Capabilities and Limitations of 3D-CFD Simulation of Anode Flow Fields of High-Pressure PEM Water Electrolysis
Abstract
1. Introduction
Relevance of CFD with Regard to PEM Water Electrolysis
2. Materials and Methods
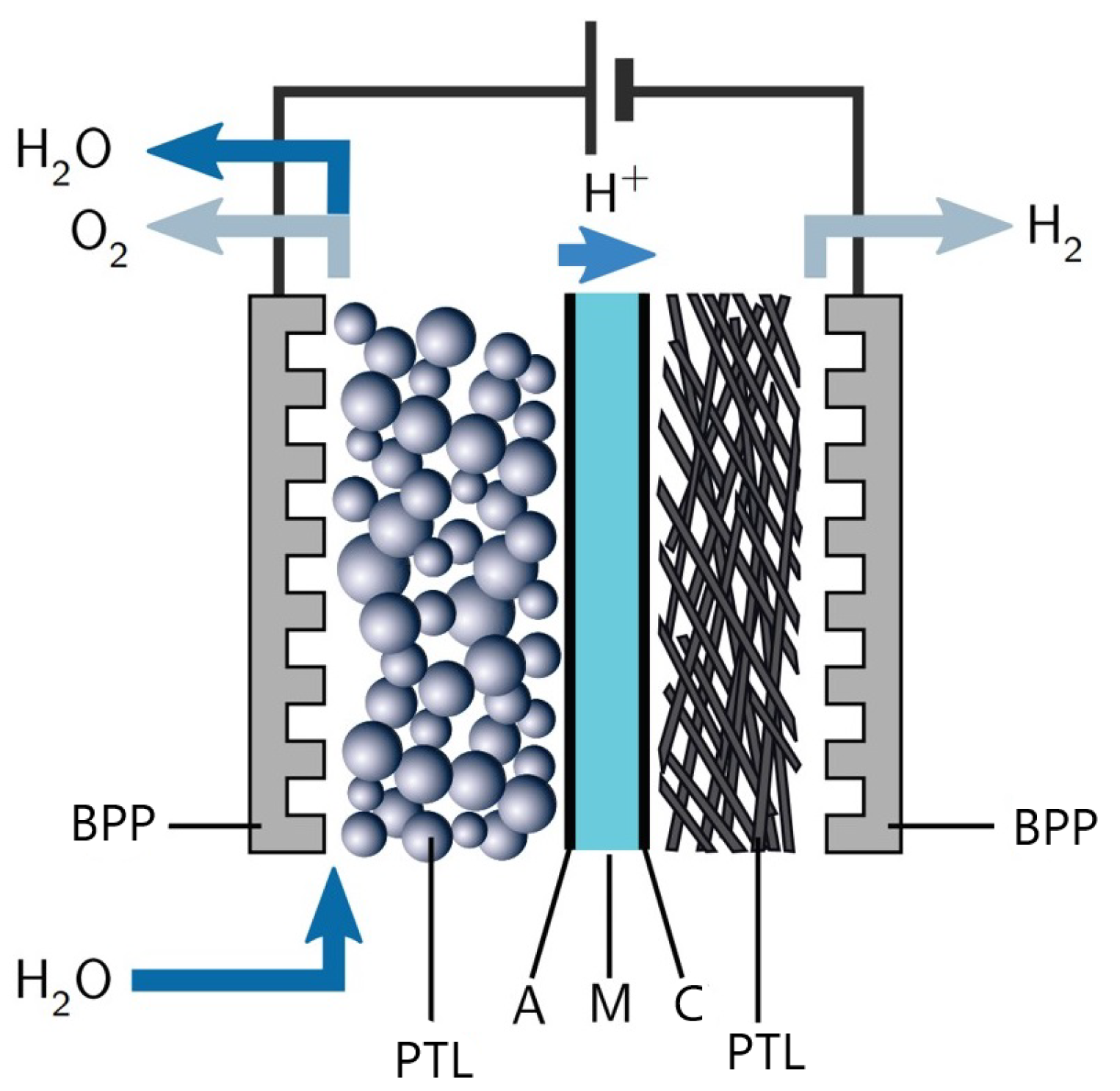
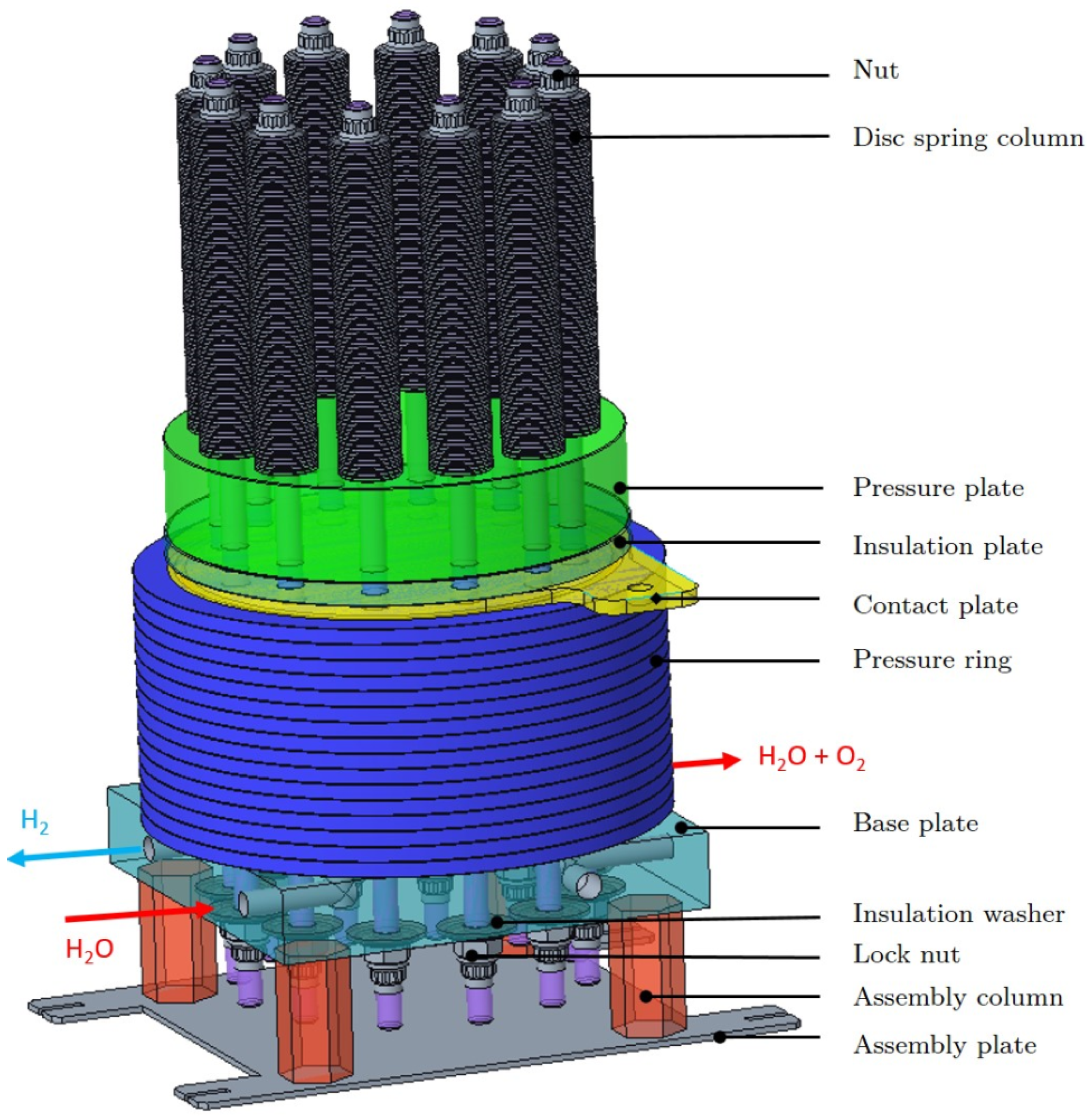
2.1. Layout of PEM Water Electrolyzer Stack
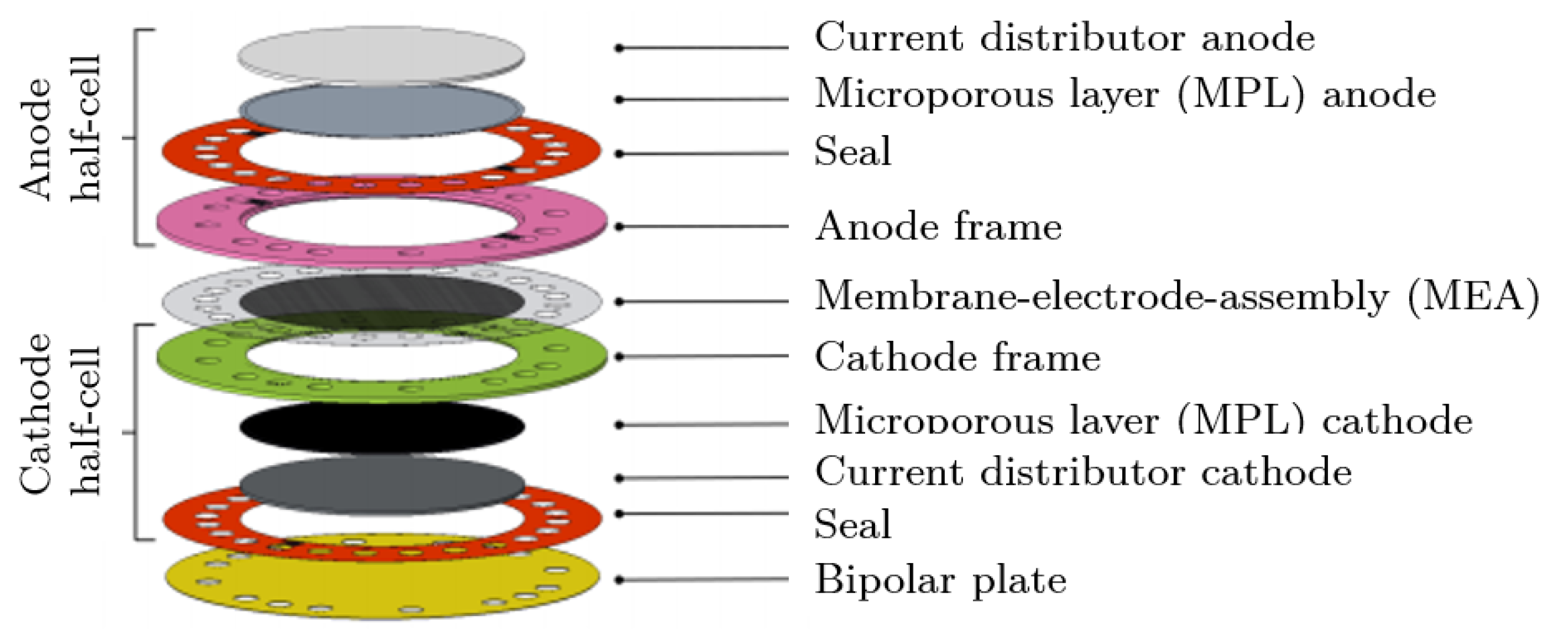
2.2. PEM Cell Assembly
2.3. Mathematical Model
Mathematical Description of the Boundary Conditions
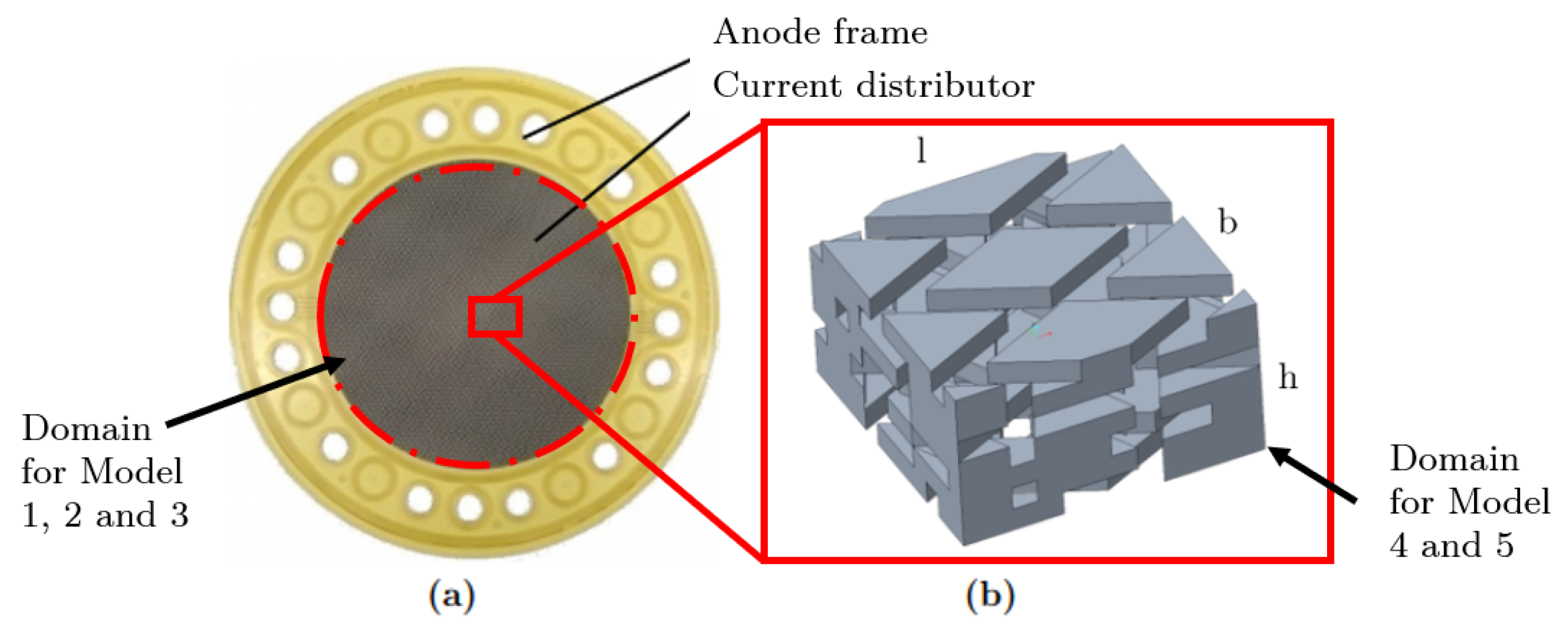
2.4. Simulation Model
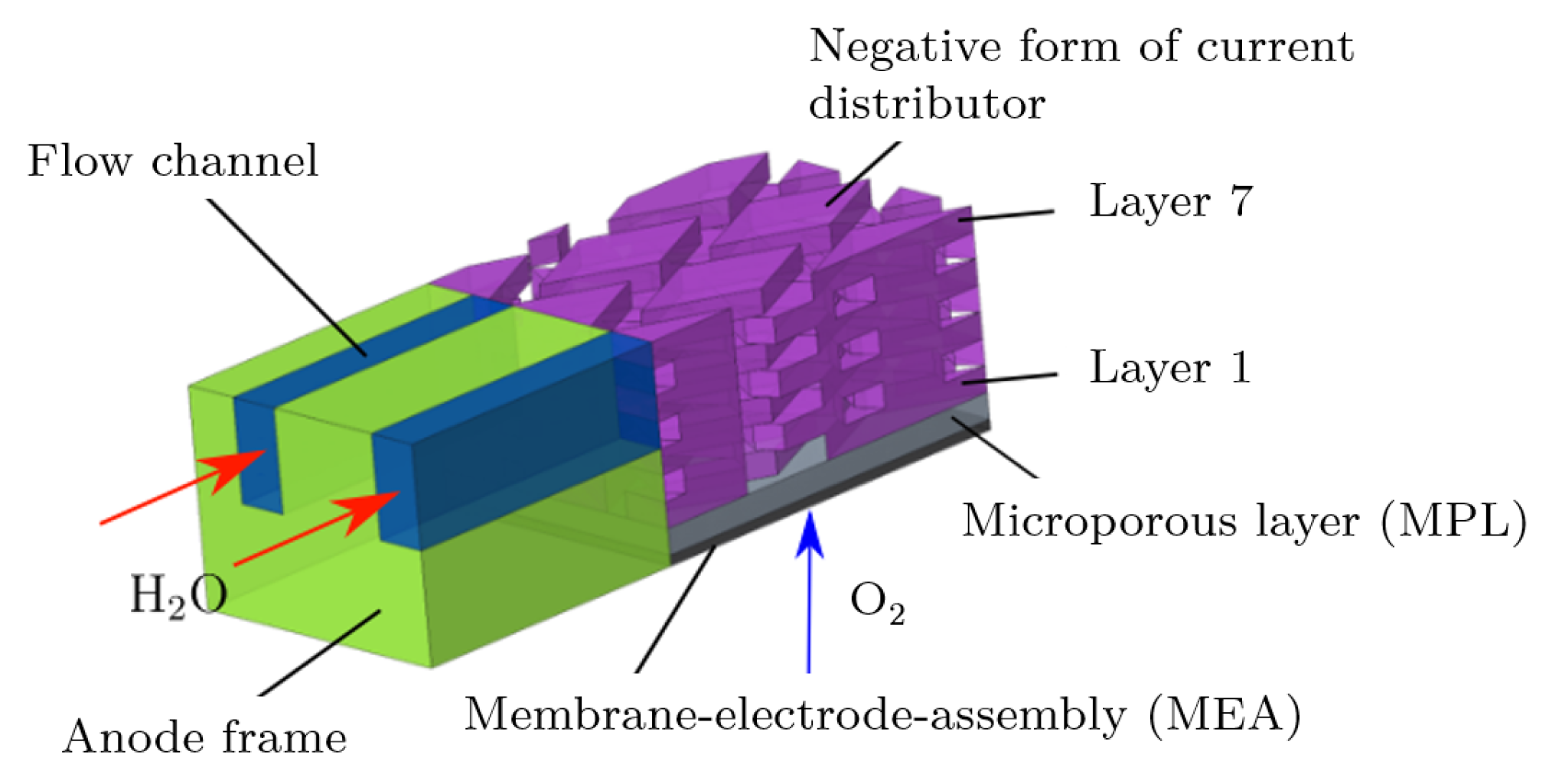
Mesh Generations of PTL and Detail Geometry
2.5. Initial and Boundary Conditions
2.5.1. Inlet Boundary Condition
- Velocity (u, v, w);
- Temperature T;
- Turbulent properties (turbulent kinetic energy k, length scale L, dissipation );
- Volume fraction (for the multiphase case).
2.5.2. Outlet Boundary Condition
2.5.3. Wall Boundary Condition
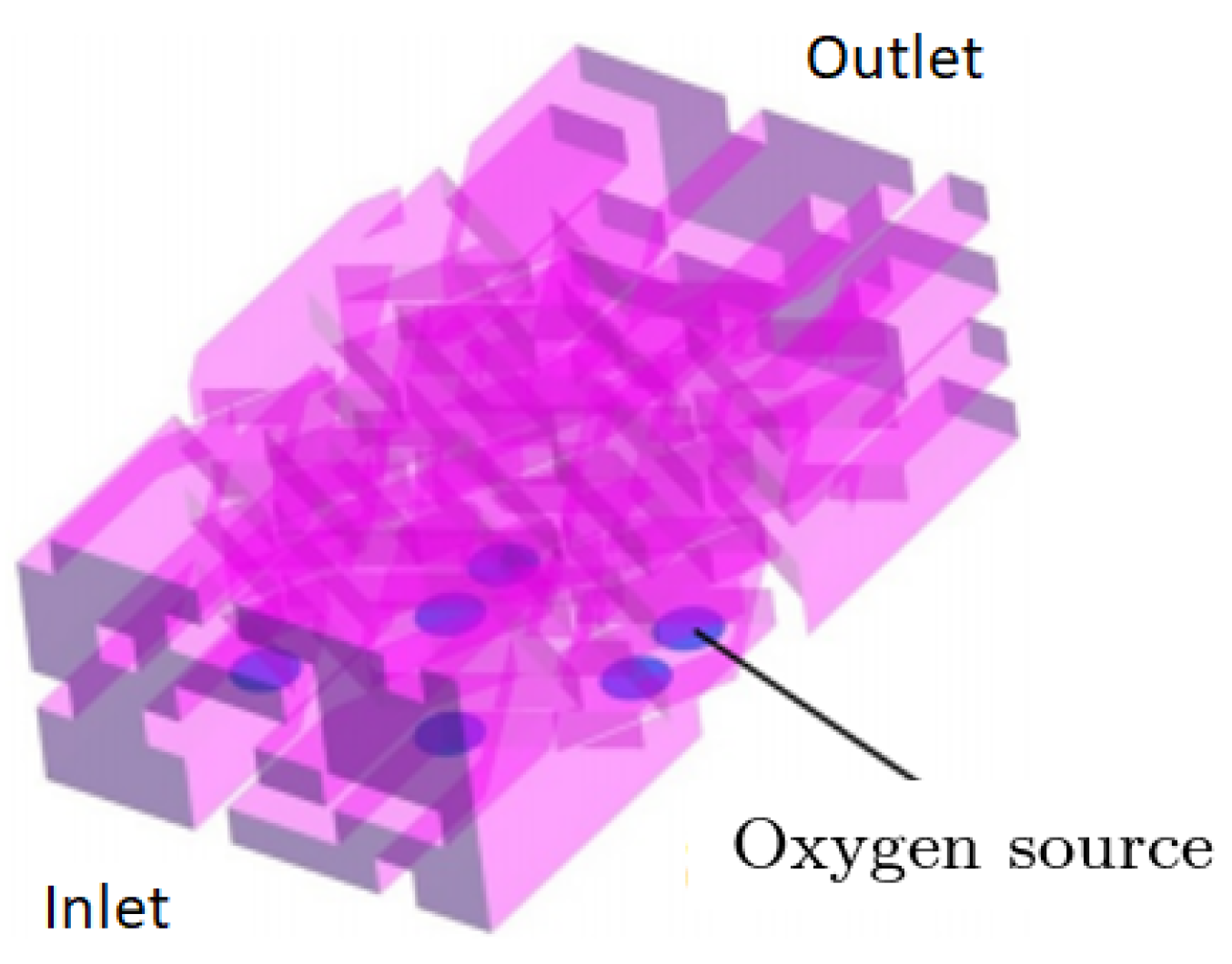
2.5.4. Oxygen Source Boundary Condition
2.5.5. Boundary Conditions for Detailed Section
2.6. Multiphase Numerical Models
2.6.1. Mixture Model
2.6.2. Volume-of-Fluid
- = 0, no continuous phase in the cell;
- = 1, only continuous phase in the cell;
- 0 < < 1, phase boundary interface.
2.6.3. Eulerian Model
3. Results and Discussion
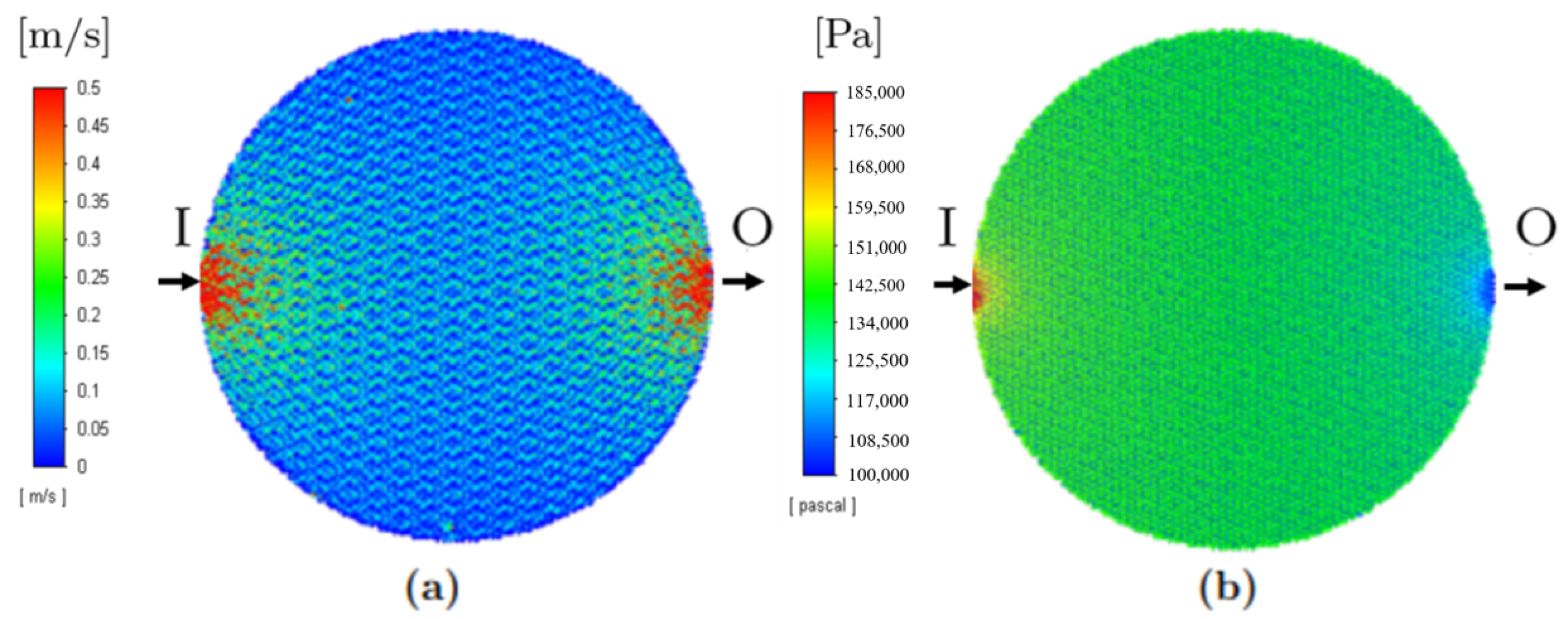
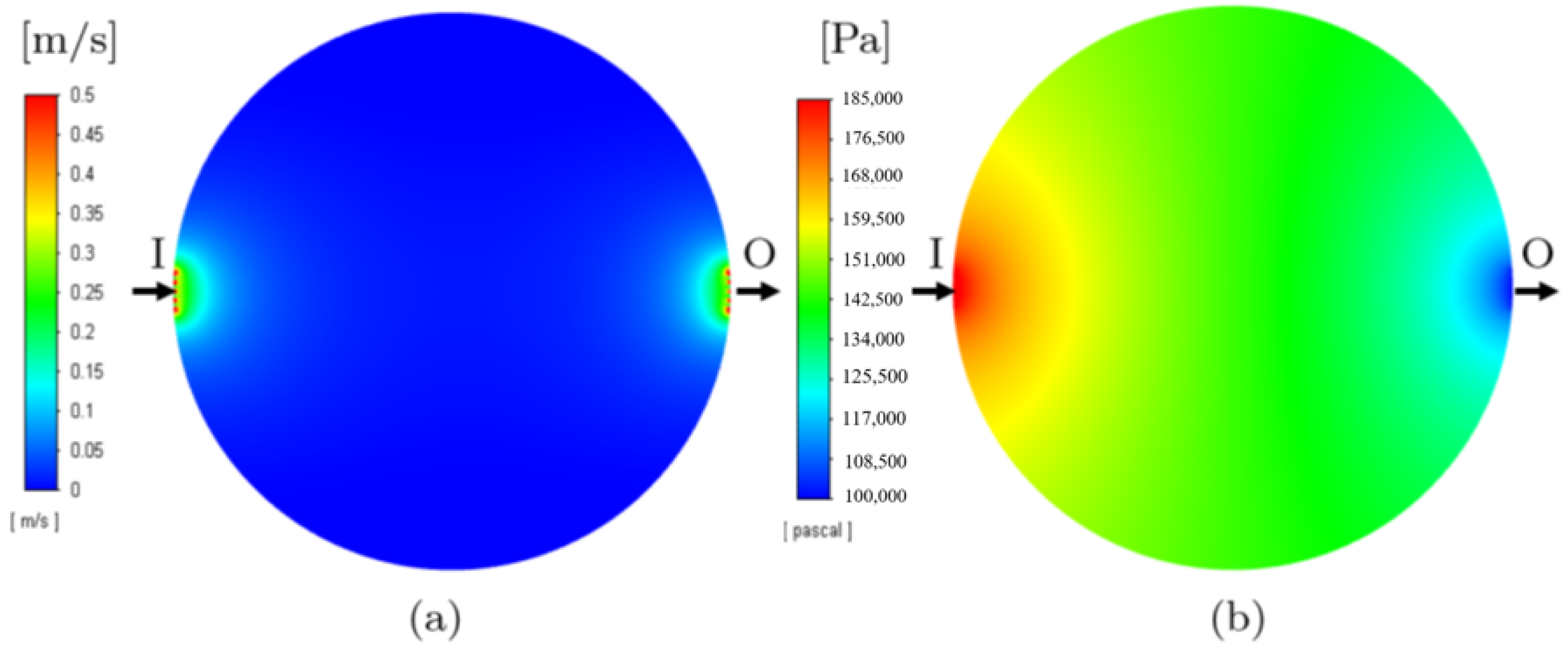
3.1. Single-Phase Models
3.2. Two-Phase Models
3.3. Detailed Geometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEM | proton exchange membrane |
| CFD | computational fluid dynamics |
| BPP | bipolar plate |
| PTL | porous transport layer |
| VOF | volume-of-fluid |
References
- Klell, M.; Eichlseder, H.; Trattner, A. Wasserstoff in der Fahrzeugtechnik. Erzeugung, Speicherung, Anwendung, 4th ed.; Springer: Berlin, Germany, 2017. [Google Scholar]
- Nie, J.; Chen, Y. Numerical modeling of three-dimensional two-phase gas–liquid flow in the flow field plate of a PEM electrolysis cell. Int. J. Hydrogen Energy 2010, 35, 3183–3197. [Google Scholar] [CrossRef]
- Hoeh, M.A.; Arlt, T.; Kardjilov, N.; Manke, I.; Banhart, J.; Fritz, D.L.; Ehlert, J.; Lüke, W.; Lehnert, W. In-Operando Neutron Radiography Studies of Polymer Electrolyte Membrane Water Electrolyzers. ECS Trans. 2015, 69, 1135–1140. [Google Scholar] [CrossRef]
- Arbabi, F.; Montazeri, H.; Abouatallah, R.; Wang, R.; Bazylak, A. Three-Dimensional Computational Fluid Dynamics Modelling of Oxygen Bubble Transport in Polymer Electrolyte Membrane Electrolyzer Porous Transport Layers. J. Electrochem. Soc. 2016, 163, F3062–F3069. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Arbabi, F. Oxygen Bubble Propagation in Polymer Electrolyte Membrane Electrolyzer Porous Transport Layers. Ph.D. Thesis, University of Toronto, Toronto, ON, USA, 2017. [Google Scholar]
- Nie, J.; Chen, Y.; Cohen, S.; Carter, B.D.; Boehm, R.F. Numerical and experimental study of three-dimensional fluid flow in the bipolar plate of a PEM electrolysis cell. Int. J. Therm. Sci. 2009, 48, 1914–1922. [Google Scholar] [CrossRef]
- Olesen, A.C.; Rømer, C.; Kær, S.K. A numerical study of the gas-liquid, two-phase flow maldistribution in the anode of a high pressure PEM water electrolysis cell. Int. J. Hydrogen Energy 2016, 41, 52–68. [Google Scholar] [CrossRef]
- Han, B.; Mo, J.; Kang, Z.; Yang, G.; Barnhill, W.; Zhang, F.Y. Modeling of two-phase transport in proton exchange membrane electrolyzer cells for hydrogen energy. Int. J. Hydrogen Energy 2017, 42, 4478–4489. [Google Scholar] [CrossRef]
- Aubras, F.; Deseure, J.; Kadjo, J.J.; Dedigama, I.; Majasan, J.; Grondin-Perez, B.; Chabriat, J.P.; Brett, D.J.L. Two-dimensional model of low-pressure PEM electrolyser: Two-phase flow regime, electrochemical modelling and experimental validation. Int. J. Hydrogen Energy 2017, 42, 26203–26216. [Google Scholar] [CrossRef]
- Zinser, A.; Papakonstantinou, G.; Sundmacher, K. Analysis of mass transport processes in the anodic porous transport layer in PEM water electrolysers. Int. J. Hydrogen Energy 2019, 42, 28077–28087. [Google Scholar] [CrossRef]
- ANSYS, Inc. Ansys Fluent Theory Guide; 2020R1; ANSYS, Inc.: Canonsburg, PA, USA, 2020. [Google Scholar]
- Bessarabov, D.; Wang, H.; Li, H.; Zhao, N. PEM Electrolysis for Hydrogen Production: Principles and Applications; Taylor-Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Haas, C. Entwicklung Eines 3D-CFD Simulationsmodells Eines PEM-Elektrolysemoduls. Master’s Thesis, Technische Universität Graz, Institut für Verbrennungskraftmaschinen und Thermodynamik, Graz, Austria, 2018. [Google Scholar]
- Rangel-Cardenas, A.L.; Koper, G.J.M. Transport in Proton Exchange Membranes for Fuel Cell Applications—A Systematic Non-Equilibrium Approach. Materials 2017, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Fellinger, T. Entwicklung Eines Simulationsmodells für Eine PEM-Hochdruckelektrolyse. Master’s Thesis, Technische Universität Graz, Institut für Verbrennungskraftmaschinen und Thermodynamik, Graz, Austria, 2016. [Google Scholar]
- Sartory, M.; Wallnöfer-Ogris, E.; Salman, P.; Fellinger, T.; Justl, M.; Trattner, A.; Klell, M. Theoretical and experimental analysis of an asymmetric high pressure PEM water electrolyser up to 155 bar. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]
- Toray Industries, Inc. Available online: http://www.toray.com (accessed on 11 May 2018).
- Moseley, P.-T.; Garche, J. Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bargel, H.-J.; Schulze, G. Werkstoffkunde; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2013. [Google Scholar]
- Sarkar, S.; Balakrishnan, L. Application of a Reynolds-Stress Turbulence Model to the Compressible Shear Layer; Final Report; Institute for Computer Applications in Science and Engineering: Hampton, VA, USA, 1990. [Google Scholar]
- AVL List GmbH. Fire R2017.1 Documentation; Documentation AVL FIRE Version R2017.1; AVL List GmbH: Graz, Austria, 2018. [Google Scholar]
- AVL List GmbH. Porosity; Documentation AVL FIRE Version R2017.1; AVL List GmbH: Graz, Austria, 2018. [Google Scholar]
- AVL List GmbH. Fire CFD Solver User Manual; Documentation AVL FIRE Version R2020.1; AVL List GmbH: Graz, Austria, 2020. [Google Scholar]
- Argyropoulos, C.D.; Markatos, N.C. Recent advances on the numerical modelling of turbulent flows. Appl. Math. Model. 2015, 39, 693–732. [Google Scholar] [CrossRef]
- Tu, J.; Yeoh, G.-H.; Liu, C. Computational Fluid Dynamics—A Practical Approach, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2012. [Google Scholar]
- Atkins, P.-W.; de Paula, J. Physikalische Chemie, 5th ed.; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- AVL List GmbH. Eulerian Multiphase; Documentation AVL FIRE Version R2017.1; AVL List GmbH: Graz, Austria, 2018. [Google Scholar]
- NIST Chemistry Webbook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 1 March 2018).
- Ferziger, J.-H.; Peric, M. Numerische Strömungsmechanik, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2002. [Google Scholar]

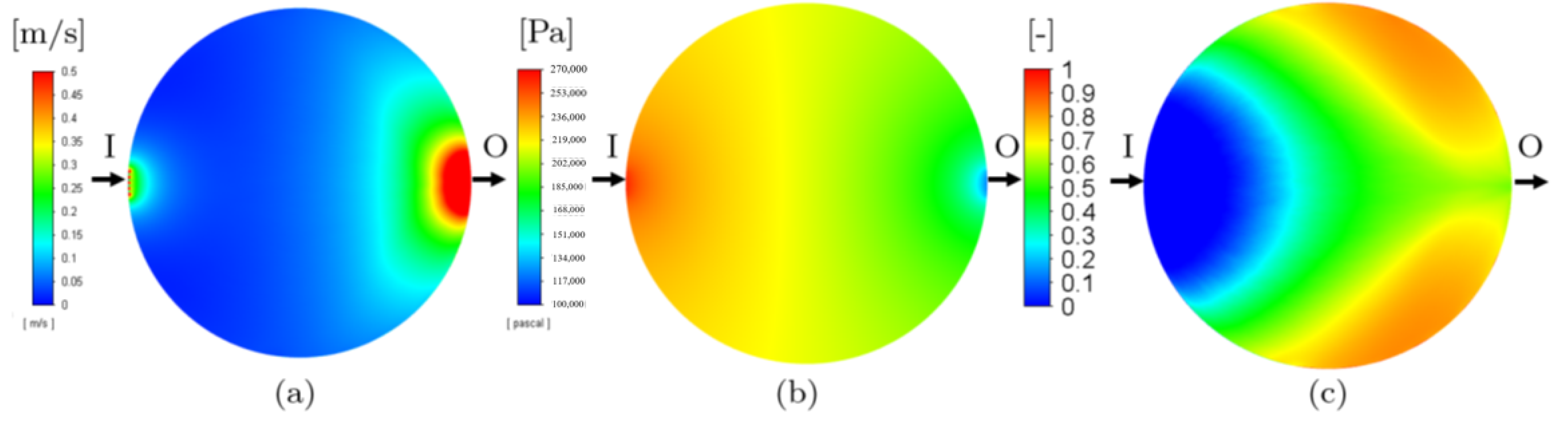
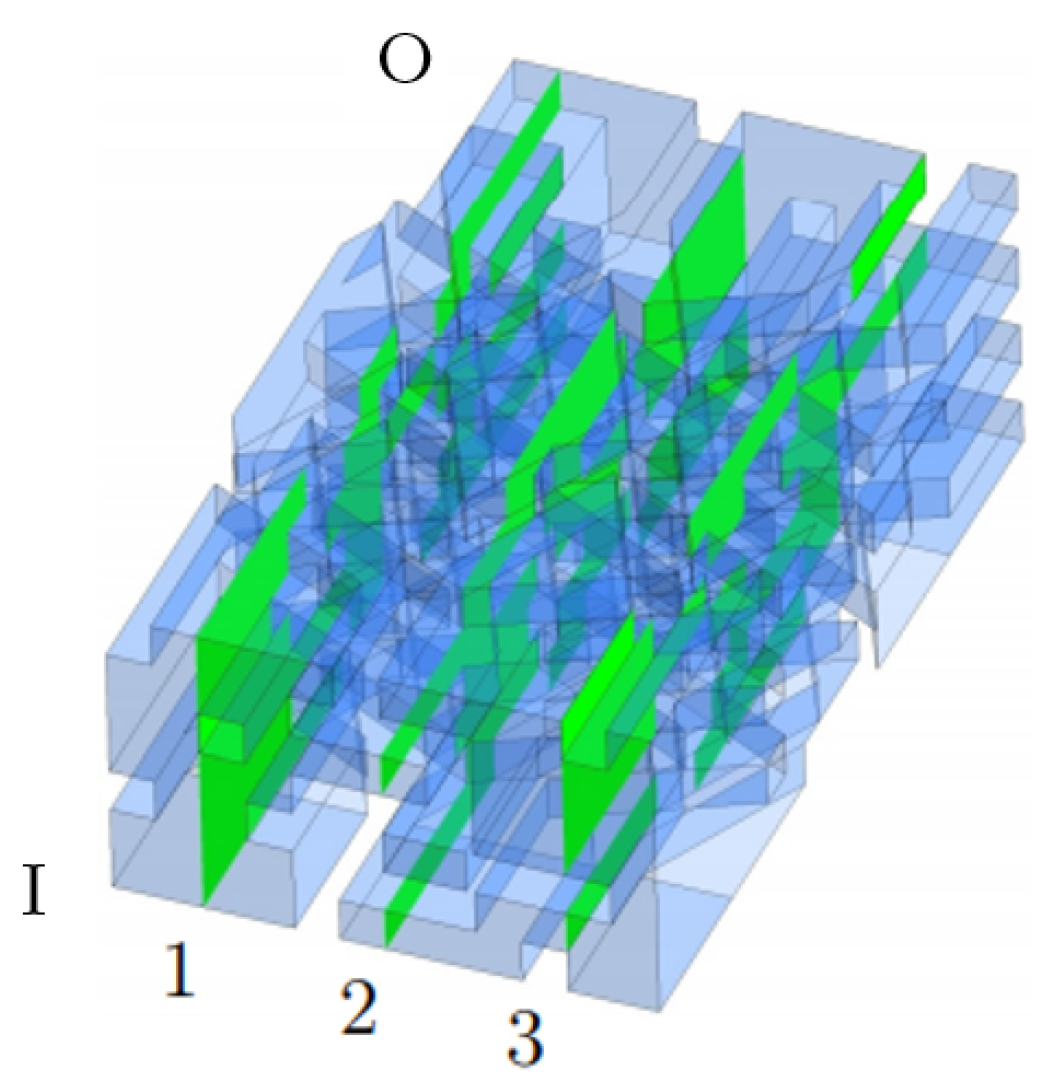

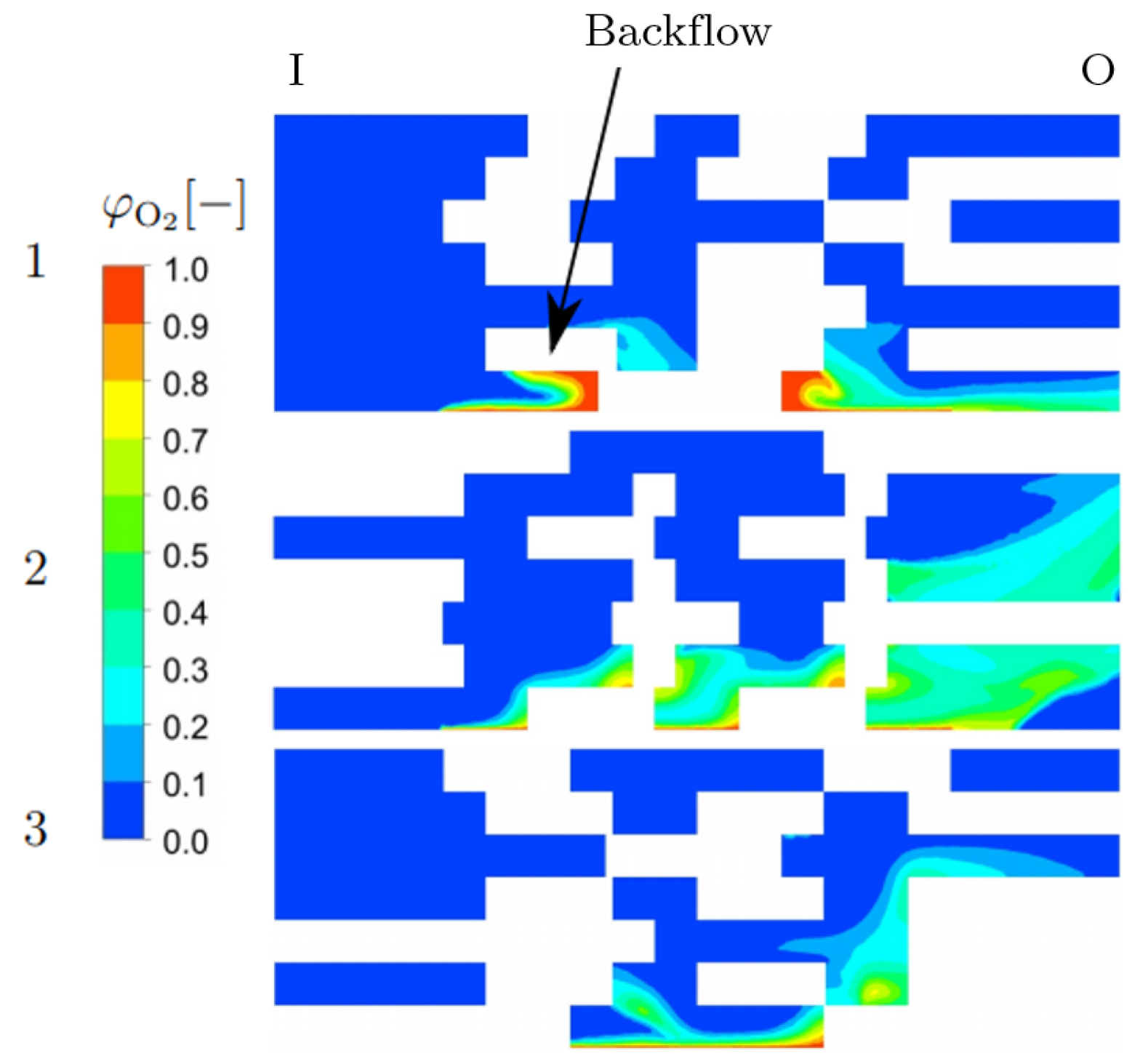
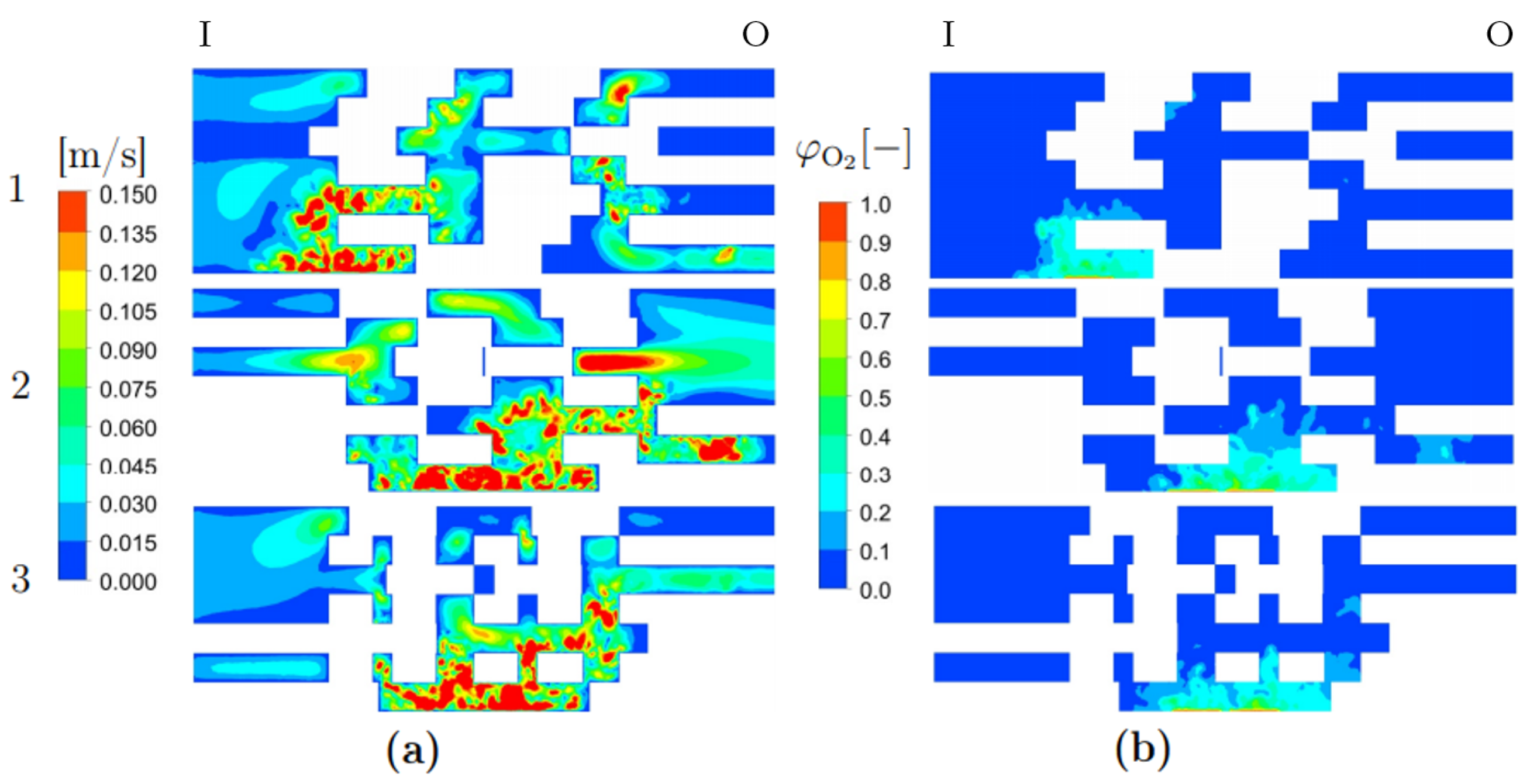
| Porosity type | Homogeneous |
| Porosity (=/) | 40% |
| Pressure drop model | Forchheimer |
| -values (1/m) | x = 0, y = 0, z = 0 |
| -values (1/m2) | x = 1010, y = 1010, z = 104 |
| Model 1 | Model 2 | |
|---|---|---|
 |  | |
| Description | Basic model | Porosity model |
| Simulation | Single phase | Single and multiphase |
| Structure | 7 layers, structured | Unstructured |
| Connection | Arbitrary | - |
| Element | Parallelogram | - |
| Element discretization | 5 × 5 × 4 | - |
| Cell type | Hexahedron | Polyhedron |
| Mesh size (cells) | 2,917,888 | 222,425 |
| Nr | Domain | Geometry | Phases | Goal |
|---|---|---|---|---|
| Model 1 | Whole PTL | Exact geometry | Single | Investigation of temperature, pressure and velocity distributions inside the PTL |
| Model 2 | Whole PTL | Porosity model | Single | Reproduction of results from Model 1 with significant reduction in computation time |
| Model 3 | Whole PTL | Porosity model | Two-phase Eulerian | Expansion of Model 2 to include the gas phase to investigate gas bubble distribution inside the PTL |
| Model 4 | Detailed section of the PTL | Exact geometry of the section | Two-phase Eulerian | Detailed investigation of local phenomena regarding gas bubble entrapment using the Eulerian approach |
| Model 5 | Detailed section of the PTL | Exact geometry of the section | Two-phase VOF | Detailed investigation of local phenomena regarding gas bubble entrapment using the VOF approach |
| Quantity | Value | Unit |
|---|---|---|
| Velocity (normal to inlet face) | 2.25 | m/s |
| Volume fraction of water | 100 | % |
| Temperature | 80 | °C |
| Turbulence intensity | 10 | % |
| Turbulence length scale in % of | 10 | % |
| Turbulent kinetic energy | 0.029 | m2/s2 |
| Turbulence length scale | 0.071 | mm |
| Turbulent dissipation rate | 11.2 | m2/s3 |
| Quantity | Value | Unit |
|---|---|---|
| Molar flux O2 | 0.025 | mol/min |
| Mass flux O2 | 13.3 | mg/s |
| Volume flux O2 | m3/s | |
| Surface area of the BC face | 5.67 | cm2 |
| Velocity (normal to inlet face) | m/s | |
| Volume fraction of oxygen | 100 | % |
| Temperature | 80 | °C |
| Turbulence intensity | 5 | % |
| Turbulence length scale in % of | 5 | % |
| Turbulent kinetic energy | 1.727 | m2/s2 |
| Turbulence length scale | 0.069 | mm |
| Turbulent dissipation rate | 5.33 | m2/s3 |
| Quantity | Inlet | Oxygen Source | Unit |
|---|---|---|---|
| Velocity (normal to inlet face) | 0.05 | m/s | |
| Volume fraction | 100% water | 100% oxygen | % |
| Temperature | 80 | 80 | °C |
| Turb. length scale in % of d_h | 10 | 5 | % |
| Turb. kin. energy | 0.085 | 0.085 | m2/s2 |
| Turb. length scale | 0.089 | 0.067 | mm |
| Turb. dissipation rate | 9.33 | 2.33 | m2/s3 |
| Fluid | Density (kg/m3) | Viscosity (kg/m·s) |
|---|---|---|
| Water | 971.79 | 35.435 |
| Oxygen | 1.0900 | 2.3395 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haas, C.; Macherhammer, M.-G.; Klopcic, N.; Trattner, A. Capabilities and Limitations of 3D-CFD Simulation of Anode Flow Fields of High-Pressure PEM Water Electrolysis. Processes 2021, 9, 968. https://doi.org/10.3390/pr9060968
Haas C, Macherhammer M-G, Klopcic N, Trattner A. Capabilities and Limitations of 3D-CFD Simulation of Anode Flow Fields of High-Pressure PEM Water Electrolysis. Processes. 2021; 9(6):968. https://doi.org/10.3390/pr9060968
Chicago/Turabian StyleHaas, Christoph, Marie-Gabrielle Macherhammer, Nejc Klopcic, and Alexander Trattner. 2021. "Capabilities and Limitations of 3D-CFD Simulation of Anode Flow Fields of High-Pressure PEM Water Electrolysis" Processes 9, no. 6: 968. https://doi.org/10.3390/pr9060968
APA StyleHaas, C., Macherhammer, M.-G., Klopcic, N., & Trattner, A. (2021). Capabilities and Limitations of 3D-CFD Simulation of Anode Flow Fields of High-Pressure PEM Water Electrolysis. Processes, 9(6), 968. https://doi.org/10.3390/pr9060968






