An Atmospheric Origin for HCN-Derived Polymers on Titan
Abstract
1. Introduction
CNH3 + HCN + 2H2O → C2NH5O2 (Glycine) + NH3
C2N2H4 + 2H2O → C2NH5O2 (Glycine) + NH3
2. Materials and Methods
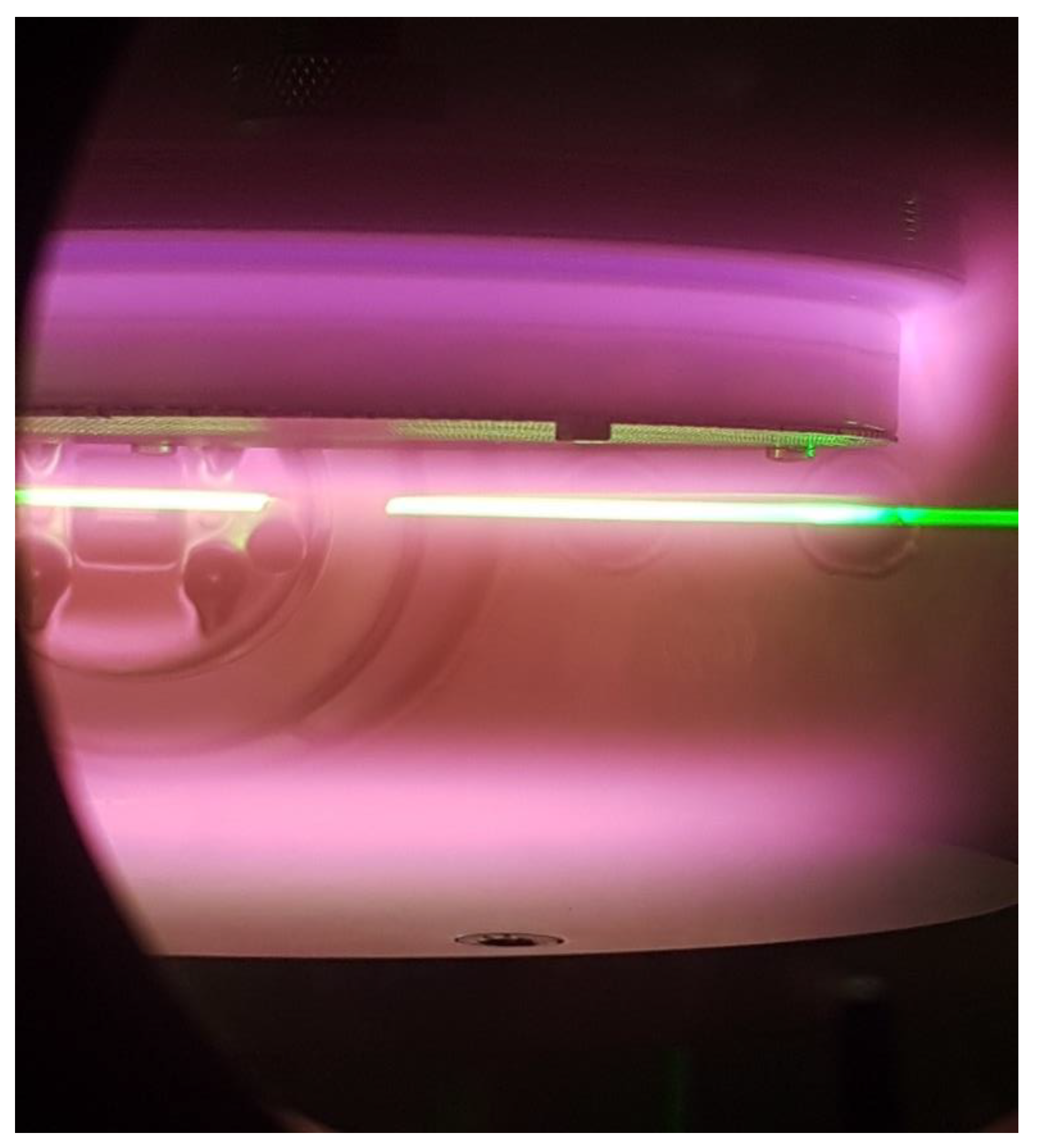
2.1. Experimental Simulation of Titan’s Upper Atmospheric Chemistry
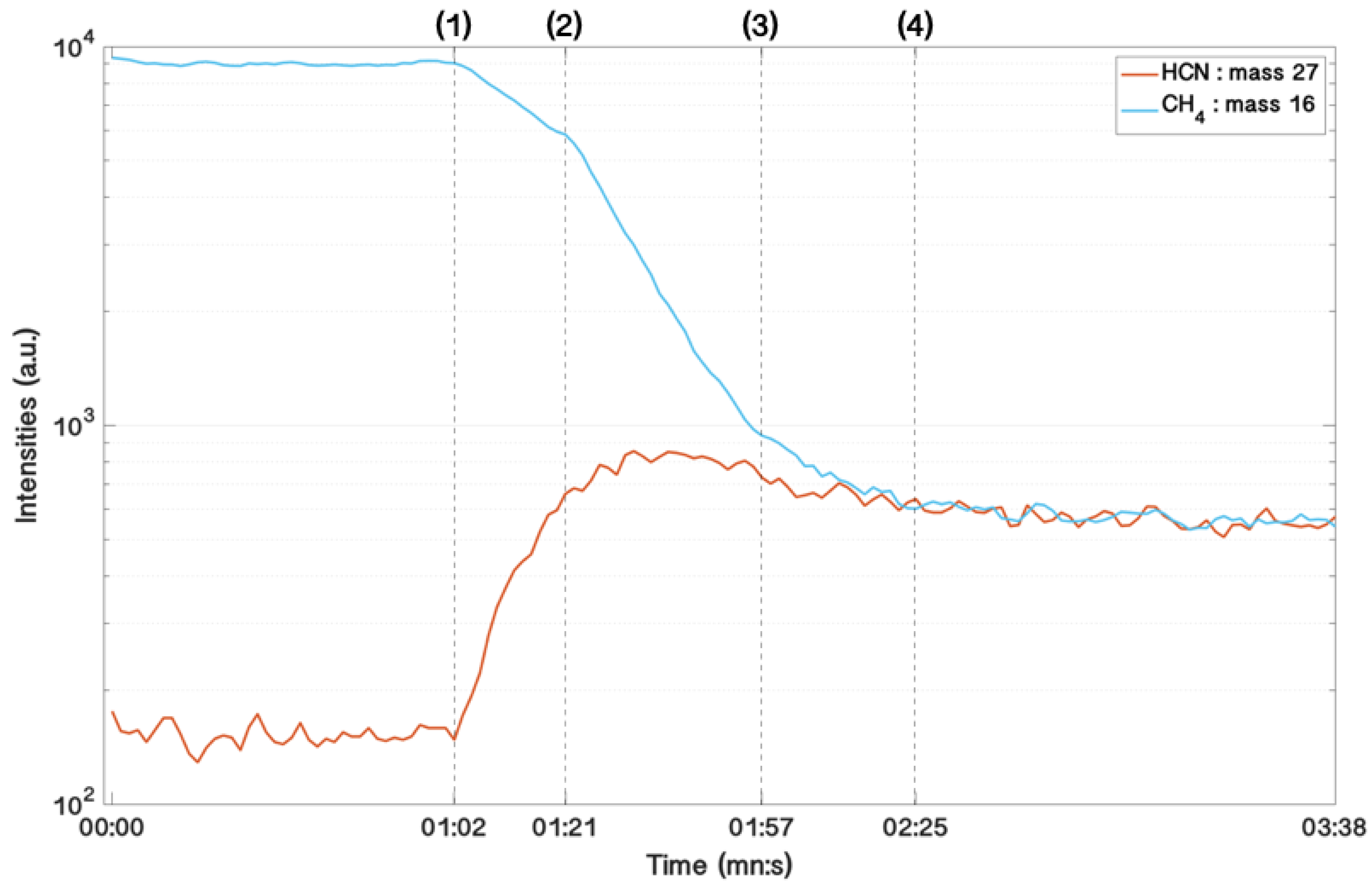
2.2. Gas Phase Diagnosis with Mass Spectrometry
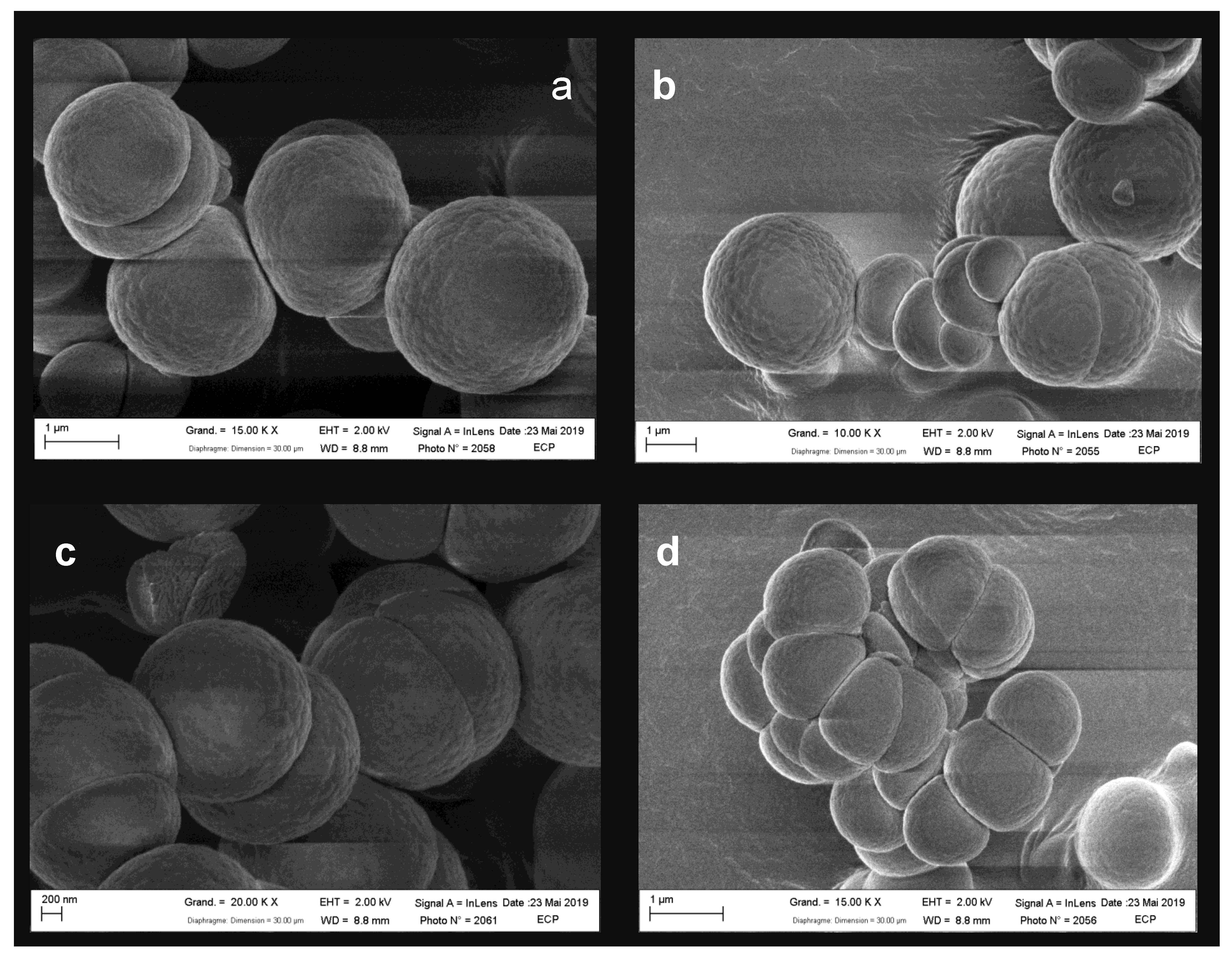
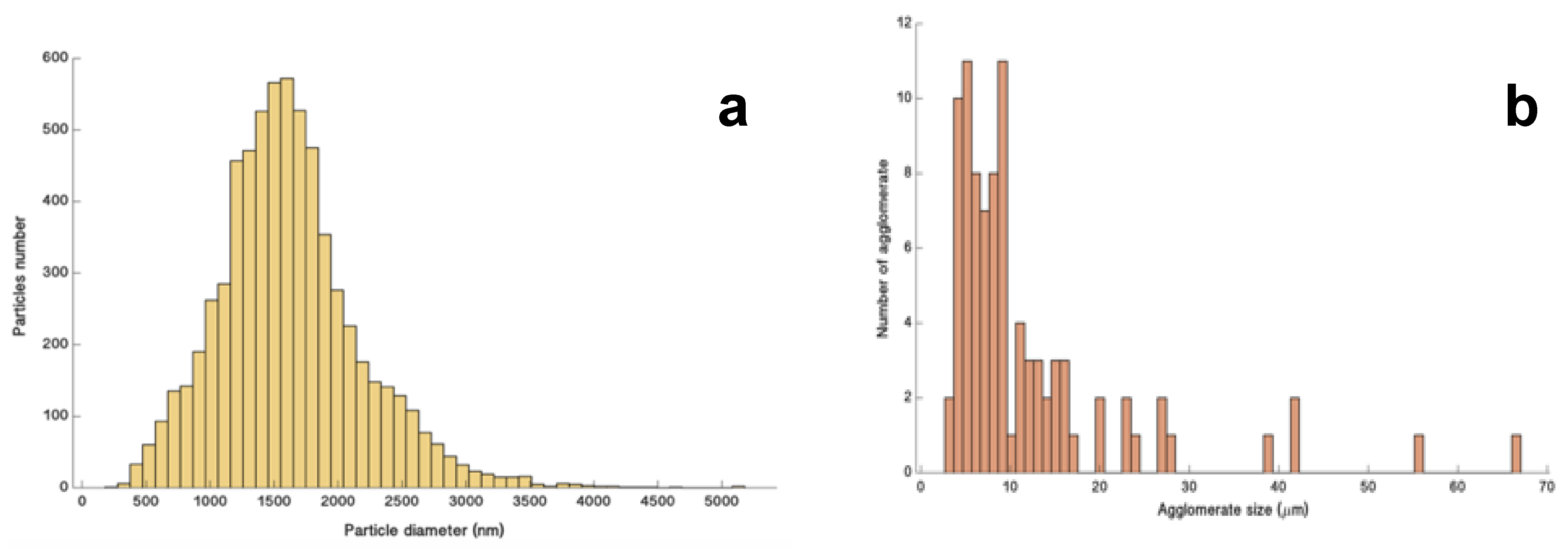
2.3. Size and Morphology of Titan’s Tholins: SEM Analysis
2.4. Chemical Analysis of Titan’s Tholins: Mid-infrared Spectroscopy
3. Results
3.1. Size and Morphology of Titan’s Tholins
3.2. Chemical Composition of Titan’s Tholins
3.3. Time Evolution of the Gas Phase and Formation and Growth of Titan’s Tholins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Di Mauro, E. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef]
- Powner, M.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. The mechanism of synthesis of amino acids by electric discharges. Biochim. Biophys. Acta 1957, 23, 480–489. [Google Scholar] [CrossRef]
- Peltzer, E.; Bada, J.; Schlesinger, G.; Miller, S. The chemical conditions on the parent body of the murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv. Space Res. 1984, 4, 69–74. [Google Scholar] [CrossRef]
- Hanel, R.; Conrath, B.; Flasar, F.M.; Kunde, V.; Maguire, W.; Pearl, J.; Pirraglia, J.; Samuelson, R.; Herath, L.; Allison, M.; et al. Infrared Observations of the Saturnian System from Voyager 1. Science 1981, 212, 192–200. [Google Scholar] [CrossRef]
- Hebrard, E.; Dobrijevic, M.; Loison, J.; Bergeat, A.; Hickson, K.M. Neutral production of hydrogen isocyanide (HNC) and hydrogen cyanide (HCN) in Titan’s upper atmosphere. Astron. Astrophys. 2012, 541, A21. [Google Scholar] [CrossRef]
- Lara, L.M.; Lellouch, E.; López-Moreno, J.J.; Rodrigo, R. Vertical distribution of Titan’s atmospheric neutral constituents. J. Geophys. Res. Space Phys. 1996, 101, 23261–23283. [Google Scholar] [CrossRef]
- Tomasko, M.; Doose, L.; Engel, S.; Dafoe, L.; West, R.; Lemmon, M.; Karkoschka, E.; See, C. A model of Titan’s aerosols based on measurements made inside the atmosphere. Planet. Space Sci. 2008, 56, 669–707. [Google Scholar] [CrossRef]
- Liang, M.-C.; Yung, Y.L.; Shemansky, D.E. Photolytically Generated Aerosols in the Mesosphere and Thermosphere of Titan. Astrophys. J. 2007, 661, L199–L202. [Google Scholar] [CrossRef]
- Waite, J.H., Jr.; Young, D.T.; Cravens, T.E.; Coates, A.J.; Crary, F.J.; Magee, B.; Westlake, J. The Process of Tholin Formation in Titan’s Upper Atmosphere. Science 2007, 316, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Lavvas, P.; Sander, M.; Kraft, M.; Imanaka, H. Surface chemistry and particle shape: Processes for the evolution of aerosols in titan’s atmosphere. Astrophys. J. 2011, 728, 80. [Google Scholar] [CrossRef]
- Lavvas, P.; Yelle, R.V.; Koskinen, T.; Bazin, A.; Vuitton, V.; Vigren, E.; Galand, M.; Wellbrock, A.; Coates, A.; Wahlund, J.-E.; et al. Aerosol growth in Titan’s ionosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Israël, G.; Szopa, C.; Raulin, F.; Cabane, M.; Niemann, H.B.; Atreya, S.K.; Bauer, S.; Brun, J.-F.; Chassefière, E.; Coll, P.; et al. Complex organic matter in Titan’s atmospheric aerosols from in situ pyrolysis and analysis. Nature 2005, 438, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Morisson, M.; Szopa, C.; Carrasco, N.; Buch, A.; Gautier, T. Titan’s organic aerosols: Molecular composition and structure of laboratory analogues inferred from pyrolysis gas chromatography mass spectrometry analysis. Icarus 2016, 277, 442–454. [Google Scholar] [CrossRef]
- Khare, B.; Sagan, C.; Thompson, W.; Arakawa, E.; Suits, F.; Callcott, T.; Williams, M.; Shrader, S.; Ogino, H.; Willingham, T.; et al. The organic aerosols of Titan. Adv. Space Res. 1984, 4, 59–68. [Google Scholar] [CrossRef]
- Coll, P.; Navarro-González, R.; Szopa, C.; Poch, O.; Ramírez, S.; Coscia, D.; Raulin, F.; Cabané, M.; Buch, A.; Israel, G. Can laboratory tholins mimic the chemistry producing Titan’s aerosols? A review in light of ACP experimental results. Planet. Space Sci. 2013, 77, 91–103. [Google Scholar] [CrossRef]
- Chatain, A.; Carrasco, N.; Ruscassier, N.; Gautier, T.; Vettier, L.; Guaitella, O. Interaction dust–plasma in Titan’s ionosphere: An experimental simulation of aerosols erosion. Icarus 2020, 345, 113741. [Google Scholar] [CrossRef]
- Maillard, J.; Carrasco, N.; Schmitz-Afonso, I.; Gautier, T.; Afonso, C. Comparison of soluble and insoluble organic matter in analogues of Titan’s aerosols. Earth Planet. Sci. Lett. 2018, 495, 185–191. [Google Scholar] [CrossRef]
- Pernot, P.; Carrasco, N.; Thissen, R.; Schmitz-Afonso, I. Tholinomics—Chemical Analysis of Nitrogen-Rich Polymers. Anal. Chem. 2010, 82, 1371–1380. [Google Scholar] [CrossRef]
- Bonnet, J.-Y.; Thissen, R.; Frisari, M.; Vuitton, V.; Quirico, É.; Orthous-Daunay, F.-R.; Dutuit, O.; Le Roy, L.; Fray, N.; Cottin, H.; et al. Compositional and structural investigation of HCN polymer through high resolution mass spectrometry. Int. J. Mass Spectrom. 2013, 354–355, 193–203. [Google Scholar] [CrossRef]
- Vuitton, V.; Bonnet, J.-Y.; Frisari, M.; Thissen, R.; Quirico, E.; Dutuit, O.; Schmitt, B.; Le Roy, L.; Fray, N.; Cottin, H.; et al. Very high resolution mass spectrometry of HCN polymers and tholins. Faraday Discuss. 2010, 147, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Gautier, T.; Carrasco, N.; Buch, A.; Szopa, C.; Sciamma-O’Brien, E.; Cernogora, G. Nitrile gas chemistry in Titan’s atmosphere. Icarus 2011, 213, 625–635. [Google Scholar] [CrossRef]
- Szopa, C.; Cernogora, G.; Boufendi, L.; Correia, J.J.; Coll, P. PAMPRE: A dusty plasma experiment for Titan’s tholins production and study. Planet. Space Sci. 2006, 54, 394–404. [Google Scholar] [CrossRef]
- Alcouffe, G.; Cavarroc, M.; Cernogora, G.; Ouni, F.; Jolly, A.; Boufendi, L.; Szopa, C. Capacitively coupled plasma used to simulate Titan’s atmospheric chemistry. Plasma Sources Sci. Technol. 2009, 19, 015008. [Google Scholar] [CrossRef]
- Alves, L.L.; Marques, L.; Pintassilgo, C.; Wattieaux, G.; Es-Sebbar, E.; Berndt, J.; Kovačević, E.; Carrasco, N.; Boufendi, L.; Cernogora, G. Capacitively coupled radio-frequency discharges in nitrogen at low pressures. Plasma Sources Sci. Technol. 2012, 21, 045008. [Google Scholar] [CrossRef]
- Fulchignoni, M.; Ferri, F.; Angrilli, F.; Ball, A.; Bar-Nun, A.; Barucci, M.A.; Bettanini, C.; Bianchini, G.; Borucki, W.; Colombatti, G.; et al. In situ measurements of the physical characteristics of Titan’s environment. Nature 2005, 438, 785–791. [Google Scholar] [CrossRef]
- Waite, J.H.W., Jr.; Niemann, H.; Yelle, R.V.; Kasprzak, W.T.; Cravens, T.E.; Luhmann, J.G.; McNutt, R.L.; Ip, W.-H.; Gell, D.; De La Haye, V.; et al. Ion Neutral Mass Spectrometer Results from the First Flyby of Titan. Science 2005, 308, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Anicich, V. An Index of the Literature for Bimolecular Gas Phase Cation-Molecule Reaction Kinetics; JPL Publication: Pasadena, CA, USA, 2003; pp. 1–1194. [Google Scholar]
- Imanaka, H.; Smith, M.A. EUV Photochemical Production of Unsaturated Hydrocarbons: Implications to EUV Photochemistry in Titan and Jovian Planets. J. Phys. Chem. A 2009, 113, 11187–11194. [Google Scholar] [CrossRef]
- Mahjoub, A.; Carrasco, N.; Dahoo, P.-R.; Fleury, B.; Gautier, T.; Cernogora, G. Effect of the Synthesis Temperature on the Optical Indices of Organic Materials Produced by N2-CH4RF Plasma. Plasma Process. Polymers 2014, 11, 409–417. [Google Scholar] [CrossRef]
- Sciamma-O’Brien, E.; Carrasco, N.; Szopa, C.; Buch, A.; Cernogora, G. Titan’s atmosphere: An optimal gas mixture for aerosol production? Icarus 2010, 209, 704–714. [Google Scholar] [CrossRef]
- Hadamcik, E.; Renard, J.B.; Alcouffe, G.; Cernogora, G.; Levasseur-Regourd, A.C.; Szopa, C. Light scattering measurements on Titan’s aerosols analogues produced by a dusty plasma. Planet. Space Sci. 2009, 57, 1631–1641. [Google Scholar] [CrossRef]
- Imanaka, H.; Khare, B.N.; Elsila, J.E.; Bakes, E.L.; McKay, C.P.; Cruikshank, D.P.; Sugita, S.; Matsui, T.; Zare, R.N. Laboratory experiments of Titan tholin formed in cold plasma at various pressures: Implications for nitrogen-containing polycyclic aromatic compounds in Titan haze. Icarus 2004, 168, 344–366. [Google Scholar] [CrossRef]
- Gautier, T.; Carrasco, N.; Mahjoub, A.; Vinatier, S.; Giuliani, A.; Szopa, C.; Anderson, C.M.; Correia, J.-J.; Dumas, P.; Cernogora, G. Mid- and far-infrared absorption spectroscopy of Titan’s aerosols analogues. Icarus 2012, 221, 320–327. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2010. [Google Scholar]
- Yung, Y.L.; Allen, M.; Pinto, J.P. Photochemistry of the atmosphere of Titan—Comparison between model and observations. Astrophys. J. Suppl. Ser. 1984, 55, 465–506. [Google Scholar] [CrossRef] [PubMed]
- Courtin, R.; Wagener, R.; McKay, C.P.; Caldwell, J.; Fricke, K.-H.; Raulin, F.; Bruston, P. UV spectroscopy of Titan’s atmosphere, planetary organic chemistry and prebiological synthesis: II. Interpretation of new IUE observations in the 220–335 nm Range. Icarus 1991, 90, 43–56. [Google Scholar] [CrossRef]
- Bakes, E.L.O.; Lebonnois, S.; Bauschlicher, C.W.; McKay, C.P. The role of submicrometer aerosols and macromolecules in H2 formation in the titan haze. Icarus 2003, 161, 468–473. [Google Scholar] [CrossRef]
- Lebonnois, S.; Bakes, E.; McKay, C.P. Atomic and molecular hydrogen budget in Titan’s atmosphere. Icarus 2003, 161, 474–485. [Google Scholar] [CrossRef]
- Wattieaux, G.; Carrasco, N.; Henault, M.; Boufendi, L.; Cernogora, G. Transient phenomena during dust formation in a N2–CH4capacitively coupled plasma. Plasma Sources Sci. Technol. 2015, 24, 015028. [Google Scholar] [CrossRef]
- Tanguy, L.; Bézard, B.; Marten, A.; Gautier, D.; Gérard, E.; Paubert, G.; Lecacheux, A. Stratospheric profile of HCN on Titan from millimeter observations. Icarus 1990, 85, 43–57. [Google Scholar] [CrossRef]
- Hidayata, T.; Arten, A.M.; Ezard, B.B.; Gautiera, D.; Owenb, T.; Matthews, H.E.; Paubertd, G. Millimeter and Submillimeter Heterodyne Observations of Titan: Retrieval of the Vertical Profile of HCN and the12C/13C Ratio. Icarus 1997, 126, 170–182. [Google Scholar] [CrossRef]
- Marten, A. New Millimeter Heterodyne Observations of Titan: Vertical Distributions of Nitriles HCN, HC3N, CH3CN, and the Isotopic Ratio 15N/14N in Its Atmosphere. Icarus 2002, 158, 532–544. [Google Scholar] [CrossRef]
- Sekine, Y.; Imanaka, H.; Matsui, T.; Khare, B.N.; Bakes, E.L.; McKay, C.P.; Sugita, S. The role of organic haze in Titan’s atmospheric chemistry: I. Laboratory investigation on heterogeneous reaction of atomic hydrogen with Titan tholin. Icarus 2008, 194, 186–200. [Google Scholar] [CrossRef]


| Parameters | Titan (1000 km Altitude) | PAMPRE Experience | Sources |
|---|---|---|---|
| Temperature (K) | 200 | 300 | HASI instrument (Huygens probe) [27] |
| Pressure (mbar) | 10−6 | 0.9 | |
| Chemical composition | 97 % N2–2% CH4 | 95 % N2–5% CH4 (injected initial gas mixture) | INMS instrument (Cassini Orbiter) [28] |
| Ionization Energy | -Solar photons -Energetic particles from Saturn | Electrons initiated by radiofrequency discharge * | [24,26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrin, Z.; Carrasco, N.; Chatain, A.; Jovanovic, L.; Vettier, L.; Ruscassier, N.; Cernogora, G. An Atmospheric Origin for HCN-Derived Polymers on Titan. Processes 2021, 9, 965. https://doi.org/10.3390/pr9060965
Perrin Z, Carrasco N, Chatain A, Jovanovic L, Vettier L, Ruscassier N, Cernogora G. An Atmospheric Origin for HCN-Derived Polymers on Titan. Processes. 2021; 9(6):965. https://doi.org/10.3390/pr9060965
Chicago/Turabian StylePerrin, Zoé, Nathalie Carrasco, Audrey Chatain, Lora Jovanovic, Ludovic Vettier, Nathalie Ruscassier, and Guy Cernogora. 2021. "An Atmospheric Origin for HCN-Derived Polymers on Titan" Processes 9, no. 6: 965. https://doi.org/10.3390/pr9060965
APA StylePerrin, Z., Carrasco, N., Chatain, A., Jovanovic, L., Vettier, L., Ruscassier, N., & Cernogora, G. (2021). An Atmospheric Origin for HCN-Derived Polymers on Titan. Processes, 9(6), 965. https://doi.org/10.3390/pr9060965






