Activated Carbon for Pharmaceutical Removal at Point-of-Entry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbates
2.2. Activated Carbons
2.3. BET Surface Area, Total Pore Volume, and Average Pore Size
2.4. Analytical Methods and Calibration Curve
2.5. pH Measurements
2.6. Adsoroption Experimental Design
3. Results
3.1. Characterization of Materials
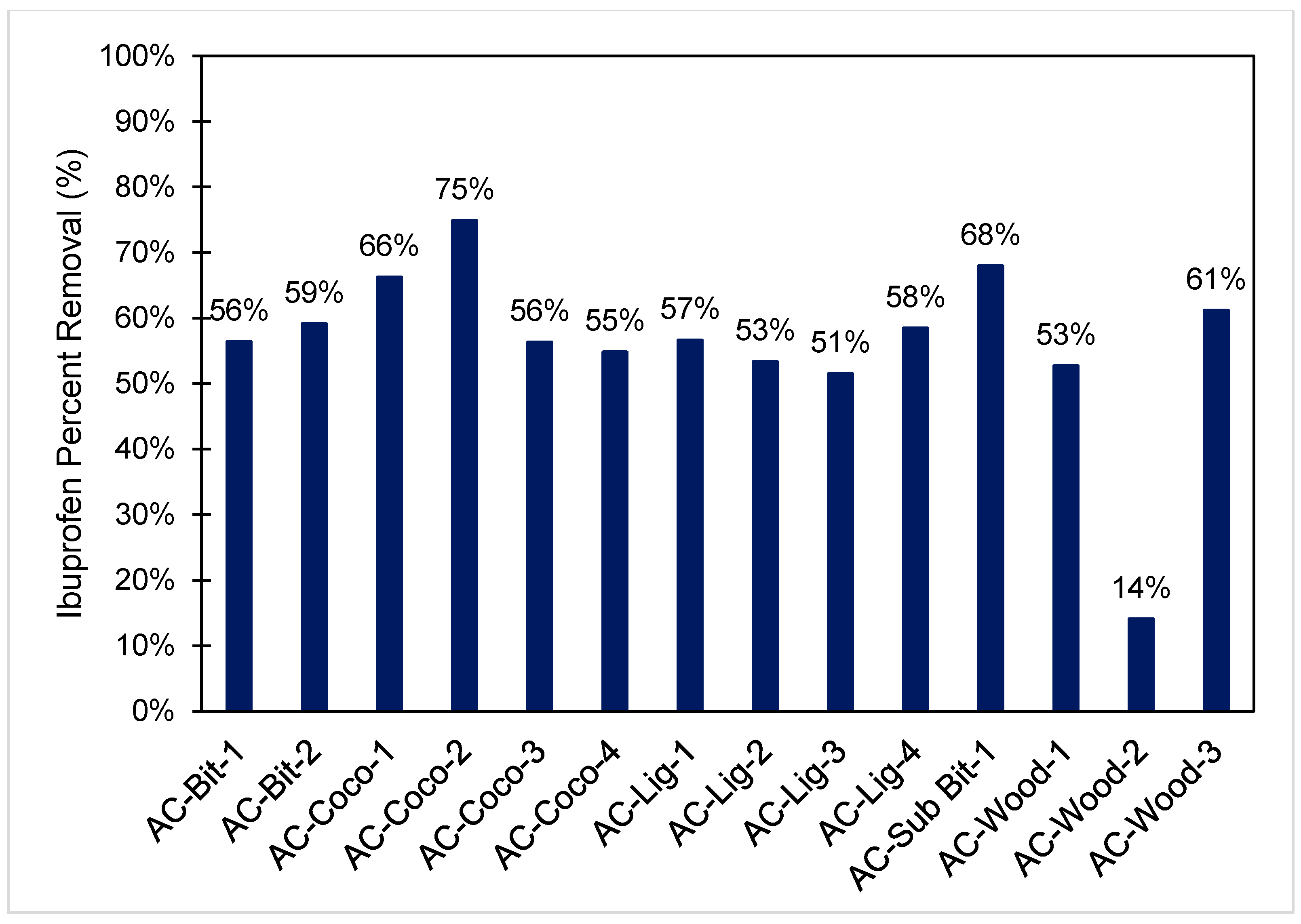
3.2. Activated Carbon Removal of Ibuprofen at Equilibrium
3.3. Effect of Particle Size Distribution on Ibuprofen Adsorption
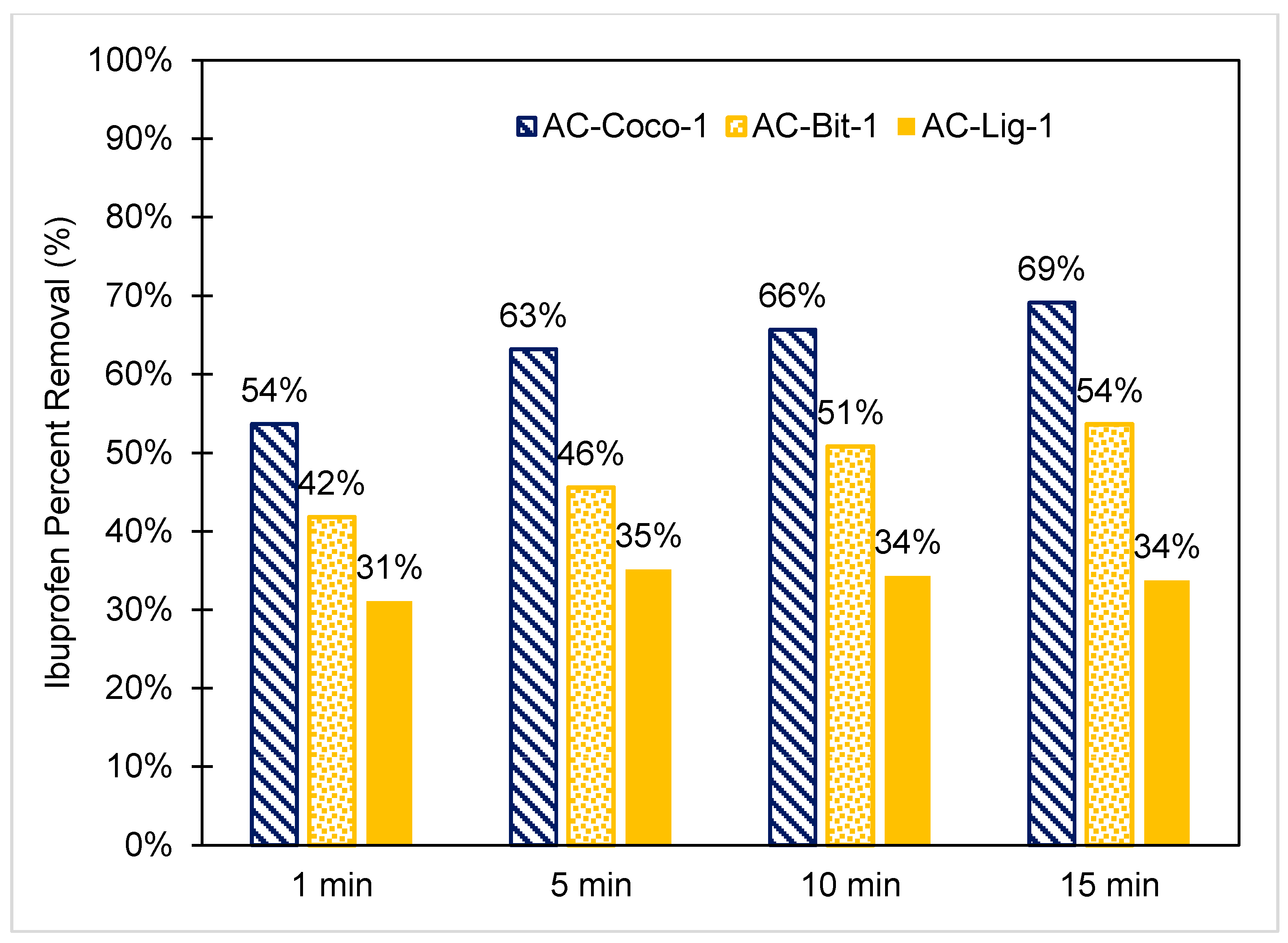
3.4. Rapid Ibuprofen Adsorption
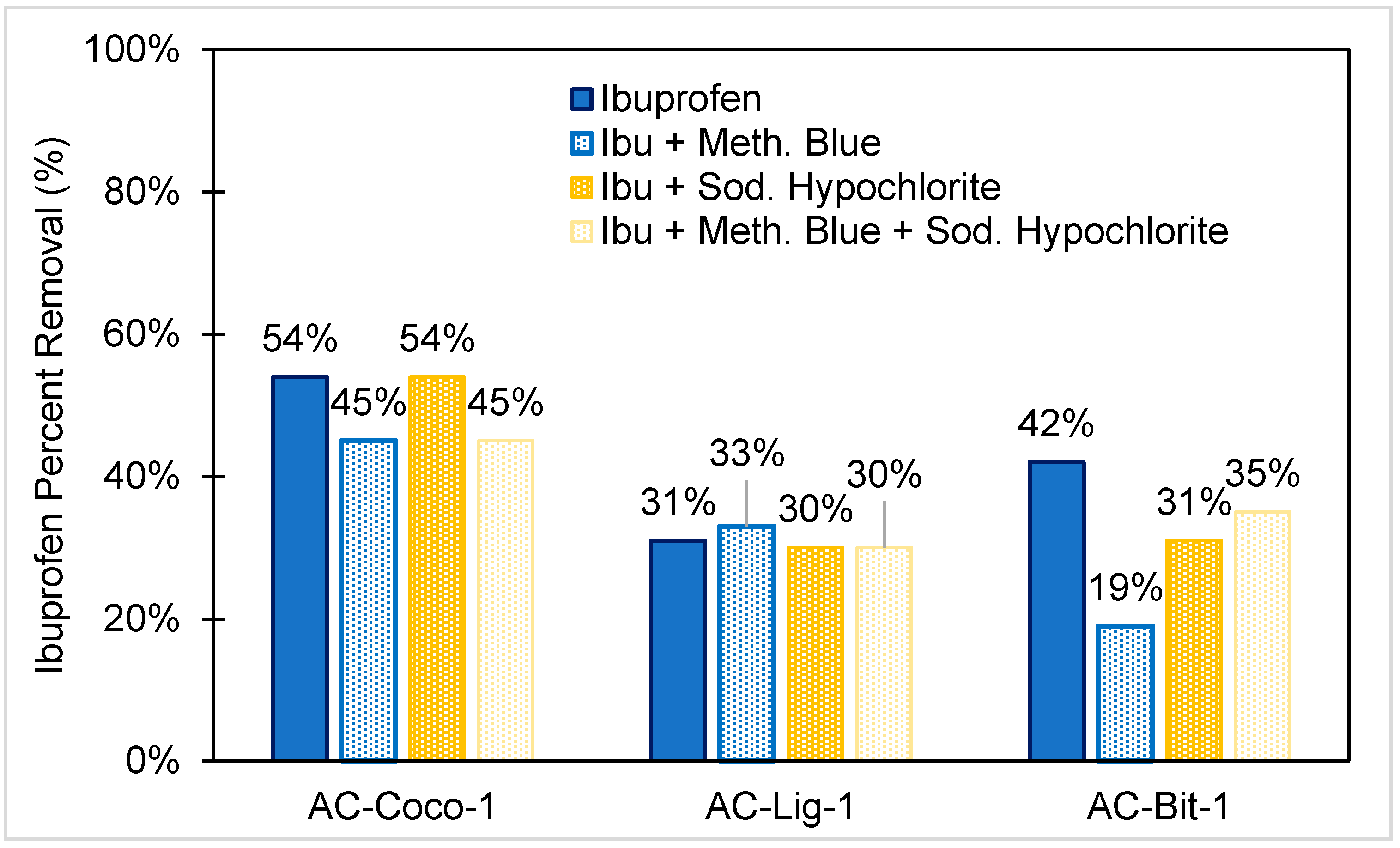
3.5. Competitive Ibuprofen Adsorption with Methylene Blue and Sodium Hypochlorite
3.6. Impact of Background Water Constituents on Ibuprofen Adsorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Food and Drug Administration: Center for Drug Evaluation and Research. Drug Disposal: Drug Take Back Locations. 2020. Available online: https://www.fda.gov/drugs/disposal-unused-medicines-what-you-should-know/drug-disposal-drug-take-back-locations (accessed on 4 April 2021).
- Lienert, J.; Bürki, T.; Escher, B. Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Sci. Technol. 2007, 56, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sheta, S.M.; Abd-Elzaher, M.M.; El-Sheikh, S.M. A novel nano-lanthanum complex: Synthesis, characterization and application as a macrofuran chemosensor in pharmaceutical, biological and environmental samples. RSC Adv. 2021, 11, 9675–9681. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Alba, A.R.F.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Dos Santos, C.; Nardocci, A. Prioritization of pharmaceuticals in drinking water exposure based on toxicity and environmental fate assessment by in silico tools: An integrated and transparent ranking. Comput. Toxicol. 2019, 9, 12–21. [Google Scholar] [CrossRef]
- Webb, S.; Ternes, T.; Gibert, M.; Olejniczak, K. Indirect human exposure to pharmaceuticals via drinking water. Toxicol. Lett. 2003, 142, 157–167. [Google Scholar] [CrossRef]
- Arnold, K.E.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the environment: Assessing risks of pharmaceuticals to wildlife and ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130569. [Google Scholar] [CrossRef] [Green Version]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- 2020 Water Quality Report—Manatee County. Available online: https://www.mymanatee.org/departments/utilities/manatee_county_water_division/water_quality_information/2020_water_quality_report (accessed on 4 June 2021).
- Olivieri, A.W.; Pecson, B.; Crook, J.; Hultquist, R. California water reuse—Past, present and future perspectives. Adv. Chem. Pollut. Environ. Manag. Prot. 2020, 5, 65–111. [Google Scholar] [CrossRef]
- Schimmoller, L. Fit for Purpose Water: The Cost of Overtreating Reclaimed Water. 2014. Available online: https://watereuse.org/watereuse-research/fit-for-purpose-water/ (accessed on 31 May 2021).
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2020, 1–48. [Google Scholar] [CrossRef]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Faulconer, E.; Mazyck, D.W. Influence of activated carbon surface oxygen functionality on elemental mercury adsorption from aqueous solution. J. Environ. Chem. Eng. 2017, 5, 2879–2885. [Google Scholar] [CrossRef] [Green Version]
- Tennant, M.F.; Mazyck, D.W. The role of surface acidity and pore size distribution in the adsorption of 2-methylisoborneol via powdered activated carbon. Carbon 2007, 45, 858–864. [Google Scholar] [CrossRef]
- Casasús, A.I.; Hagan-Rogers, A.M.; Rodriguez, R.; Mazyck, D.W. Novel photo-activated method for removal of mercury from industrial wastewater. J. Water Process. Eng. 2020, 38, 101667. [Google Scholar] [CrossRef]
- Jaman, S.; Rodriguez, R.; Mazyck, D.W. Evaluation of Common Granular Activated Carbon Parameters for Trace Contaminant Removal. J. Environ. Eng. 2019, 145, 04019035. [Google Scholar] [CrossRef]
- Valcarce, C.O.; Gonzaga, E.W.; Mazyck, D.W. Evaluating the Efficacy of PAC and Water Parameters to Remove Trace Organics. J. Am. Water Work. Assoc. 2017, 109, E50–E60. [Google Scholar] [CrossRef]
- Singh, S.K.; Townsend, T.G.; Mazyck, D.; Boyer, T.H. Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro- and meso-porous activated carbon. Water Res. 2012, 46, 491–499. [Google Scholar] [CrossRef]
- Rodriguez, R.; Contrino, D.; Mazyck, D.W. Role of Activated Carbon Precursor in Mercury Removal. Ind. Eng. Chem. Res. 2020, 59, 17740–17747. [Google Scholar] [CrossRef]
- Alves, T.C.; Cabrera-Codony, A.; Barceló, D.; Rodriguez-Mozaz, S.; Pinheiro, A.; Gonzalez-Olmos, R. Influencing factors on the removal of pharmaceuticals from water with micro-grain activated carbon. Water Res. 2018, 144, 402–412. [Google Scholar] [CrossRef]
- Mansour, F.; Al-Hindi, M.; Yahfoufi, R.; Ayoub, G.M.; Ahmad, M.N. The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: A review. Rev. Environ. Sci. Bio/Technol. 2018, 17, 109–145. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Boyer, T.H. Physical-chemical interactions between pharmaceuticals and biochar in synthetic and real urine. Chemosphere 2019, 218, 818–826. [Google Scholar] [CrossRef]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Gasperi, J.; Rocher, V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 2019, 218, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Boyer, T.H. Pharmaceutical removal in synthetic human urine using biochar. Environ. Sci. Water Res. Technol. 2017, 3, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Kitak, T.; Dumičić, A.; Planinšek, O.; Šibanc, R.; Srčič, S. Determination of Solubility Parameters of Ibuprofen and Ibuprofen Lysinate. Molecules 2015, 20, 21549–21568. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, I.M. Thermodynamic Evaluation of Ibuprofen Solubility in Aqueous and Non-Aqueous Cosolvent Systems; Purdue University: West Lafayette, IN, USA, 2000. [Google Scholar]
- Yu, Q.; Li, M.; Ji, X.; Qiu, Y.; Zhu, Y.; Leng, C. Characterization and methanol adsorption of walnut-shell activated carbon prepared by KOH activation. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 260–268. [Google Scholar] [CrossRef]
- Zeng, Y.; Prasetyo, L.; Nguyen, V.T.; Horikawa, T.; Do, D.; Nicholson, D. Characterization of oxygen functional groups on carbon surfaces with water and methanol adsorption. Carbon 2015, 81, 447–457. [Google Scholar] [CrossRef]
- Dun, W.; Wu, S.; Tang, W.; Wang, X.; Sun, D.; Du, S.; Gong, J. Solubility of Ibuprofen Sodium Dihydrate in Acetone + Water Mixtures: Experimental Measurement and Thermodynamic Modeling. J. Chem. Eng. Data 2014, 59, 3415–3421. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Surface Area Analysis from the Langmuir and BET Theories. In Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2004; Volume 16, pp. 58–81. [Google Scholar] [CrossRef]
- ASTM International. ASTM D6851-20, Standard Test Method for Determination of Contact pH with Activated Carbon. 2020. Available online: https://compass.astm.org/EDIT/html_annot.cgi?D6851+20 (accessed on 1 May 2021).
- Real, F.J.; Benitez, F.J.; Acero, J.L.; Casas, F. Adsorption of selected emerging contaminants onto PAC and GAC: Equilibrium isotherms, kinetics, and effect of the water matrix. J. Environ. Sci. Health Part A 2017, 52, 727–734. [Google Scholar] [CrossRef]
- Sodeinde, O.A. Preparation of a Locally Produced Activated Carbon from Coconut Shells and Its Use in Reducing Hexamine Cobalt(III). Int. J. Chem. Eng. Appl. 2012, 2012, 67–71. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Dynamic analysis of sorption of Methylene Blue dye on granular and powdered activated carbon. Chem. Eng. J. 2008, 144, 400–406. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G. Adsorption of Methylene Blue onto activated carbon produced from steam activated bituminous coal: A study of equilibrium adsorption isotherm. Chem. Eng. J. 2006, 124, 103–110. [Google Scholar] [CrossRef]
- Arias, M.; López, E.; Nunez, A.; Rubinos, D.; Soto, B.; Barral, M.T.; Diaz-Fierros, F. Adsorption of Methylene Blue by Red Mud, An Oxide- Rich Byproduct of Bauxite Refining. In Effect of Mineral-Organic-Microorganism Interactions on Soil and Freshwater Environments; Berthelin, J., Huang, P.M., Bollag, J.M., Andreux, F., Eds.; Springer: Boston, MA, USA, 1999; pp. 361–365. [Google Scholar]
- Liu, W.; Zhang, J.; Cheng, C.; Tian, G.; Zhang, C. Ultrasonic-assisted sodium hypochlorite oxidation of activated carbons for enhanced removal of Co(II) from aqueous solutions. Chem. Eng. J. 2011, 175, 24–32. [Google Scholar] [CrossRef]
- Haydar, S.; Ferro-García, M.; Rivera-Utrilla, J.; Joly, J. Adsorption of p-nitrophenol on an activated carbon with different oxidations. Carbon 2003, 41, 387–395. [Google Scholar] [CrossRef]
- Gillogly, T.E.; Snoeyink, V.L.; Holthouse, A.; Wilson, C.M.; Royal, E.P. Effect of chlorine on PAC’s ability to adsorb MIB. J. Am. Water Work. Assoc. 1998, 90, 107–114. [Google Scholar] [CrossRef]
- Pelekani, C.; Snoeyink, V. Competitive adsorption in natural water: Role of activated carbon pore size. Water Res. 1999, 33, 1209–1219. [Google Scholar] [CrossRef]
- Aschermann, G.; Neubert, L.; Zietzschmann, F.; Jekel, M. Impact of different DOM size fractions on the desorption of organic micropollutants from activated carbon. Water Res. 2019, 161, 161–170. [Google Scholar] [CrossRef]
- Newcombe, G.; Drikas, M. Adsorption of NOM onto activated carbon: Electrostatic and non-electrostatic effects. Carbon 1997, 35, 1239–1250. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Influence of ionic strength, anions, cations, and natural organic matter on the adsorption of pharmaceuticals to silica. Chemosphere 2010, 80, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Review of Disintegrants and the Disintegration Phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedidi, H.; Reinert, L.; Lévêque, J.-M.; Soneda, Y.; Bellakhal, N.; Duclaux, L. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon 2013, 54, 432–443. [Google Scholar] [CrossRef]
- Oh, S.; Shin, W.S.; Kim, H.T. Effects of pH, dissolved organic matter, and salinity on ibuprofen sorption on sediment. Environ. Sci. Pollut. Res. Int. 2016, 23, 22882–22889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woermann, M.; Sures, B. Ecotoxicological effects of micropollutant-loaded powdered activated carbon emitted from wastewater treatment plants on Daphnia magna. Sci. Total Environ. 2020, 746, 141104. [Google Scholar] [CrossRef]





| Carbon ID | BET Surface Area (m2/g) | Average Pore Size (Å) | Total Pore Volume (cc/g) | Micro-Pore Volume (cc/g) | Water Contact pH |
|---|---|---|---|---|---|
| AC-Bit-1 | 784 | 20.1 | 0.39 | 0.27 | 9.78 |
| AC-Bit-2 | 797 | 24.2 | 0.48 | 0.27 | 10.3 |
| AC-Coco-1 | 1139 | 16.9 | 0.48 | 0.40 | 10.9 |
| AC-Coco-2 | 1252 | 13.9 | 0.59 | 0.53 | 11.3 |
| AC-Coco-3 | 1008 | 17.0 | 0.42 | 0.35 | 9.57 |
| AC-Coco-4 | 1184 | 17.7 | 0.52 | 0.42 | 10.8 |
| AC-Lig-1 | 689 | 41.5 | 0.71 | 0.18 | 3.25 |
| AC-Lig-2 | 427 | 51.0 | 0.54 | 0.12 | 11.8 |
| AC-Lig-3 | 440 | 42.5 | 0.47 | 0.20 | 9.65 |
| AC-Lig-4 | 461 | 42.6 | 0.49 | 0.20 | 11.4 |
| AC-Sub Bit-1 | 1072 | 25.0 | 0.67 | 0.35 | 9.24 |
| AC-Wood-1 | 1450 | 33.9 | 1.23 | 0.41 | 5.41 |
| AC-Wood-2 | 426 | 25.6 | 0.27 | 0.18 | 9.17 |
| AC-Wood-3 | 1529 | 31.2 | 1.19 | 0.45 | 3.08 |
| Carbon ID | Final Ibuprofen Concentration (mg/L) | Ibuprofen Capacity (mg ibu/g Carbon) |
|---|---|---|
| AC-Bit-1 | 440 | 108 |
| AC-Bit-2 | 402 | 111 |
| AC-Coco-1 | 340 | 127 |
| AC-Coco-2 | 247 | 145 |
| AC-Coco-3 | 429 | 108 |
| AC-Coco-4 | 444 | 103 |
| AC-Lig-1 | 438 | 109 |
| AC-Lig-2 | 470 | 102 |
| AC-Lig-3 | 477 | 99 |
| AC-Lig-4 | 409 | 112 |
| AC-Sub Bit-1 | 315 | 130 |
| AC-Wood-1 | 465 | 100 |
| AC-Wood-2 | 845 | 26 |
| AC-Wood-3 | 382 | 114 |
| Particle Size Distribution of Coconut-Based Activated Carbon | Ibuprofen Final Concentration in Solution (mg/L) | Ibuprofen Capacity (mg ibu/g Carbon) | Final Solution pH |
|---|---|---|---|
| PSD A:2 mm-1 mm | 776 | 7.0 | 9.97 |
| PSD B:1 mm-850 µm | 710 | 9.0 | 10.44 |
| PSD C:850 µm-500 µm | 616 | 12.0 | 10.53 |
| PSD D:500 µm-212 µm | 354 | 19.0 | 10.63 |
| PSD E:212 µm-75 µm | 90 | 27.0 | 10.83 |
| PSD F:75 µm-45 µm | 102 | 27.0 | 10.91 |
| PSD G:45 µm-20 µm | 108 | 27.0 | 10.37 |
| Water ID | TOC (ppm) | pH | ORP (mV) |
|---|---|---|---|
| Natural Water | 5.2 | 7.1 | 411 |
| Natural Water + 1 g/L ibu | 443.1 | 7.7 | 406 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finn, M.; Giampietro, G.; Mazyck, D.; Rodriguez, R. Activated Carbon for Pharmaceutical Removal at Point-of-Entry. Processes 2021, 9, 1091. https://doi.org/10.3390/pr9071091
Finn M, Giampietro G, Mazyck D, Rodriguez R. Activated Carbon for Pharmaceutical Removal at Point-of-Entry. Processes. 2021; 9(7):1091. https://doi.org/10.3390/pr9071091
Chicago/Turabian StyleFinn, Michelle, Gabrielle Giampietro, David Mazyck, and Regina Rodriguez. 2021. "Activated Carbon for Pharmaceutical Removal at Point-of-Entry" Processes 9, no. 7: 1091. https://doi.org/10.3390/pr9071091
APA StyleFinn, M., Giampietro, G., Mazyck, D., & Rodriguez, R. (2021). Activated Carbon for Pharmaceutical Removal at Point-of-Entry. Processes, 9(7), 1091. https://doi.org/10.3390/pr9071091







