In Situ Modification of Activated Carbons by Oleic Acid under Microwave Heating to Improve Adsorptive Removal of Naphthalene in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Starch-Based Carbon (SC), Starch-Based Activated Carbon (SAC), Pulverized Coal (PC), and Pulverized Coal-Based Activated Carbon (PCAC)
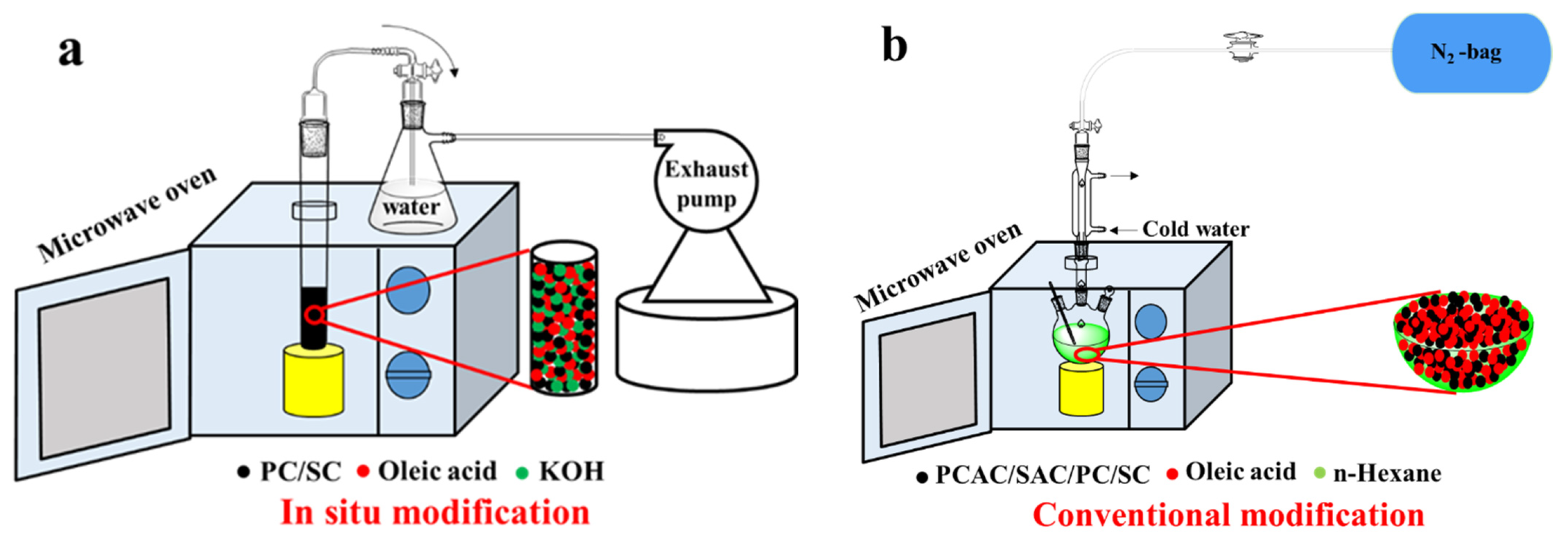
2.3. One-Pot Preparation of Activated Carbons (ACs) by Simultaneous Oleic Acid (OA) Modification and KOH Activation
2.4. Modification of Materials with OA in n-Hexane
2.5. Characterization
2.6. Adsorption Experimental Studies
2.6.1. Batch Adsorption
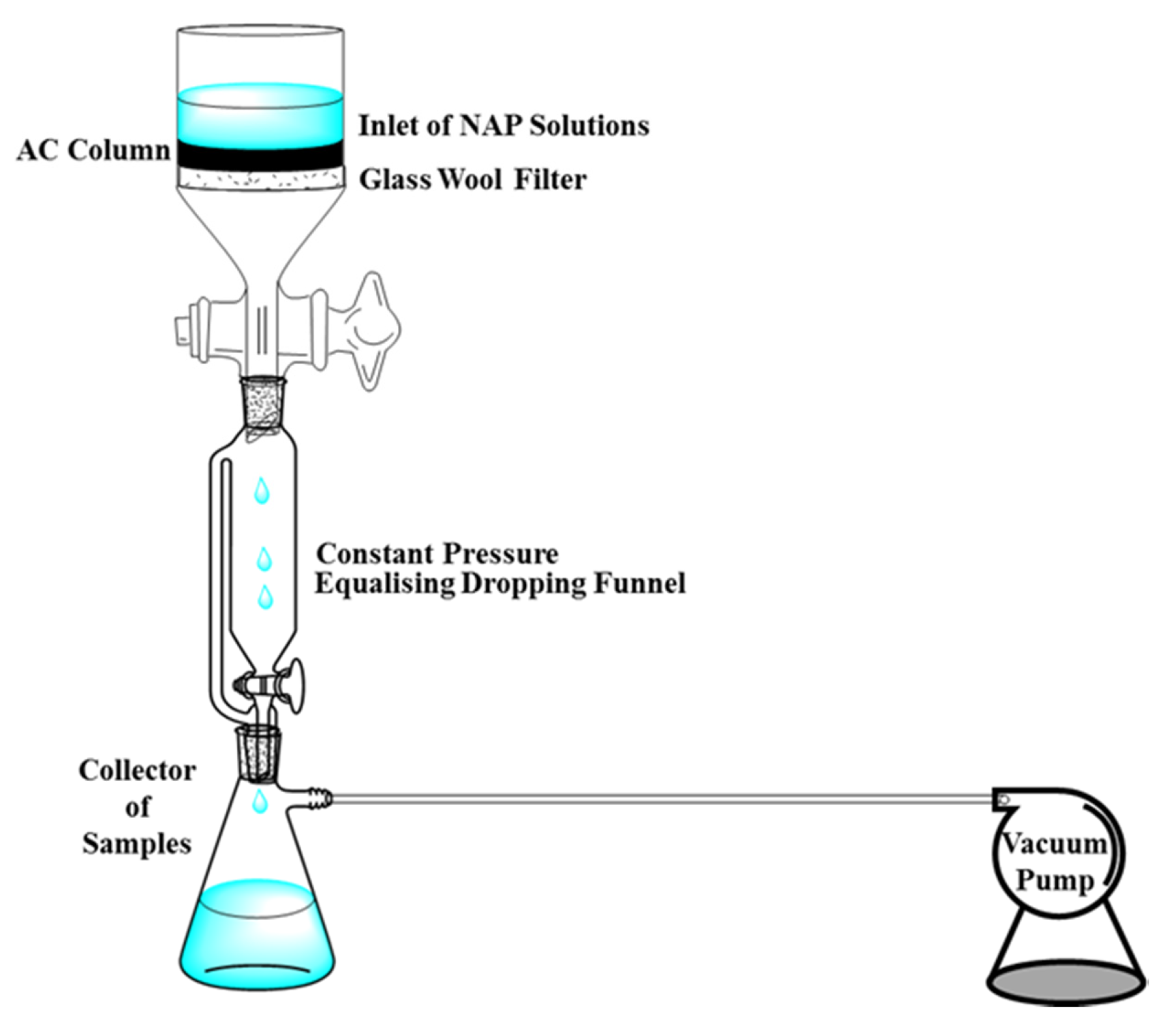
2.6.2. Column Adsorption
2.7. Data Analysis
3. Results
3.1. Characterization of Materials
3.2. Batch Adsorption
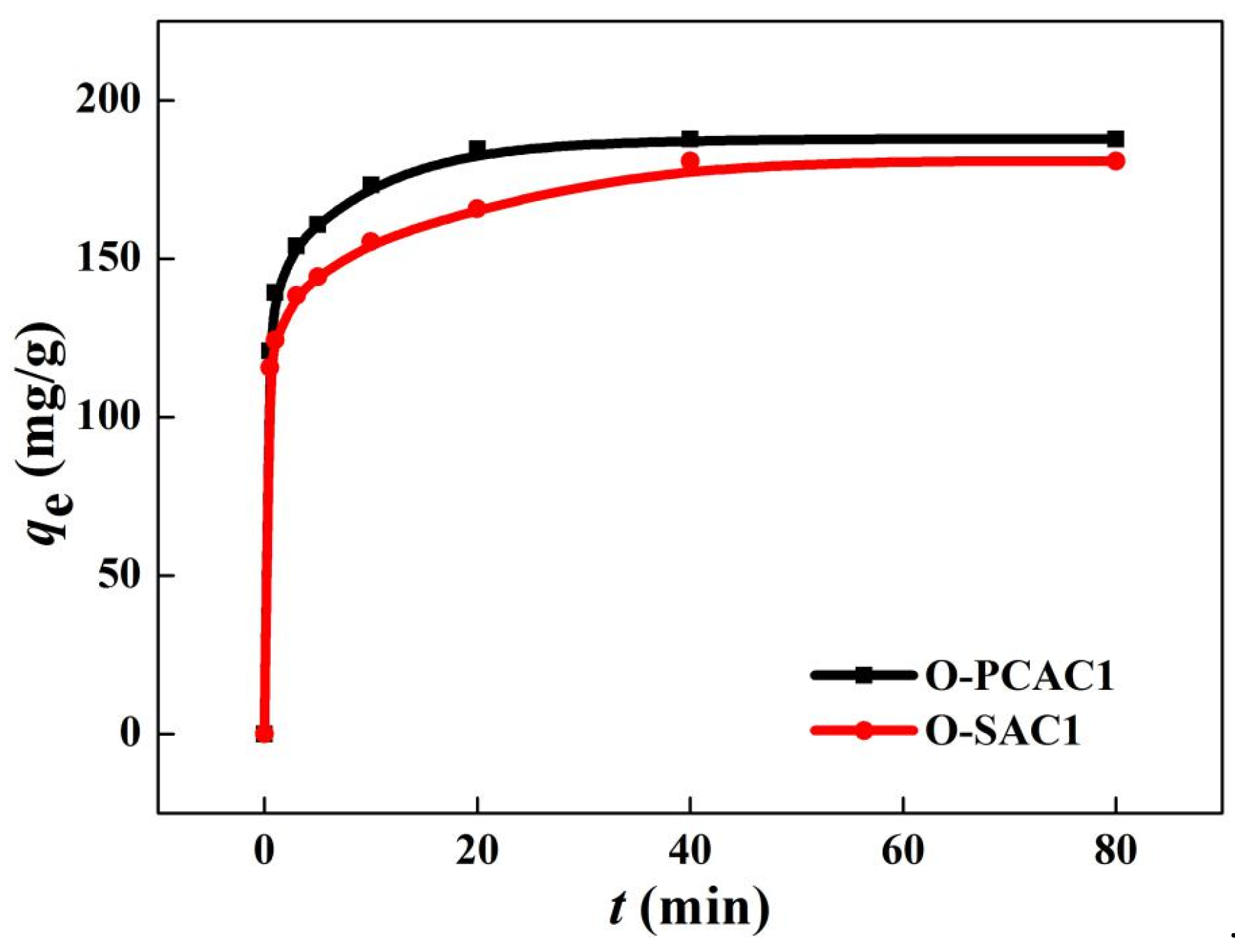
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
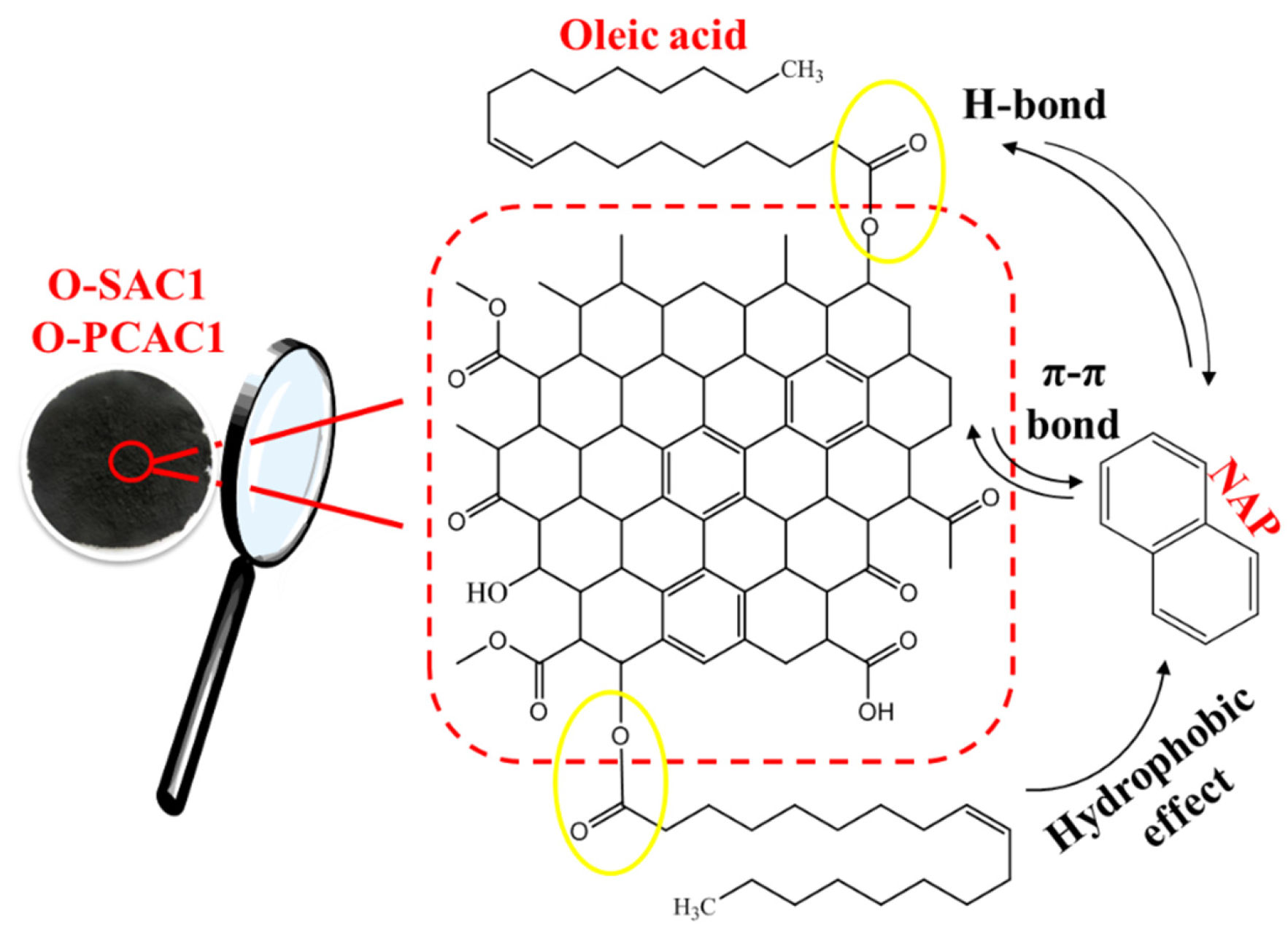
3.2.3. Adsorption Mechanism
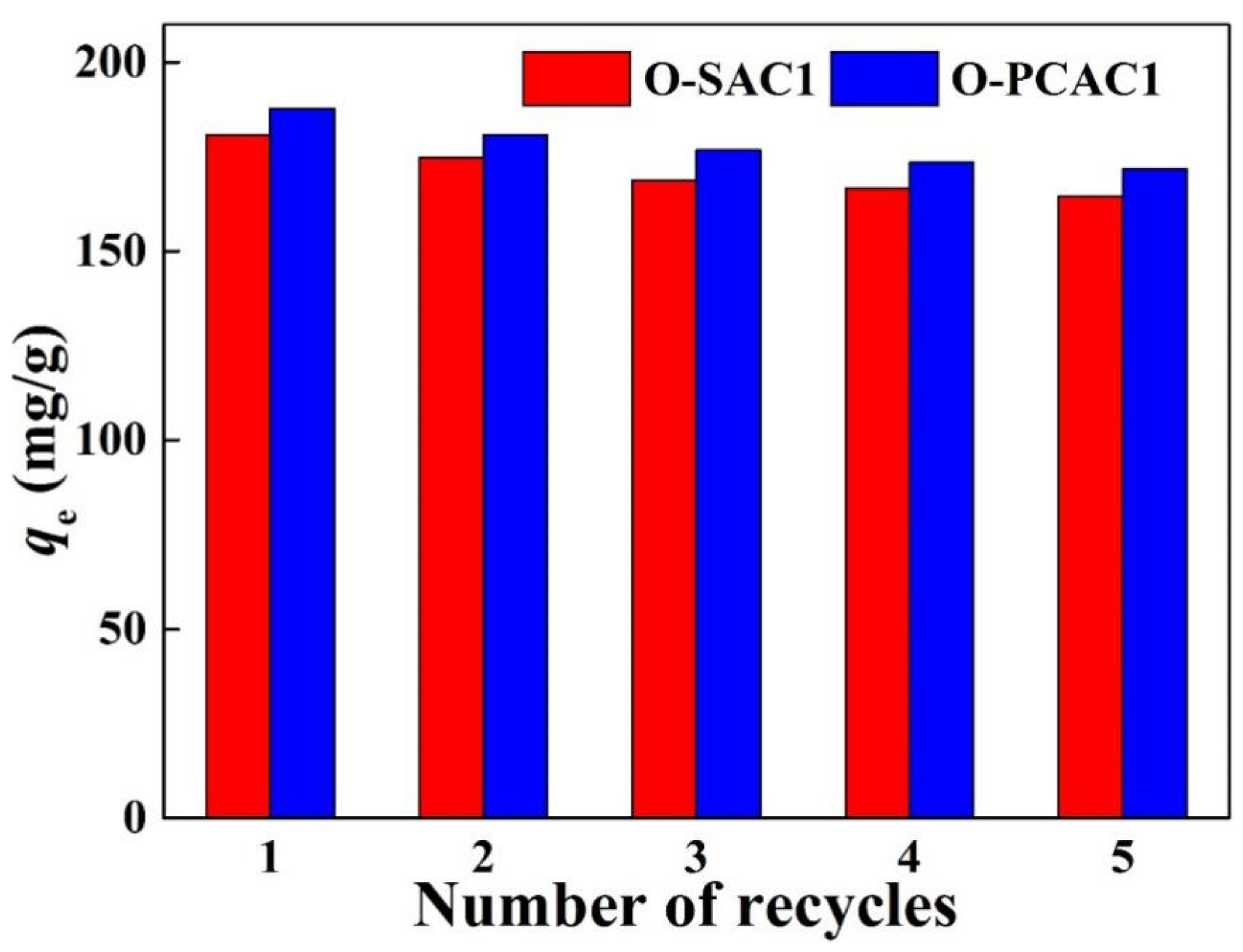
3.2.4. Regeneration of In Situ Modified Activated Carbons (IMACs)
3.3. Column Adsorption
3.3.1. Effect of Column Height
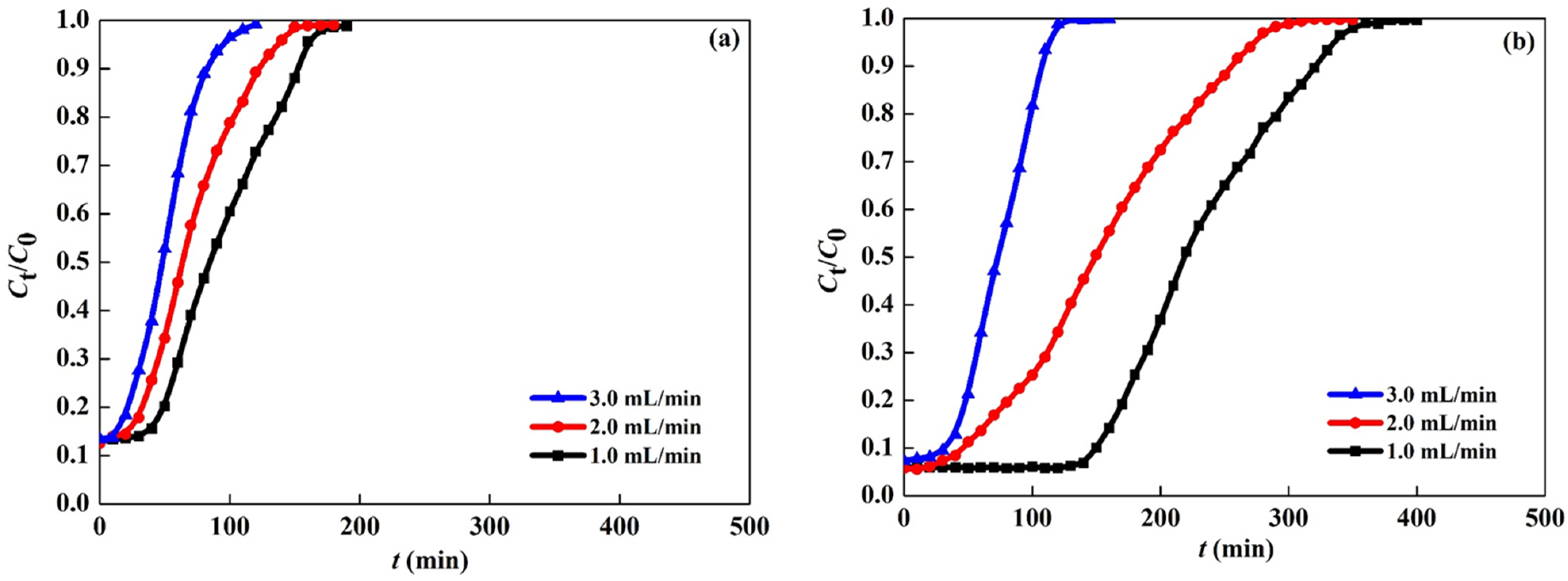
3.3.2. Effect of Flow Rate
3.3.3. Effect of Initial NAP Concentration
3.3.4. Breakthrough Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| AC | activated carbon |
| ABET | BET specific surface area |
| AMicro | micropore specific surface area |
| AE | external specific surface area |
| APS | average pore size |
| BTF | banana trunk fibers |
| BET | Brunauer-Emmett-Teller |
| BJH | Barrett-Joyner-Halenda |
| EDAX | Energy Dispersive X-ray Analysis |
| FT-IR | Fourier Transmission InfraRed spectroscopy |
| IMACs | in situ modified ACs |
| NAP | naphthalene |
| OA | Oleic acid |
| OBTF | OA modified BTF |
| OMC | ordered mesoporous carbon |
| O-PCAC1 | in situ modified PCAC |
| O-PCAC2 | conventional- modified PCAC with OA |
| O-SAC1 | in situ modified SAC |
| O-SAC2 | conventional- modified SAC with OA |
| PC | pulverized coal |
| PCAC | pulverized coal-based ACs |
| PFO | pseudo-first-order |
| PSO | pseudo-second-order |
| RL | reflection loss |
| RMSE | Root mean square error |
| SAC | Starch-based AC |
| SC | starch-based carbon |
| SEM | Scanning Electron Microscopy |
| VMeso | mesopore volume |
| VMicro | micropore volume |
| VTotol | total pore volume |
| XPS | X-ray photoelectron spectroscopy |
| Symbols | |
| Ac | the area under the breakthrough curve |
| C | a constant related to the boundary layer thickness |
| C0 | initial/inlet NAP concentration |
| Ce | equilibrium NAP concentration |
| Ct | the outlet NAP concentration |
| C0-Ct | the adsorbed NAP concentration |
| H | column height |
| K1 | pseudo-first-order rate constant |
| K2 | pseudo-second-order rate constant |
| kAB | the kinetic constant |
| Kbf | liquid-film diffusion constant |
| KF | a constant related to the bonding energy |
| KL | a constant related to the free energy |
| KP | the intraparticle diffusion constant |
| KPi | the calculated diffusion rate parameter at different stages |
| kTh | the Thomas rate constant |
| m | the mass of adsorbents used |
| N0 | the saturation concentration |
| Q | the flow rate |
| Q0 | low rate |
| q0 | the adsorption capacity |
| qe | the equilibrium adsorption capacity |
| qEq | the amount of NAP adsorbed per unit dry weight |
| qm | the maximal adsorption capacity |
| qtotal | the maximum column capacity |
| R2 | correlation coefficients |
| T | the temperature of adsorption system |
| te | the exhaustion time |
| tb | breakthrough time |
| ttotal | the total flow time |
| V | the volume of the NAP solution |
| W | the total amount of IMACs in the column |
| α | initial sorption constant |
| β | initial desorption constant |
| v | the feed flow rate |
| U0 | the superficial velocity |
| z | the bed depth of the fixed bed column |
| 1/n | a constant related to adsorption intensity |
References
- Yang, W.; Wang, Y.; Sharma, P.; Li, B.; Liu, K.; Liu, J.; Flury, M.; Shang, J.Y. Effect of naphthalene on transport and retention of biochar colloids through saturated porous media. Colloid Surface A 2017, 530, 146–154. [Google Scholar] [CrossRef]
- Lair, A.; Ferronato, C.; Chovelon, J.M.; Herrmann, J.M. Naphthalene degradation in water by heterogeneous photocatalysis: An investigation of the influence of inorganic anions. J. Photochem. Photobiol. A Chem. 2008, 193, 193–203. [Google Scholar] [CrossRef]
- Ohnishi, S.; Hiraku, Y.; Hasegawa, K.; Hirakawa, K.; Oikawa, S.; Murata, M.; Kawanishi, S. Mechanism of oxidative DNA damage induced by metabolites of carcinogenic naphthalene. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 827, 42–49. [Google Scholar] [CrossRef]
- Abo-State, M.A.M.; Riad, B.Y.; Bakr, A.A.; Abdel, A.M.F. Biodegradation of naphthalene by Bordetella avium isolated from petroleum refinery wastewater in Egypt and its pathway. J. Radiat. Res. Appl. Sci. 2018, 11, 1–9. [Google Scholar]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Mirando, N.; Levchenko, A. Liquid and vapor phase adsorption of BTX in lignin derived activated carbon: Equilibrium and kinetics study. J. Clean. Prod. 2018, 182, 372–378. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ateia, M.; Yoshimura, C. Natural organic matter undergoes different molecular sieving by adsorption on activated carbon and carbon nanotubes. Chemosphere 2018, 203, 345–352. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.Z.; Zhai, G.Y.; Zhang, J.; Zhang, C.L.; Bao, N.; Cheng, C. Preparation of activated carbon from lotus stalks with the mixture of phosphoric acid and pentaerythritol impregnation and its application for Ni (II) sorption. Chem. Eng. J. 2012, 209, 155–162. [Google Scholar] [CrossRef]
- Liu, H.; Liang, S.; Gao, J.H.; Ngo, H.H.; Guo, W.S.; Guo, Z.Z.; Wang, J.; Li, Y.R. Enhancement of Cr (VI) removal by modifying activated carbon developed from Zizania caduciflora with tartaric acid during phosphoric acid activation. Chem. Eng. J. 2014, 246, 168–174. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Farhang, M.; Alimohammadi, M.; Afsharnia, M.; Mckay, G. Adsorptive removal of fluoride from water by activated carbon derived from CaCl2-modified Crocus sativus leaves: Equilibrium adsorption isotherms, optimization, and influence of anions. Chem. Eng. Commun. 2018, 205, 955–965. [Google Scholar] [CrossRef]
- Peng, X.M.; Hu, F.P.; Zhang, T.; Qiu, F.X.; Dai, H.L. Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofloxacin and norfloxacin: A batch and fixed-bed column study. Bioresour. Technol. 2018, 249, 24–934. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; El-Sayed, M.; Moustafa, Y.M. Modeling and optimization of oil adsorption from wastewater using an amorphous carbon thin film fabricated from wood sawdust waste modified with palmitic acid. Environ. Process. 2017, 4, 147–168. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Joshi, M.V.; Jayaram, R.V. Treatment of oil spill by sorption technique using fatty acid grafted sawdust. Chemosphere 2006, 64, 1026–1031. [Google Scholar] [CrossRef]
- Sathasivam, K.; Haris, M.R.H.M. Adsorption kinetics and capacity of fatty acid-modified banana trunk fibers for oil in water. Water Air Soil Poll. 2010, 213, 413–423. [Google Scholar] [CrossRef]
- Zhu, M.; Yao, J.; Dong, L.; Sun, J.J. Adsorption of naphthalene from aqueous solution onto fatty acid modified walnut shells. Chemosphere 2016, 144, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.S.; Deng, Y.L. Superhydrophobic surface fabricated from fatty acid-modified precipitated calcium carbonate. Ind. Eng. Chem. Res. 2010, 49, 5625–5630. [Google Scholar] [CrossRef]
- Kumar, J.A.; Amarnath, D.J.; Kumar, P.S.; Kaushik, C.S.; Varghese, M.E.; Saravanan, A. Mass transfer and thermodynamic analysis on the removal of naphthalene from aqueous solution using oleic acid modified palm shell activated carbon. Desalin Water Treat. 2018, 106, 238–250. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N. Microwave plasma induced grafting of oleic acid on cotton fabric surfaces. Appl. Surf. Sci. 2012, 258, 4636–4641. [Google Scholar] [CrossRef]
- Liu, P.Y.; Wu, Z.S.; Sun, Z.H.; Ye, J. Comparison study of Naphthalene Adsorption on activated carbons prepared from different raws. Korean J. Chem. Eng. 2018, 35, 2086–2096. [Google Scholar] [CrossRef]
- Liu, P.Y.; Wu, Z.S.; Ge, X.Y.; Yang, X. Hydrothermal synthesis and microwave-assisted activation of starch-derived carbons as an effective adsorbent for naphthalene removal. RSC. Adv. 2019, 9, 11696–11706. [Google Scholar] [CrossRef]
- Ge, X.Y.; Tian, F.; Wu, Z.S.; Yan, Y.J.; Cravotto, G.; Wu, Z.L. Adsorption of naphthalene from aqueous solution on coal-based activated carbon modified by microwave induction: Microwave power effects. Chem. Eng. Process. 2015, 91, 67–77. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Eränen, K.; Leveneur, S.; Salmi, T. Epoxidation of oleic acid under conventional heating and microwave radiation. Chem. Eng. Process. 2016, 102, 70–87. [Google Scholar] [CrossRef]
- Kim, D.; Choi, J.; Kim, G.-J.; Seol, S.K.; Ha, Y.-C.; Vijayan, M.; Jung, S.; Kim, B.H.; Lee, G.D.; Park, S.S. Microwave-accelerated energy-efficient esterification of free fatty acid with a heterogeneous catalyst. Bioresour. Technol. 2011, 102, 3639–3641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.C.; Zhuang, J.D.; Liu, Q. A covalent route for efficient surface modification of ordered mesoporous carbon as high performance microwave absorbers. Nanoscale 2013, 5, 12502–12511. [Google Scholar] [CrossRef]
- Liao, L.Q.; Zhang, C.; Gong, S.Q. Preparation of Poly (varepsilon-caprolactone)/Clay Nanocomposites by Microwave-Assisted in situ Ring-Opening Polymerization. Macromol. Rapid Commun. 2007, 28, 1148–1154. [Google Scholar] [CrossRef]
- Pan, J.M.; Huang, X.B.; Gao, L.; Peng, Y.X.; Liu, S.C.; Gu, R.X. Experimental investigation of a natural favonoid adsorption on macroporous polymers with intrinsic cis-diol moieties recognition function: Static and dynamic methods. Chem. Eng. J. 2017, 312, 263–274. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Acid modified local clay beads as effective low-cost adsorbent for dynamic adsorption of methylene blue. J. Ind. Eng. Chem. 2013, 19, 1153–1161. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Dynamic adsorption behavior of methylene blue onto oil palm shell granular activated carbon prepared by microwave heating. Chem. Eng. J. 2012, 203, 81–87. [Google Scholar] [CrossRef]
- Hassan, A.F.; Hrdina, R. Chitosan/nanohydroxyapatite composite based scallop shells as an efficient adsorbent for mercuric ions: Static and dynamic adsorption studies. Int. J. Biol. Macromol. 2018, 109, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Iruretagoyena, D.; Bikane, K.; Sunny, N.; Lu, H.; Kazarian, S.G.; Chadwick, D.; Pini, R.; Shah, N. Enhanced selective adsorption desulfurization on CO2 and steam treated activated carbons: Equilibria and kinetics. Chem. Eng. J. 2020, 379, 1–6. [Google Scholar] [CrossRef]
- Li, N.; Cheng, W.Y.; Pan, Y.Z. Adsorption of naphthalene on modified zeolite from aqueous solution. J. Environ. Prot. 2017, 8, 416–425. [Google Scholar] [CrossRef][Green Version]
- Iannicelli-Zubiani, E.M.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 2018, 341, 75–82. [Google Scholar] [CrossRef]
- Niazi, L.; Lashanizadegan, A.; Sharififard, H. Chestnut oak shells activated carbon: Preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J. Clean. Prod. 2018, 185, 554–561. [Google Scholar] [CrossRef]
- Wei, X.H.; Wu, Z.S.; Wu, Z.L.; Ye, B.C. Adsorption behaviors of atrazine and Cr (III) onto different activated carbons in single and co-solute systems. Powder Technol. 2018, 329, 207–216. [Google Scholar] [CrossRef]
- Ghaedi, M.; Daneshyar, A.; Asfaram, A.; Purkait, M.K. Adsorption of naphthalene onto high-surface-area nanoparticle loaded activated carbon by high performance liquid chromatography: Response surface methodology, isotherm and kinetic study. RSC Adv. 2016, 6, 54322–54330. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, W.; Chai, H.K.; Yao, J. Acid-hydrolyzed agricultural residue: A potential adsorbent for the decontamination of naphthalene from water bodies. Korean J. Chem. Eng. 2017, 34, 1073–1080. [Google Scholar] [CrossRef]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-Moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr (VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour. Technol. Rep. 2019, 7, 1–10. [Google Scholar]
- Marrakchi, F.; Bouaziz, M.; Hameed, B.H. Adsorption of acid blue 29 and methylene blue on mesoporous K2CO3-activated olive pomace boiler ash. Colloid Surface A 2017, 535, 157–165. [Google Scholar] [CrossRef]
- Liu, N.; Charrua, A.B.; Weng, C.H.; Yuan, X.L.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Ying, D.Y.; Sanguansri, L.; Cai, Y.X.; Le, X.Y. Adsorption of catechin onto cellulose and its mechanism study: Kinetic models, characterization and molecular simulation. Food Res. Int. 2018, 112, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Lu, A.; Liu, S.; Pei, Y.; Luo, X. An easy and unique design strategy for insoluble humic acid/cellulose nanocomposite beads with highly enhanced adsorption performance of low concentration ciprofloxacin in water. Bioresour. Technol. 2020, 302, 122812. [Google Scholar] [CrossRef] [PubMed]
- Mthombeni, N.H.; Mbakop, S.; Ray, S.C.; Leswifi, T.; Ochieng, A.; Onyango, M.S. Highly efficient removal of chromium (VI) through adsorption and reduction: A column dynamic study using magnetized natural zeolite-polypyrrole composite. J. Environ. Chem. Eng. 2018, 6, 4008–4017. [Google Scholar] [CrossRef]



| Samples | ABET m2/g | AMicro m2/g | AE m2/g | VTotol cm3/g | VMicro cm3/g | VMeso cm3/g | APS nm | Ref. |
|---|---|---|---|---|---|---|---|---|
| SC | 8.90 | – | 8.53 | 0.006 | – | – | 2.62 | [19] |
| SAC | 616.80 | 522.65 | 94.18 | 0.327 | 0.255 | – | 2.12 | [19] |
| O-SAC1 | 912.89 | 481.16 | 431.73 | 0.588 | 0.232 | 0.356 | 2.58 | This study |
| O-SAC2 | 284.25 | – | 284.25 | 0.298 | 0.280 | 0.018 | 4.80 | This study |
| PC | 4.19 | 1.46 | 2.73 | 0.014 | 0.001 | 0.013 | 12.97 | This study |
| PCAC | 401.70 | 337.30 | 64.40 | 0.210 | 0.160 | – | 2.34 | [19] |
| O-PCAC1 | 725.00 | 435.34 | 289.66 | 0.484 | 0.214 | 0.270 | 2.67 | This study |
| O-PCAC2 | 30.58 | 25.03 | 5.55 | 0.020 | 0.007 | 0.013 | 2.67 | This study |
| Component Assignment Binding Energy (eV) | Peak I | Peak II | Peak III | Peak IV | |
|---|---|---|---|---|---|
| C=O | C–O | R–O*–C=O | O*=C–OH | ||
| 531.2 | 532.4 | 533.3 | 534.3 | ||
| Concentration (%) | SC | 14.7 | 26.7 | 30.4 | 28.2 |
| SAC | 21.0 | 22.7 | 26.0 | 30.4 | |
| O-SAC1 | 29.6 | 30.0 | 28.0 | 12.3 | |
| O-SAC2 | 31.1 | 29.7 | 29.1 | 10.0 | |
| PC | 9.3 | 43.3 | 18.3 | 29.0 | |
| PCAC | 21.4 | 52.4 | 17.5 | 8.7 | |
| O-PCAC1 | 27.5 | 25.5 | 26.1 | 20.9 | |
| O-PCAC2 | 18.4 | 29.7 | 15.5 | 36.5 | |
| Samples | N% | C% | H% | S% | O,diff% | N/C | H/C | O/C | [O+N]/C |
|---|---|---|---|---|---|---|---|---|---|
| SC | 0.02 | 62.98 | 6.78 | 0.40 | 29.82 | 0.000 | 0.108 | 0.474 | 0.480 |
| SAC | 0.07 | 67.84 | 4.68 | 0.26 | 27.15 | 0.010 | 0.069 | 0.400 | 0.405 |
| O-SAC1 | 0.53 | 57.78 | 1.85 | 0.73 | 39.11 | 0.009 | 0.032 | 0.677 | 0.686 |
| O-SAC2 | 0.09 | 56.85 | 1.40 | 0.32 | 41.34 | 0.002 | 0.025 | 0.727 | 0.729 |
| PC | 0.65 | 68.33 | 0.48 | 0.34 | 30.20 | 0.010 | 0.007 | 0.442 | 0.451 |
| PCAC | 0.21 | 67.10 | 3.81 | 0.23 | 28.65 | 0.003 | 0.057 | 0.427 | 0.433 |
| O-PCAC1 | 0.60 | 76.81 | 1.74 | 0.65 | 20.21 | 0.008 | 0.023 | 0.263 | 0.271 |
| O-PCAC2 | 0.57 | 57.61 | 1.03 | 1.22 | 39.59 | 0.010 | 0.018 | 0.687 | 0.697 |
| Models | Parameter | O-SAC1 | O-PCAC1 |
|---|---|---|---|
| C0 (mg/L) | 30 | 30 | |
| qexp (mg/g) | 180.8 | 187.8 | |
| PFO | qe, cal (mg/g) | 70.93 | 62.87 |
| K1 (1/min) | 0.0756 | 0.1156 | |
| R2 | 0.8688 | 0.8911 | |
| RMSE | 11.9 | 13.0 | |
| PSO | qe, cal (mg/g) | 181.82 | 188.68 |
| K2 (g/mg min) | 0.0047 | 0.0091 | |
| R2 | 0.9988 | 0.9999 | |
| RMSE | 3.6 | 3.9 | |
| Elovich | qe, cal (mg/g) | 184.46 | 196.94 |
| (1/β)ln(αβ) (mg/g) | 124.18 | 137.44 | |
| 1/β | 13.7570 | 13.5780 | |
| R2 | 0.9870 | 0.9508 | |
| RMSE | 16.1 | 15.4 |
| Adsorbents | C0 (mg/L) | T (K) | qe (mg/g) | Ref. |
|---|---|---|---|---|
| Modified walnut shells | 25 | 298 | 7.20 | [15] |
| Modified coal-based AC | 30 | 298 | 131.20 | [21] |
| Modified zeolite | 1 | 303 | 0.30 | [31] |
| ZnS-NPs-AC | 50 | 298 | 142.70 | [35] |
| Modified hazelnut shell | 25 | 298 | 17.30 | [36] |
| SC | 30 | 303 | 52.9 | [19] |
| SAC | 30 | 303 | 158.9 | [19] |
| PC | 30 | 303 | 75.26 | This study |
| PCAC | 30 | 303 | 160.7 | [19] |
| O-SAC1 | 30 | 303 | 180. 8 | This study |
| O-PCAC1 | 30 | 303 | 187.8 | This study |
| O-SAC2 * | 30 | 303 | 116.2 | This study |
| O-PCAC2 * | 30 | 303 | 120.5 | This study |
| Models | Parameters | O-SAC1 | O-PCAC1 |
|---|---|---|---|
| Langmuir | qmax (mg/g) | 270.27 | 333.33 |
| KL (L/mg) | 0.5873 | 0.6122 | |
| R2 | 0.9889 | 0.9820 | |
| RMSE | 9.7 | 9.6 | |
| Freundlich | KF (L/mg) | 103.52 | 123.48 |
| 1/n | 0.3433 | 0.4156 | |
| R2 | 0.9934 | 0.9926 | |
| RMSE | 2.9 | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Liu, P.; Wu, Z.; Cravotto, G. In Situ Modification of Activated Carbons by Oleic Acid under Microwave Heating to Improve Adsorptive Removal of Naphthalene in Aqueous Solutions. Processes 2021, 9, 391. https://doi.org/10.3390/pr9020391
Wu Z, Liu P, Wu Z, Cravotto G. In Situ Modification of Activated Carbons by Oleic Acid under Microwave Heating to Improve Adsorptive Removal of Naphthalene in Aqueous Solutions. Processes. 2021; 9(2):391. https://doi.org/10.3390/pr9020391
Chicago/Turabian StyleWu, Zhansheng, Pengyun Liu, Zhilin Wu, and Giancarlo Cravotto. 2021. "In Situ Modification of Activated Carbons by Oleic Acid under Microwave Heating to Improve Adsorptive Removal of Naphthalene in Aqueous Solutions" Processes 9, no. 2: 391. https://doi.org/10.3390/pr9020391
APA StyleWu, Z., Liu, P., Wu, Z., & Cravotto, G. (2021). In Situ Modification of Activated Carbons by Oleic Acid under Microwave Heating to Improve Adsorptive Removal of Naphthalene in Aqueous Solutions. Processes, 9(2), 391. https://doi.org/10.3390/pr9020391







