involved in the production of nitric oxide. Thus, the objective of this study is to evaluate the effect of different potassium nitrate concentrate supplementation on Drosophila melanogaster longevity imitating a human carbohydrate-based diet with relationship to possible cause of oxidative stress. Influence of 0.5–3% potassium nitrate medium on the lifespan and motor function in different groups consisting of 100 fruit fly females in each was analyzed. In this assay, female fly species supplemented with potassium nitrate diet showed life span increase by 18.6% and 5.1% with 1% and 2% KNO3, respectively, with a positive impact on locomotor function. In conclusion, we found that low concentration of potassium nitrate medium increased lifespan and locomotor function in Drosophila melanogaster.
1. Introduction
Aging is a natural but imminent decline of physiological functions that eventually leads to death. Age is a major risk factor for the most common chronic degenerative, metabolic, and cardiovascular diseases [1,2]. Thus, healthy aging is a relevant and decisive process to develop and maintain functional ability for wellbeing at older age [3]. Use of dietary KNO3− may improve the aerobic capacity during increasing cycling exercise (ICE) in young physically active male persons [4]. External environmental factors such as diet, sleep quality, stress management, physical activity, and psychosocial aspects have an important impact on healthy aging and longevity [5,6]. Some dietary patterns and supplements and/or drug treatment can slow down the aging process and/or extend the lifespan [7]. All of them as well as regular exercise have shown to reduce the risk of neurodegenerative, cardiovascular, and metabolic diseases in mammalian models [8].
Dietary nitrate and nitrite supplementation can positively affect the production of nitric oxide (NO) [9]. NO has been identified as a major participant in regulative processes acting as signaling molecules and is relevant to a vast variety of different physiological processes—including mitochondrial function, blood stream regulation, heart and skeletal muscle contraction [10], neural protection and development, and immune activation, as well as signal transmission [11]. Scientific data show that nitrite protects against heart, brain, kidney, and liver cell injuries. NO can be produced by two pathways:
- (a)
- enzymatic pathway—when the special enzyme NO-synthase endogenously converts L-arginine into NO [10];
- (b)
- non-enzymatic recently discovered nitrate (KNO3−)-nitrite (NO2)-nitric oxide (NO) pathway.
The production of NO via the NO-synthase pathway is oxygen dependent, while the NO3− > NO2− > NO pathway is activated under hypoxic conditions [12]. Consumed dietary NO3− is broken down to bioactive NO2− by oral bacteria (saliva’s anaerobic bacteria) and further reduced to NO in the stomach and/or in peripheral blood vessels [9,12,13]. Aging interferes with NO signaling at every possible biochemical stage, from its production to inactivation [10,14,15]. Scientists agree that the bioavailability of NO decreases with age. In young and healthy organisms, the endothelial production of NO through the L-arginine pathway is effective and adequate, but aging inhibits the synthesis of endothelial NO production [11,16,17]. Deficiency of NO production increases the risk of hypertension, atherosclerosis, peripheral artery disease, heart failure, heart attack, stroke [10,14], and impaired cognitive function [18]. All of these conditions have been positively affected by dietary nitrite and nitrate intake, which can boost nitrate-nitrite-nitric oxide pathway and could have cardiovascular and metabolic benefits [14,19,20].
In recent decades, various model organisms, including the fruit fly Drosophila melanogaster, have been used to study the processes of aging and longevity. Drosophila melanogaster is an excellent model to analyze age-related decline and has a number of advantages as a species of short lifespan (60–80 days) [21] as well as great accessibility to different genetics tools [22]. NO plays an essential signaling role for these species as well [23,24]. Nitrite reduction to NO in the fruit fly was demonstrated in the newest trial, indicating its important role in the fly’s physiology [25]. Assuming that lower NO bioavailability is one of the hallmarks of aging and longevity and is related to cardiovascular and metabolic diseases as well, a boost of dietary nitrate-nitrite-NO pathway could appear to be an effective option to prevent age-related diseases [26].
We hypothesized that stimulating Drosophila via potassium nitrate supplementation could extend the lifespan of this species. Testing this hypothesis and observing food flies under experimental conditions, we gained more understanding of the safe dosing and evaluated possible synergetic effects in relationship with other ingredients used in the medium from the point of view of oxidative stress. To test this hypothesis, we carried out an experiment lasting 39 days where fruit flies were supplemented with different potassium nitrate concentrate solutions added to the medium. None of the research so far has yet hypothesized and analyzed the possible relationship between nitrates (which eventually acts as a source of nitric oxide) in relationship to longevity and (yet questionably) oxidative stress in living organisms. We believe that the novelty of this research is presented in our findings where, despite the “negative background” that has followed nitrates (in general) during recent decades, we present a whole different aspect of the possible application of the analyzed compound as well as raise multiple questions for future research. We also managed to define the “optimal/good” proportion—which flips the overall perspective of nitrates, bringing light to a whole new idea of using this compound in relationship to longevity and possibly oxidative stress.
2. Materials and Methods
2.1. Fruit Flies Culture
The fruit fly Drosophila melanogaster (female species only) Berlin-K wild strains kindly provided by Vilnius University and originally obtained from Bloomington Drosophila Stock Center (catalogue line Nr. 8522) were used in this study. Flies were cultivated under standard laboratory conditions. Unmated female species were transferred into the standard Drosophila vial (25 × 95 mm) with an oxygen permeable plug/cap, kept in a vertical position at all times (plug side). Test vials were placed on the standard tray in incubators for the whole duration of the experiment. Each tube contained 10 food flies and each group consisted of 10 vials, totaling 100 food flies in each (a total of 4 experimental + 1 control group) group, for a total of 500 fruit flies. All flies were randomly divided into one of 5 groups: control group, 0.5% potassium nitrate (KNO3), 1% KNO3, 2% KNO3, and 3% KNO3 solution groups. The solutions were prepared immediately before use. Flies were separated into 5 different groups on the same day (10 h after hatching), right after the media cooled down to room temperature, which accounted as the first day/start of experiment.
2.2. Feeding Assay
Flies were maintained on the media consisting of: water 750 g, agar-agar 15 g, dry yeast 150 g, raisins 40 g, melissa 50 g, and corn flour 50 g. Flies were separated 10 h after hatching and reared under standard laboratory conditions with a 12-h light/dark cycle at 25 °C. All experimental vials contained on average 20 mL of media, supplemented with 0.5%, 1%, 2%, or 3% of KNO3, which was added after cooling the media to 50 °C. Distilled water was used in the preparation of the corresponding solutions for all experimental groups, which served as an addition to a regular diet. Dead flies as well as locomotion assessment was carried out on the daily basis (same time or day 10:00 CET).
2.3. RING Protocol (Locomotor Behavior)
Locomotor behavior of fruit flies was evaluated using a rapid iterative negative geotaxis (RING) protocol [26]. Female fruit flies were placed in the same conditions with test tubes containing agar-dextrose-yeast (ADY) diet without or with the addition of 0.5% KNO3, 1% KNO3−, 2% KNO3, and 3% KNO3 solutions. A RING assay was repeated 7, 14, and 20 days after the ADY diet was started. Twenty fruit flies without anesthetizing and randomly were transferred to each of the prepared 5 tubes, forming all of them into the RING apparatus on the experimental day. Fruit flies were allowed to acclimatize in the new environment for 15 min. During this time, a digital camera was placed 1 m in front of the RING apparatus. The digital camera was focused, zoomed, and a timer was set to 3.0 s. The RING apparatus with fruit flies was sharply tapped three times on the surface of the bench, ensuring that the flies were knocked down to the bottom of the tubes. A picture was taken at the third second after the last (third) tap of the apparatus. A 1 min break was given to the fruit flies before repeating the same procedure. The whole experiment was performed in five sets (for control and four different potassium nitrate groups), and a total of five pictures was done for each set of the experiment. All pictures were analyzed, and a mean value was calculated from the pictures.
2.4. Statistical Analysis
Survival days estimates were expressed as the median ± percentage difference from the control group. Data normality is checked using the Shapiro–Wilk criterion [27]. Based on the results, the distribution of survival days does not follow a normal distribution, so the nonparametric Kruskal–Wallis (KW) criterion [28] is used because we are comparing more than two independent samples. The results of KW were found to be statistically significant, so the post hoc Mann–Whitney criterion [29] was applied to determine the impact of the salts on the survived population lifespan. Test results were statistically significant when probability p < 0.05. Survival assays for all conditions were done once.
3. Results
This experiment showed 1% and 2% concentrations of potassium nitrate significantly extended lifespan in female flies (Figure 1B,C). Precisely, median lifespan has significantly increased by 18.6% and 5.1% with 1% and 2% KNO3, respectively (Figure 1B,C,E; Table 1).
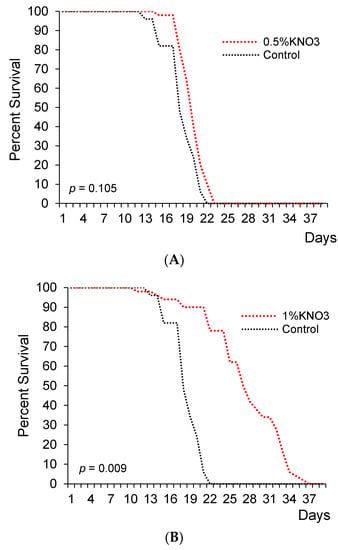
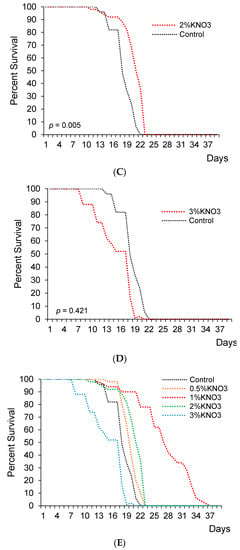
Figure 1.
The impact of potassium nitrate on the lifespan of female flies. Female flies supplemented with 0.5% ((A); n = 50; p = 0.105), 1% KNO3 ((B); n = 50; p = 0.009), 2% KNO3 ((C); n = 50; p = 0.005), and 3% KNO3− ((D); n = 50; p = 0.421). Cumulative survival of females (E).

Table 1.
KNO3- extends the median and mean lifespan of Drosophila melanogaster.
In the control group, the median lifespan was 29.5 days for female fruit flies. Different KNO3 supplementation diversely changed fruits flies’ lifespans. The measure of 0.5% KNO3 had no significant effect on the lifespan of fruit flies (Figure 1A,E). The median lifespan appeared to extend by 1.7% with 0.5% nitrate (p > 0.05), while 3% KNO3 medium concentration shortened the lifespan (p < 0.05) if compared to the control group (Figure 1A,D,E and Table 1).
To assess the potassium nitrate impact on fruit fly’s lifespan, we additionally used a negative geotaxis assay to measure locomotor ability in fruit flies [29]. The same groups of Drosophila were tested 7, 14, and 20 days from the whole experiment beginning. Aging significantly decreased climbing height in all groups of fruit flies (p < 0.05). The 1% KNO3 solution significantly maintained higher climbing height in 7, 14, and even 20 days old flies compared to the control group (p < 0.05) (Figure 2).
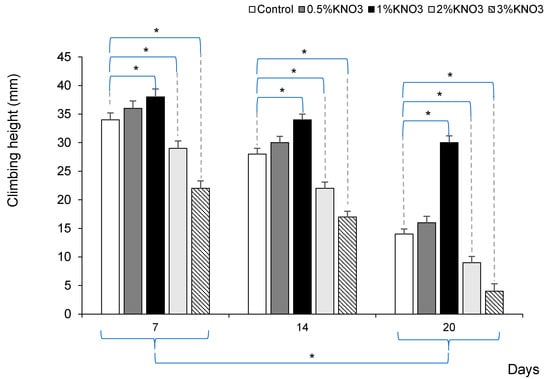
Figure 2.
Potassium nitrate impact on fruit fly’s locomotor decline. * p < 0.05 comparison between control vs. different potassium nitrate groups, and in each group between 7, 14, and 20 days.
4. Discussion & Conclusions
A potentially important and probably main limitation of this study is quality and contamination of ingredients used for medium preparation. Identifying it as the biggest and the least controlled variable, we believe that other forms of medium that could have similar amount of oxidative stress should be tested.
Nevertheless, we found a supporting evidence of potassium nitrate effect on the female fruit fly Drosophila melanogaster lifespan together with the assessment of their physical activity. Only female fruit flies were selected for this experiment due to recently confirmed scientific evidence that mating (especially with multiple partners) could have a possible effect on lifespan in Drosophila [30]. Based on that, single sex (female) food flies were selected to eliminate possible side effects and/or factors that could influence the life expectancy of flies and to purify the effect of different concentrations of KNO3 on their longevity.
Our principal findings showed that different concentrations of KNO3 supplementation had a significant effect on the fruit fly’s longevity. It was mentioned that the median lifespan duration of healthy and well-maintained fruit flies is approximately 70 days and could reach a maximum of approximately 90 days at 25 °C [31,32]. The experiment performed by our scientific team lasted only 39 days, which, in comparison to other experiments carried out on Drosophila melanogaster that averaged 70–90 days, would seem rather short. It is important to emphasize that most experiments use a synthetic, very balanced media such as instant Drosophila medium 4–24. A natural medium with ingredients widely available in practically any food store was used in our case, which, in our opinion, best represents the products used in the human diet. This leads to possible interpretations of balanced and unbalanced diets and potassium nitrate influence on oxidative stress caused by consumed food and/or lifestyle. It can only be assumed which of the factors—quality of products in the region, possible food contamination, conservation, etc.—could have caused changes in the body (accumulation of free radicals, increase in oxidative stress) causing a shorter life expectancy compared to results from other studies [28,32]. It is also essential to note that the medium used in our experiment was rather sweet and high in carbohydrates (due to raisins as one of the main ingredients). A sweet medium has also been shown to result in shorter life span of fruit flies [33,34].
We found that low doses of potassium nitrate medium—1% and 2%—significantly prolonged the lifespan of female fruit flies, but no significant effect was seen in the 0.5% group; however, 3% medium had a statistically significant negative effect causing greater amounts of early death during the first weeks of the experiment, followed by vague locomotor function. We could explain this by hypothesizing a possible inverted “U shape” curve effect for this given compound, assuming that too small of a concentration has very little or no effect and too high of a concentration is/could be lethal. As it was previously reported—low doses of nitrite, but not higher doses, had protective effects in vivo and in vitro models of vascular dysfunction, myocardial ischemic injury [35,36], and liver ischemia-reperfusion injury [35,37]. Our results demonstrate that additional research with different species and compounds is required to establish the “optimal” point of the hypothesized inverted “U curve.” The locomotion analysis was performed only in 1% KNO3 to keep the same experimental format as was in Moretti and colleagues’ (2020) study. Nitrate toxicity to humans and animals has been known and analyzed for quite some time now [38,39,40]. Based on available scientific information, we assume that 3% KNO3 resulted in higher death rate and shorter lifespan due to overdose and possibly occurring toxicity. Plenty of various aging-related markers can be observed in the Drosophila species—from genetic to environmental factors. One of the most relevant aging-related factors is physiological decline with changes of metabolism, behavior, reproductive and immune capacity, physical activity, neuronal, gut, and cardiac functions [41]. In the present study, physical activity or more detailed, negative geotaxis of fruit flies was evaluated. Experiments showed that climbing was significantly higher in those fruit flies, which were fed 1% KNO3 medium.
Aging is related to organism functions, capacity decline, and impairment. Aging is the main risk factor for life-menacing conditions such as cardiovascular diseases, neurodegeneration, cancer, and sarcopenia—which demonstrate a significant increase in the number of cases [2,42]. Sarcopenia is one of the most principal health problems associated with aging [43,44], described as age-related muscle mass decline and associated with physical disability, metabolic impairment, and frailty in older persons [45,46]. Drosophila and human muscles, despite their differences, demonstrate morphological and functional changes due to the aging process [44]. Locomotor activity significantly decreased with aging in Drosophila [47,48,49,50]. With age, heart muscle functional and performance decline is also observable in food flies [51] as in humans [52]. Simultaneously, this dysfunction in humans increases the risk of cardiovascular disease, and this impairment is conventionally associated with a risk of cognitive impairment in later life [53,54]. Thus, we need to precisely understand the aging mechanisms and how this process enhances the risk of disease to help this growing problem. As one of the possible solutions to the problem, one might consider nitrate consumption via available salts (e.g., NaNO3, KNO3, Ca(NO3)2) or green leafy or root vegetables (e.g., beetroots concentrate or their juice, red spinach, etc.) [55,56]. Nitrate and nitrite supplements and their consumption in therapeutic strategies are effective in enhancing NO concentrations in vivo [57,58,59]. NO is a signaling molecule crucial to the regulation of various physiological processes and preservation of physiological functions and health with advancing age [13]. The NO pathway through numerous biochemical and physiological cascades regulates vascular, metabolic, immune, and cognitive functions [60]. We support the opinion that NO pathway by boosting nitrate-nitrite supplementation may be an acceptable option to respond directly to age-related impairments [61].
This manuscript provides results (as they are) from the first experiment carried out by our scientific team in a possibly practical/extended nitrate application in the future. Findings presented by our study demonstrated increased lifespan as a result of low dosage supplementation of potassium nitrate solutions (1% and 2%) in Drosophila melanogaster. However, a higher dosage (3%) resulted in an increased number of early deaths that occurred at the early stages of the experiment, followed by vague behavior and a rapid decline of locomotor function. Too small dosage (0.5%) showed no significant effect on the longevity of the analyzed species. Both longevity and locomotion function were statistically significant in the experimental groups of 1% and 2% potassium nitrate supplementation.
This is the first kind of experiment carried out with nitrates in relationship to longevity. For decades “nitrates” have been carrying a negative reputation and have been a subject to multiple health warnings. Based on this research, we believe that it is important to carry on further experiments in relationship to aging and its concomitant conditions such as: sarcopenia, motor function decline, cognitive function, etc. Only 20 years ago, the discovery of nitric oxide was awarded the Nobel prize “The molecule of the year,” and today, the possibility to access this molecule via recently discovered nitrate (KNO3−)-nitrite (NO2)-nitric oxide (NO) pathway fascinates and gives hope to whole new horizon of discoveries. Potassium salts are major fertilizers used in modern agriculture—giving life to seeds and enchasing growth. Understanding these processes and applying them to human physiology could shed some light to yet unknown forms of possible applications.
Author Contributions
Conceptualization, T.L., J.P. and P.V.; Methodology, T.L. and Z.V.; Validation, S.C. and J.P.; Formal Analysis, J.P., S.C. and T.L.; Investigation, T.L.; Resources, T.L.; Data Curation, S.C. and Z.V.; Writing—Original Draft Preparation, S.C.; Writing—Review & Editing, T.L., S.C., J.P. and P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the article.
Acknowledgments
The work is partly attributed to the project “Innovative application of biologically active substances for the prevention of cardiovascular insufficiency and sarcopenia” (Nr. 01.2.1-LVPA-K-856-01-0065) under grant agreement with the Lithuanian Business Support Agency (LVPA). The authors wish to thank the Lithuanian Research Centre for Agriculture and Forestry for the support of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaeberlein, M. Longevity and aging. F1000prime Rep. 2013, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Sherlock, P.; Kalache, A.; Kirkwood, T.; McKee, M.; Prince, M. WHO’s proposal for a decade of healthy ageing. Lancet 2019, 394, 2152–2153. [Google Scholar] [CrossRef] [Green Version]
- Liubertas, T.; Kairaitis, R.; Stasiule, L.; Capkauskiene, S.; Stasiulis, A.; Viskelis, P.; Viškelis, J.; Urbonaviciene, D. The influence of amaranth (Amaranthus hypochondriacus) dietary nitrates on the aerobic capacity of physically active young persons. J. Int. Soc. Sports Nutr. 2020, 17. [Google Scholar] [CrossRef]
- Simmons, D. Epigenetic influence and disease. Nat. Educ. 2008, 1, 6. [Google Scholar]
- Kaelin, W.G., Jr.; McKnight, S.L. Influence of metabolism on epigenetics and disease. Cell 2013, 153, 56–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cabo, R.; Carmona-Gutierrez, D.; Bernier, M.; Hall, M.N.; Madeo, F. The search for antiaging interventions: From elixirs to fasting regimens. Cell 2014, 157, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. (Auckl. N.Z.) 2014, 44 (Suppl. 1), S35–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.M.; Vanhatalo, A.; Seals, D.R.; Rossman, M.J.; Piknova, B.; Jonvik, K.L. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med. Sci. Sports Exerc. 2021, 53, 280–294. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J. Geriatr. Cardiol. 2011, 8, 230. [Google Scholar]
- Lundberg, J.O.; Govoni, M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004, 37, 395–400. [Google Scholar] [CrossRef]
- Duncan, C.; Dougall, H.; Johnston, P.; Green, S.; Brogan, R.; Leifert, C.; Smith, L.; Golden, M.; Benjamin, N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995, 1, 546–551. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Ahluwalia, A.; Benjamin, N.; Bryan, N.S.; Butler, A.; Cabrales, P.; Fago, A.; Feelisch, M.; Ford, P.C.; et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009, 5, 865–869. [Google Scholar] [CrossRef]
- Bryan, N.S.; Loscalzo, J. Nitrite and Nitrate in Human Health and Disease; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Van Der Loo, B.; Labugger, R.; Skepper, J.N.; Bachschmid, M.; Kilo, J.; Powell, J.M.; Palacios-Callender, M.; Erusalimsky, J.D.; Quaschning, T.; Malinski, T.; et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 2000, 192, 1731–1744. [Google Scholar] [CrossRef] [Green Version]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Toda, N.; Ayajiki, K.; Okamura, T. Cerebral blood flow regulation by nitric oxide in neurological disorders. Can. J. Physiol. Pharmacol. 2009, 87, 581–594. [Google Scholar] [CrossRef]
- Moretti, C.; Zhuge, Z.; Zhang, G.; Haworth, S.M.; Paulo, L.L.; Guimarães, D.D.; Cruz, J.; Montenegro, M.F.; Cordero-Herrera, I.; Braga, V.; et al. The obligatory role of host microbiota in bioactivation of dietary nitrate. Free Radic. Biol. Med. 2019, 145, 342–348. [Google Scholar] [CrossRef]
- Carlström, M.; Lundberg, J.O.; Weitzberg, E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol. 2018, 224, e13080. [Google Scholar] [CrossRef]
- Grotewiel, M.S.; Martin, I.; Bhandari, P.; Cook-Wiens, E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005, 4, 372–397. [Google Scholar] [CrossRef]
- Iliadi, K.G.; Boulianne, G.L. Age-related behavioral changes in Drosophila. Ann. N. Y. Acad. Sci. 2010, 1197, 9–18. [Google Scholar] [CrossRef]
- Davies, S.A.; Stewart, E.J.; Huesmann, G.R.; Skaer, N.J.; Maddrell, S.H.; Tublitz, N.J.; Dow, J.A. Neuropeptide stimulation of the nitric oxide signaling pathway in Drosophila melanogaster Malpighian tubules. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 273, R823–R827. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kounatidis, I.; Ligoxygakis, P. Drosophila as a model to study the role of blood cells in inflammation, innate immunity and cancer. Front. Cell. Infect. Microbiol. 2014, 3, 113. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, C.H.; Schiffer, T.A.; Montenegro, M.F.; Larsen, F.J.; Tsarouhas, V.; Carlström, M.; Lundberg, J.O. Dietary nitrite extends lifespan and prevents age-related locomotor decline in the fruit fly. Free Radic. Biol. Med. 2020, 160, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Peat, J.; Barton, B. Medical Statistics: A Guide to Data Analysis and Critical Appraisal; Blackwell Publishing: Hoboken, NJ, USA, 2005. [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014; pp. 157–164. [Google Scholar]
- Richardson, J.T.E. Mann-Whitney Test. In The SAGE Encyclopedia of Educational Research, Measurement, and Evaluation; Frey, B.B., Ed.; SAGE Publications: Thousand Oaks, CA, USA, 2018; pp. 1005–1008. [Google Scholar]
- Nichols, C.D.; Becnel, J.; Pandey, U.B. Methods to assay Drosophila behavior. J. Vis. Exp. 2012, 61, e3795. [Google Scholar]
- Koliada, A.; Gavrilyuk, K.; Burdylyuk, N.; Strilbytska, O.; Storey, K.B.; Kuharskii, V.; Lushchak, O.; Vaiserman, A. Mating status affects Drosophila lifespan, metabolism and antioxidant system. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 246, 110716. [Google Scholar] [CrossRef]
- Ziehm, M.; Thornton, J.M. Unlocking the potential of survival data for model organisms through a new database and online analysis platform: SurvCurv. Aging Cell 2013, 12, 910–916. [Google Scholar] [CrossRef] [Green Version]
- Ziehm, M.; Piper, M.D.; Thornton, J.M. Analysing variation in Drosophila aging across independent experimental studies: A meta-analysis of survival data. Aging Cell 2013, 12, 917–922. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, R.; Kolli, S.; Bauer, J.H. Organically grown food provides health benefits to Drosophila melanogaster. PLoS ONE 2013, 8, e52988. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, E.; Van Leeuwen, L.A.; Dos Santos, E.; James, J.; Best, L.; Lennicke, C.; Vincent, A.J.; Marinos, G.; Foley, A.; Cochemé, H.M.; et al. Sugar-induced obesity and insulin resistance are uncoupled from shortened survival in Drosophila. Cell Metab. 2020, 31, 710–725. [Google Scholar] [CrossRef]
- Duranski, M.R.; Greer, J.J.; Dejam, A.; Jaganmohan, S.; Hogg, N.; Langston, W.; Patel, R.P.; Yet, S.-F.; Wang, X.; Lefer, D.J.; et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005, 115, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Vitturi, D.A.; Patel, R.P. Current perspectives and challenges in understanding the role of nitrite as an integral player in nitric oxide biology and therapy. Free Radic. Biol. Med. 2011, 51, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Meng, Z.; Liu, Y.; Patel, R.P.; Lang, J.D. The hepatoprotective effect of sodium nitrite on cold ischemia-reperfusion injury. J. Transpl. 2012, 2012, 635179. [Google Scholar] [CrossRef]
- Wright, M.J.; Davison, K.L. Nitrate accumulation in crops and nitrate poisoning in animals. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1964; Volume 16, pp. 197–247. [Google Scholar]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Piper, M.D.; Partridge, L. Drosophila as a model for ageing. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef]
- Partridge, L. The new biology of ageing. Phil. Trans. R. Soc. 2010, 365, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.L. Of worms and women: Sarcopenia and its role in disability and mortality. J. Am. Geriatr. Soc. 2004, 52, 1185–1190. [Google Scholar] [CrossRef]
- Augustin, H.; Partridge, L. Invertebrate models of age-related muscle degeneration. Biochim. Biophys. Acta 2009, 1790, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Yanagita, I.; Muta, K.; Nawata, H. Frailty in elderly diabetes patients. Endocr. J. 2017, 65, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Zamboni, M.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.S.; Lekkas, P.; Braddock, J.M.; Farman, G.P.; Ballif, B.A.; Irving, T.C.; Maughan, D.W.; Vigoreaux, J.O. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys. J. 2008, 95, 2391–2401. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.F.; Liang, D.T.; Krantz, D.E. Differential decline in behavioral performance of Drosophila melanogaster with age. Mech. Ageing Dev. 2006, 127, 647–651. [Google Scholar] [CrossRef]
- Martinez, V.G.; Javadi, C.S.; Ngo, E.; Ngo, L.; Lagow, R.D.; Zhang, B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev. Neurobiol. 2007, 67, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Rhodenizer, D.; Martin, I.; Bhandari, P.; Pletcher, S.D.; Grotewiel, M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp. Gerontol. 2008, 43, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Ocorr, K.; Bodmer, R.; Cartry, J. Drosophila as a model to study cardiac aging. Exp. Gerontol. 2011, 46, 326–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.L.; De Craen, A.J.; Kerse, N.; Teh, R.; Granic, A.; Davies, K.; Wesnes, K.; Den Elzen, W.P.J.; Gussekloo, J.; Stephan, B.C.; et al. Predicting Risk of Cognitive Decline in Very Old Adults Using Three Models: The Framingham Stroke Risk Profile; the Cardiovascular Risk Factors, Aging, and Dementia Model and Oxi-Inflammatory Biomarkers. J. Am. Geriatr. Soc. 2017, 65, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.I.; Aarsland, D.; Day, S.; Sønnesyn, H.; Alzheimer’s Society Vascular Dementia Systematic Review Group; Ballard, C. Hypertension is a potential risk factor for vascular dementia: Systematic review. Int. J. Geriatr. Psychiatry 2011, 26, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.M.; Corriveau, R.A.; Craft, S.; Faber, J.E.; Greenberg, S.M.; Knopman, D.; Lamb, B.T.; Montine, T.J.; Nedergaard, M.; Carrillo, M.C.; et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 2015, 11, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Fasuyi, A.O.; Dairo, F.A.S.; Adeniji, A.O. Tropical vegetable (Amaranthus cruentus) leaf meal as alternative protein supplement in broiler starter diets: Bionutritional evaluation. J. Cent. Eur. Agric. 2008, 9, 23–34. [Google Scholar]
- Johnson, L.C.; DeVan, A.E.; Justice, J.N.; Seals, D.R. Nitrate and Nitrite in Aging and Age-Related Disease. In Nitrite and Nitrate in Human Health and Disease; Humana Press: Cham, Switzerland, 2017; pp. 259–277. [Google Scholar]
- Lundberg, J.O.; Carlström, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Rocha, B.S.; Gago, B.; Pereira, C.; Barbosa, R.M.; Bartesaghi, S.; Lundberg, J.O.; Radi, R.; Laranjinha, J. Dietary nitrite in nitric oxide biology: A redox interplay with implications for pathophysiology and therapeutics. Curr. Drug Targets 2011, 12, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Montenegro, M.F. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. J. Int. Med. 2019, 285, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Stephan, B.C.; Harrison, S.L.; Keage, H.A.; Babateen, A.; Robinson, L.; Siervo, M. Cardiovascular disease, the nitric oxide pathway and risk of cognitive impairment and dementia. Curr. Cardiol. Rep. 2017, 19, 87. [Google Scholar] [CrossRef] [Green Version]
- Keeton, J.T. History of nitrite and nitrate in food. In Nitrite and Nitrate in Human Health and Disease; Humana Press: Totowa, NJ, USA, 2011; pp. 69–84. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).