Abstract
Biomass pellet fuel is one of the development directions of renewable energy. The purpose of the article is to study the combustion characteristics of five kinds of biomass pellet fuel that can be used as biomass fuel and analyze their combustion kinetics. The thermogravimetric method (TG method) was used to analyze the combustion characteristics of five kinds of biomass pellet fuel and to calculate the index S of comprehensive combustion characteristic. The Arrhenius equation and the Coats–Redfern method were used to analyze the combustion kinetics of five kinds of biomass pellet fuel. The activation energy and pre-exponential factor were obtained according to different temperature ranges. Conclusions are as follows: The pyrolysis of five kinds of biomass pellet fuel mainly includes three stages: (1) water evaporation stage, (2) volatile component combustion stage, (3) fixed carbon oxidation stage. The TG curves of five kinds of biomass pellet fuel are roughly the same at the same heating rate. The peaks of thermal weight loss rate and maximum degradation rate are both in the high temperature range. The differential thermal gravity (DTG) curves of five kinds of biomass pellet fuel have an obvious peak. The peak temperature of the largest peak in the DTG curves is 280–310 °C. The first-order reaction equation is used to obtain the kinetic parameters in stages. The correlation coefficients are bigger than the value of 0.92. The fitting results are in good agreement with the experimental results. The activation energy of each sample is basically the same in each stage. The value in the volatile matter combustion stage is 56–542 kJ/mol, and the activation energy of the carbon layer slowly increases rapidly. The five kinds of biomass pellet fuels have good combustion characteristics and kinetic characteristics, and they can be promoted and applied as biomass pellet fuels in the future.
1. Introduction
In today’s era, the calls for energy crises [1,2,3] and environmental pollution [4,5] are increasing. The reuse of agricultural and forestry waste has become the focus of attention [6]. Crop stalks, which are abundant in resource reserves, are also one of the widely used agricultural and forestry wastes. As the output of various fruit husk residues increases year by year, people have begun to pay attention to them [7].
Pyrolysis is the widely used method in converting waste biomass into valuable chemicals of biomass pellet fuels [8]. Stylianos et al. [9] studied the pyrolysis of cellulose, hemicellulose and lignin. In the process of pyrolysis, the biomass pellet fuels are transformed to the low molecular weight gases(volatiles) and solid(char). Liu et al. [10] studied the combustion behavior of corncob/bituminous coal and hardwood/bituminous coal. Sharma et al. [11] studied the thermal decomposition process and the reaction mechanism of the eucalyptus. The combustible gases can be used as biomass fuel because of high calorific value. The process can be used in many and various chemicals. This study would be beneficial for a better understanding of the pyrolytic-cracking mechanism, and it is also useful to improve its transformation and application for all kinds of biomass fuels [12]. It is very attractive for concerning environmental issues during energy production [13,14]. It can be concluded from various studies on pyrolysis analysis for different biomasses than the properties can significantly influence both heat transfer and reaction rates [15,16]. It is essential to establish efficient and reasonable processes using the kinetic analysis method. It allows control of decomposition mechanism of biomass as a function of pressure, temperature and combustion using the thermo-kinetic method [17]. It is necessary for accurately prediction of reactions’ kinetic parameters during the pyrolytic degradation [18,19,20]. Liu Xiang et al. [21] studied the kinetics of two-stage scheme for co-combustion of bituminous coal and herbaceous biomass. Maia et al. [22] studied the kinetic parameters of red peppers. Nyakuma et al. [23] studied the non-isothermal kinetic analysis of oil palm empty fruit bunch pellets by thermogravimetric analysis. Selim et al. [24] studied the Thermogravimetric–Fourier Transform Infrared (TG–FTIR) analysis and kinetic study of Ulva prolifera pyrolysis. Varma et al. [25] studied the physicochemical characterization and pyrolysis kinetics of wood sawdust. Zhihua Chen et al. [26] studied the characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Cui Yong Zhang et al. [27] studied the hot air ignition characteristics of cotton and corn straw pellet based on Thermogravimetric–Differential thermal gravity (TG-DTG). Wang X et al. [28] studied the combustion characteristics and kinetic analysis of bio-oil from fast pyrolysis of biomass. Harun, et al. [29] studied the chemical and mechanical properties of pellets made from agricultural and woody biomass blends.
In order to find a better way to use five kinds of biomass pellet fuel (masson pine, Chinese fir, willow, slash pine, and poplar), it is useful to study these kinds of biomass pellet fuel. We have fully understood the properties of the raw materials, which provides a theoretical basis for the utilization of five kinds of biomass pellet fuel. In this paper, an automatic industrial analyzer was used to determine the main chemical components in five kinds of biomass pellet fuel, such as moisture, ash, and volatile [30]. The thermogravimetric analysis method and the Coats-Redfern method were used to study kinetic analysis of five kinds of biomass pellet fuel. The research results can provide guidance for the combustion and utilization of five kinds of biomass pellet fuel [31,32].
2. Materials and Methods
2.1. Material
Five kinds of biomass pellet fuels (namely Masson pine, Chinese fir, Willow, Slash pine and Poplar) are purchased through the market. After selecting and removing impurities, a plant pulverizer made in our laboratory was used to crush and sieve. The fines that have passed the 120-mesh sieve were stored in a dry and clean jar with a stopper.
2.2. Method
2.2.1. Determination of the Components of Five Kinds of Biomass Pellet Fuel
With reference to the specific experimental methods in the relevant national standards for the determination of the moisture, ash and volatile in five kinds of biomass pellet fuels were determined. In this paper, the HTGF-3000 automatic industrial analyzer is used to determine the moisture, ash, and volatile content in each sample. The system is automatically operated by a computer. The temperature is increased to 145 °C and the temperature is kept constant for 30 min. When the mass change is less than 0.0006 g, the moisture analysis ends, and the system reports the moisture measurement result. During the process of the experiments, the temperature is raised to 900 °C under a nitrogen atmosphere and its flow rate is 5 mL/min. After reaching the specified time, the furnace temperature is reduced to the set value. When the mass change is less than 0.0006 g, the volatile matter analysis ends, and the system reports volatile content measurement result. The temperature is raised to 845 °C, and experiments are performed in an oxygen atmosphere which the flow rate is 5 mL/min. When the change of mass is below 0.0006 g, the ash analysis ends, and the system reports the ash determination result. Each sample is performed for three experiments, and the results of the experiments will be recorded.
2.2.2. Thermal Weight Loss Analysis of Five Kinds of Biomass Pellet Fuel
The samples of five kinds of biomass pellet fuel with a 120-mesh sieve were prepared. The thermogravimetric analyzer (TGA/Pyris 6 (Perkin Elmer instruments Limited, Boston, USA), temperature sensitivity 0.01 K, mass sensitivity 1 × 10−5 mg) was used at 20 °C/min and increased from room temperature to 800 °C. Experiments were performed under a nitrogen atmosphere with a flow rate of 20 mL/min. The mass of the sample is 10 mg which was inserted into the platin crucible directly. It recorded the sample heating thermogravimetric curve (TG) and derivative thermogravimetric curve (DTG) in the process.
3. Results and Analysis
3.1. Industry Analysis of Five Kinds of Biomass Pellet Fuel
The main components of plant raw materials are generally cellulose, hemicellulose, and lignin, as well as moisture, ash, and non-structural components such as extracts [33]. Different plant species has different proportions, and extract components also have the difference. Table 1 is the industry analysis of five kinds of biomass pellet fuel.

Table 1.
Industry analysis of five kinds of biomass pellet fuel (%).
It can be seen from Table 1 that the moisture and ash content of the five kinds of biomass pellet fuels are similar. Slash pine has the highest moisture content, with a content of 15.46%. Chinese fir has the lowest ash content, with a content of 0.52%. The volatile content is very different. The volatile content of Masson pine is only 62.62%. The combustion stage is mainly for the volatile to burn. The five kinds of biomass pellet fuels have high volatile content and are suitable for combustion.
3.2. Analysis of Thermal Weight Loss Characteristics of Five Kinds of Biomass Pellet Fuel
Thermal weight loss analysis was performed on five kinds of biomass pellet fuel which its heating rate is 20 °C/min. The TG and DTG curves were obtained (Figure 1 and Figure 2). It is found from Figure 1 and the TG and DTG curves have changed significantly during the process of heating from room temperature to 100 °C. The reason is that the sample had not dried completely before the experiment and the moisture content was high. The figures show that the pyrolysis of five kinds of biomass pellet fuel mainly includes three stages. They can be concluded as follows: (1) water evaporation stage (2) volatile component combustion stage (3) fixed carbon oxidation stage. The thermal weight loss analysis was carried out at the heating rate of 20 °C/min. In the first stage of drying, from room temperature to about 170 °C, the peak temperature of weight loss is at 100 °C, and the weight loss at this stage is about 10%. During the heating process, it absorbs heat to evaporate water (free water, crystal water, absorbed water, etc.). This is the drying stage of five kinds of biomass pellet fuel, and the composition has not changed significantly. The second stage is the volatile combustion stage, from about 190 to 450 °C. It is the stage of the main weight loss of five kinds of biomass pellet fuel and the formation of the initial carbon layer. The main chemical components in the husk of five kinds of biomass pellet fuel are cellulose, hemicellulose and lignin, etc. Five kinds of biomass pellet fuels are heated to decompose to generate volatiles at this stage. The heat is carried away by the nitrogen gas stream, and the weight is greatly reduced. The weight loss at this stage is about 75%. The third stage is after 450 °C, which is the slow oxidation and decomposition stage of the carbon layer. The weight loss at this stage is about 10%. The TG curve tends to be flat, and the pyrolysis of the organic matter in the five kinds of biomass pellet fuels are basically completed. The residual ash and other non-decomposable substances are left finally. The burning results of five kinds of biomass pellet fuel are roughly the same. The results of the other five forestry biomass are also roughly the same.
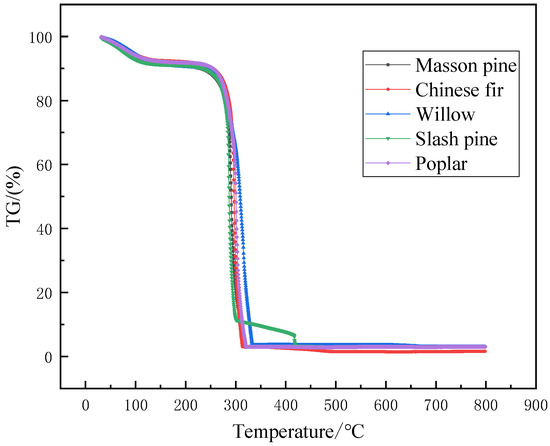
Figure 1.
TG curves of five kinds of biomass pellet fuel.
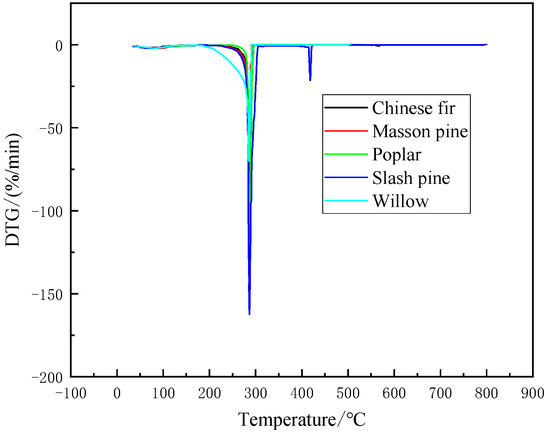
Figure 2.
DTG curves of five kinds of biomass pellet fuel.
It can be seen from Figure 1 and Figure 2 that there is an obvious peak in the DTG curve. The trend is that there is a minimum weight loss first. After reaching the ignition temperature, the violent reaction reaches the maximum weight loss peak and then falls back until the reaction ends. This is because the content of hemicellulose is small. Therefore, in the low-temperature combustion stage, the thermal analysis of hemicellulose and cellulose shows volatile matter in the combustion process is superimposed and there is a peak in the DTG curve. This is because early thermal decomposition of a few hemicellulose generated volatile. It reveals the reason for the ignition of volatile components. It consumes a part of heat. It shows that the peak temperature in the DTG curve is 280–310 °C. The mean combustion rate, and the maximum combustion rate of samples which mainly occurs at the volatile matter combustion region. The maximum combustion rate of poplar was 117.45%/min at 301 °C. The maximum combustion rate of Slash pine is 158.25%/min at 287 °C. The maximum combustion rate of masson pine was 103.36%/min at 291 °C. The maximum combustion rate of Chinese fir is 134.1%/min at 297 °C. The maximum burning rate of willow is 68.12%/min at 309 °C.
The effect of heating rate on pyrolysis is more complicated. As the heating rate increases, the time for the sample particles to reach the pyrolysis temperature is shortened, which is beneficial to pyrolysis. However, the increase in heating rate also increases the temperature difference between the inside and outside of the particles [34].
Thus, considering Figure 1 and Figure 2, it is shown that the TG curves of five kinds of biomass pellet fuel are roughly the same at the same heating rate. The peaks of thermal weight loss rate and maximum degradation rate are both in the high temperature range. In addition, Figure 1 and Figure 2 show that the average mass loss in the second stage accounts for about 70% of the sample mass. Combining the composition of five kinds of biomass pellet fuel and the related properties of each component in Table 1, the holocellulose is basically degraded at this stage completely. The degradation of lignin runs through the entire high-temperature pyrolysis process.
3.3. Ignition Characteristics
The ignition characteristic is mainly correlated to the ignition temperature. The tangent method is usually to be used to calculate the ignition temperature. The peak point of the DTG curve is taken as a vertical line to intersect the TG curve at the point, and the tangent to the TG curve is crossed over it. The corresponding temperature is ignition temperature. When the ignition temperature is low, it is easy ignite the fuel. The ignition temperatures are calculated by the method, and the results are shown in Table 2. The highest ignition temperature of poplar wood is 271 °C. It indicates that five kinds of biomass pellet fuel are easy to ignite and is beneficial to combustion, and is suitable as a biomass fuel.

Table 2.
Ignition temperature and burn-out temperature.
3.4. Burnout Characteristics
The burn-out characteristic is related to the combustion rate. The burn-out point can reflect the burn-out characteristic, and the burn-out point can be determined in the DTG curve. When the weight loss rate is below –1%/min, its corresponding temperature is the burn-out temperature. Table 2 is the burn-out temperature of each sample.
3.5. Comprehensive Combustion Characteristics
The combustion characteristics of biomass fuel can be evaluated by the index S of comprehensive combustion characteristics. When the index S of comprehensive combustion characteristic becomes higher, the combustion performance of biomass fuel is in good condition. The index S of comprehensive combustion characteristic can be calculated Formula (1):
where S is the index of comprehensive combustion characteristic (min−2·K−3), (dX/dt)max is the maximum combustion rate (%/min), (dX/dt)mean is the average combustion rate (%/min), Ti is the ignition temperature (K), Tf is the burn-out temperature (K).
The specific calculation method is as follows:
(1) Ignition temperature Ti: The vertical line passes the peak point P of DTG curve, the TG curve intersects at point A, and the tangent line L2 of TG curve passes through point A, which intersects the horizontal line L1 when the volatiles begin to lose weight. The abscissa value corresponding to point i is the ignition temperature (°C).
(2) Burn-out temperature Tf: The intersection of the tangent line L2 and the horizontal line L3 at the end of the TG curve weight loss is point f, the abscissa value corresponding to f is the burnout temperature (°C).
(3) Maximum burning rate vp: The ordinate value on the DTG curve corresponding to point P is the maximum burning rate (%/min).
(4) Maximum burning rate temperature Tp: The abscissa value on the DTG curve corresponding to point P is the maximum burning rate temperature (°C).
(5) Average burning rate va: The calculation formula is shown in Formula (2):
where β is the heating rate, the value is 20 °C/min, αi is the remaining sample percentage corresponding to the ignition temperature point (%), αf is the remaining sample percentage corresponding to the burnout temperature point (%).
The comprehensive combustion characteristic index S is used to evaluate the burning performance of five kinds of biomass pellet fuel. The comprehensive burning characteristic index S of each sample is calculated by formula (1). The results are shown in Table 3, the comprehensive combustion characteristic index of Chinese fir is the highest, and the comprehensive combustion characteristic indexes of other samples are similar.

Table 3.
Comprehensive combustion characteristic indexes.
4. Combustion Kinetic Analysis
4.1. Combustion Mechanism
The biomass pellet fuel is mainly composed of cellulose, hemicellulose and lignin, and the main components of hemicellulose and cellulose are volatiles. The main products of lignin pyrolysis are carbon, a little volatiles and other substances [35]. The starting temperature of pyrolysis for hemicellulose is 423–623 K, it is 548–623 K for cellulose, and it is 523–773 K for lignin. It shows that the ignition temperature range of samples is in the range of hemicellulose and cellulose. The thermal decomposition process of the three major components of biomass is a process of superimposing and mixing together. In the early stage, the combustion mainly reflects the precipitation and ignition process of volatile components. The latter stage mainly reflects the formation of coke and its combustion process. The analysis of combustion mechanism of biomass pellet fuel needs to be calculated in the stages [36].
In the stage of low temperature, the hemicellulose and cellulose of biomass pellet fuel thermally desorb volatiles. When it reaches a certain level for the temperature and oxygen, the volatiles will ignite and burn. The heat released from combustion of volatiles provides the subsequent precipitation and ignition of the volatiles. In this stage, a little porous coke is formed after the biomass produces precipitated volatiles. During this time, the ignition temperature has not reached its appropriate point, and the volatilization also hinders permeation and diffusion of oxygen to the coke surface. Therefore, the main manifestation in early stage is the combustion of volatiles. At this stage, the volatiles mixed with oxygen reached a certain concentration and temperature. It is a gas phase combustion. Therefore, in this stage, it follows the typical homogeneous ignition combustion model. The reaction rate is calculated by the exhalation rate and volatile concentration. When the temperature continues to rise, the amount of coke is relatively little. The coke is produced by the high-temperature thermal decomposition of the remaining lignin, and it spans the temperature range for a long time [37]. The ash which is produced by the previous cellulose thermal decomposition, it hinders the contact between coke and oxygen. The temperature has reached the ignition point of coke, but its combustion rate is relatively slow. Therefore, the later combustion stage biomass is mainly a slow process of combustion for the coke. The multiphase reaction between solid coke and oxygen on the carbon surface is a multiphase combustion model. The reaction rate is mainly calculated by oxygen concentration. Therefore, it is useful to study the kinetic parameters of combustion process of biomass pellet fuel in different stages.
4.2. Kinetic Calculation
The combustion belongs to the weight loss reaction of biomass decomposition. For the non-isothermal thermo-gravimetric experiment of biomass combustion, the combustion reaction is carried out at the programmed heating rate of 20 K/min [38]. The thermal decomposition reaction of biomass combustion accords with:
A (solid) + B (gas) → C (solid)
The kinetic parameters are calculated by the Arrhenius equation:
where k is the reaction constant, A is the pre-exponential factor (min−1), E is the activation energy of reaction (kJ/mol), R is the universal gas constant, 8.314 J/(K·mol). T is the absolute temperature (K), t is the reaction time (min), α is the conversion rate of reaction process, α = (m0 − mt)/(m0 − mf), where m0 is the mass of sample at the initial moment of pyrolysis, mt is the mass of sample at the moment t of pyrolysis, and mf is the quality of sample at the time when the solution is terminated.
The constant heating rate β is adopted in the process of thermogravimetric analysis, and the equation (t) can be obtained by substituting β = dT/dt into Equation (3):
Let
, after integrating both sides of Equation (6), we get:
According to literature (26), the reaction order n = 1, and the equation is simplified according to the Coats–Redfern method:
Among them, 1 − 2RT/E ≈ 1, ln[AR(1 − 2RT/E)/βE] is a constant, and the straight line can be obtained by plotting ln[−ln(1 − α)/T2] versus 1/T, and then the kinetic parameters activation energy E and pre-exponential factor A also can be obtained.
Figure 3 shows that the combustion stage of volatile of Chinese fir is divided into two parts. The first stage is 260–295 °C, its activation energy and correlation coefficient are 56.15 kJ/mol and 0.9401, respectively. The second stage is 295–310 °C. Its activation energy and correlation coefficient are 542.63 kJ/mol and 0.9966, respectively.
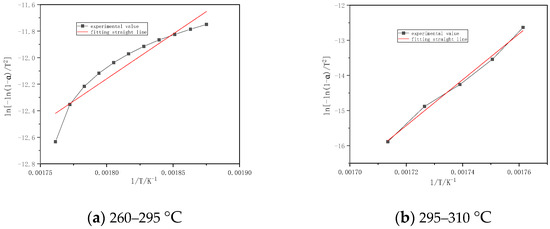
Figure 3.
Fitting straight line for burning stages of Chinese fir.
Figure 4 shows one part of the combustion stage of volatiles of masson pine. The temperature range is 260–310 °C, its activation energy and correlation coefficient are 172.99 kJ/mol and 0.9241, respectively.
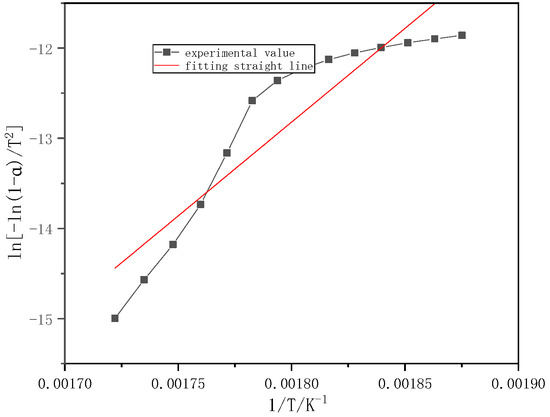
Figure 4.
Fitting straight line for burning stage of masson pine (260–310 °C).
Figure 5 shows one part of volatiles of the combustion stage of willow. The temperature range is 270–320 °C, its activation energy and correlation coefficient are 130.51 kJ/mol and 0.9252, respectively.
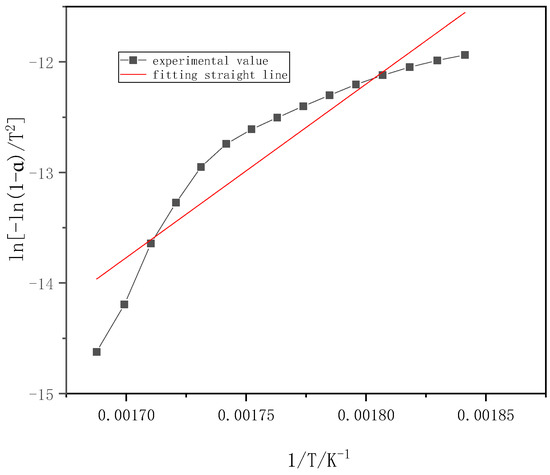
Figure 5.
Fitting straight line for burning stage of willow (270–320 °C).
Figure 6 shows that the combustion stages of volatile of Slash pine are divided into two parts. The first stage is 270–310 °C, its activation energy is 269.49 kJ/mol, and its correlation coefficient is 0.9704. The second stage is 400–415 °C, its activation energy and correlation coefficient are 80.70 kJ/mol and 0.9984, respectively.
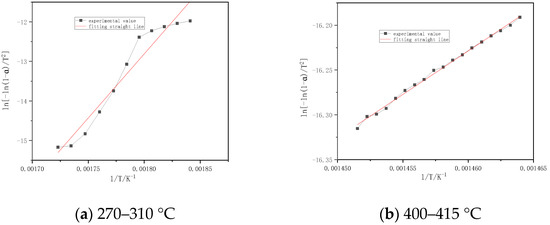
Figure 6.
Fitting straight line for burning stages of Slash pine.
Figure 7 shows that the combustion stages of volatile of poplar are divided into two parts. The first stage is 270–900 °C, its activation energy and correlation coefficient are 70.11 kJ/mol and 0.9904, respectively. The second stage is 297–310 °C, its activation energy and correlation coefficient are 509.12 kJ/mol and 0.9978, respectively.
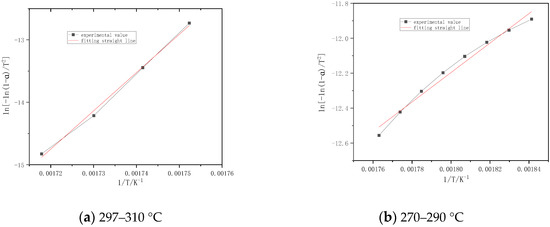
Figure 7.
Fitting straight line for burning stages of poplar.
It can be seen from Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7 that each sample begins to react at about 190 °C, and ends at around 420 °C. The temperature ranges from 190–440 °C, and the heating rate β = 20 K/min. According to different combustion reaction stages, multiple first order reaction is used for calculation. Since the combustion stage of volatile of five kinds of biomass pellet fuel is 190–420 °C, the activation energy parameters of combustion stage of volatile are calculated in one or two temperature ranges. The kinetic parameters of each sample in different temperature ranges are listed in Table 4.

Table 4.
Kinetic parameters in different temperature ranges.
Table 4 shows that the kinetic parameters are obtained by the first-order reaction equation in stages. The correlation coefficients are all higher than 0.92, and there are good fitting results. The activation energy of each sample is basically the same in the combustion stage, and the activation energy of volatile of combustion stage is 56~542 kJ/mol. The different combustion activation energy of each sample is mainly manifested in the volatile combustion stage. The decrease in activation energy of the volatile components of five kinds of biomass pellet fuel and seed during the combustion stage is due to the large amount of cellulose and lignin. The decomposition temperature of cellulose and lignin is low and volatile. The initial stage of volatile matter combustion is 190–320 °C. The first exothermic peak of five kinds of biomass pellet fuel appears at 210–320 °C. This temperature range is mainly the decomposition and combustion of the volatile matter. The middle stage of volatile matter combustion is 320–370 °C, the activation energy is lower than the first stage. This stage is volatiles continue to burn. Volatiles burned in the later stage at 370–410 °C remained stable compared with the other two stages. During the slow oxidation stage of the carbon layer, the activation energy increases sharply at 410–440 °C. This stage is mainly the secondary pyrolysis of lignin, which involves the ring-opening reaction of the aromatic ring structure. At this time, the coke content of the solid product gradually increases, and the originally disordered carbon structure gradually tends to be orderly. The reduction of active sites leads to a rapid decrease in reaction activity, which is reflected in a significant increase in activation energy value.
5. Conclusions
In order to study the combustion characteristics of five biomass pellet fuels (masson pine, Chinese fir, willow, slash pine, and poplar), five samples were analyzed and studied by using thermogravimetric analyzer. The combustion kinetics were calculated by Arrhenius equation and Coats–Redfern method, and the activation energy and pre-exponential factor of different temperature stages were calculated by the first-order reaction equation. The conclusions are as follows:
- (1)
- The pyrolysis of five kinds of biomass pellet fuel mainly includes three stages: (1) water evaporation stage; (2) volatile component combustion stage; (3) fixed carbon oxidation stage.
- (2)
- The TG curves of five kinds of biomass pellet fuel are roughly the same. The peaks of thermal weight loss rate and maximum degradation rate are both in the range of high temperature. The DTG curves of five kinds of biomass pellet fuel have an obvious peak. The range of peak temperature in the DTG curves is 280–310 °C.
- (3)
- The kinetic parameters are obtained by the first-order reaction equation in the combustion stages. The correlation coefficients are all above 0.92, and the fitting results are good. The activation energy of each sample is basically the same in the combustion stage. The activation energy of the volatile matter in combustion stage is 56~542 kJ/mol, and the activation energy of the carbon layer slowly increases rapidly.
- (4)
- The five kinds of biomass pellet fuels have good combustion characteristics and kinetic characteristics, and they can be promoted and applied as biomass pellet fuels in the future.
Funding
This research was funded by China Post-doctoral Science Foundation, grant number 2016M592455; Hunan Provincial Education Department’s Scientific Research Project, grant number 17B278; Hunan Provincial University’s Science and Technology Innovation Team Support Plan, grant number 2014207; Central South University of Forestry and Technology’s School-level Youth Science Foundation Project, grant number QJ2017006B.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Nomenclature
| A | the pre-exponential factor(s−1) |
| E | the activation energy(kj/mol) |
| S | the comprehensive combustion characteristic index(min−2·K−3) |
| R | the universal gas constant (8.314J/(K·mol)) |
| T | the absolute temperature(K) |
| m | the mass of the sample |
| Greek letter | |
| α | degree of conversion |
| β | heating rate (K/min) |
| Subscripts and superscripts | |
| 0 | initial value |
| f | burning-out value |
| t | the reaction time(min) |
| i | ignition |
References
- Zuo, H.; Tan, J.; Wei, K.; Huang, Z.; Zhong, D.; Xie, F. Effects of different poses and wind speeds on wind-induced vibration characteristics of a dish solar concentrator system. Renew. Energy 2021, 168, 1308–1326. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, W.; E, J.; Li, S.; Li, Z.; Xu, H.; Fu, G. Effects of propane addition and burner scale on the combustion characteristics and working performance. Appl. Energy 2021, 285, 116484. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, G.; E, J.; Zuo, W.; Wei, K.; Hu, W.; Tan, J.; Zhong, D. Catastrophic analysis on the stability of a large dish solar thermal power generation system with wind-induced vibration. Sol. Energy 2019, 183, 40–49. [Google Scholar] [CrossRef]
- Zhang, B.; Zuo, H.; Huang, Z.; Tan, J.; Zuo, Q. Endpoint forecast of different diesel-biodiesel soot filtration process in diesel particulate filters considering ash deposition. Fuel 2020, 271, 117678. [Google Scholar] [CrossRef]
- Zhong, C.; Tan, J.; Zuo, H.; Wu, X.; Wang, S.; Liu, J. Synergy effects analysis on CDPF regeneration performance enhancement and NOx concentration reduction of NH3–SCR over Cu–ZSM–5. Energy 2021, 230, 120814. [Google Scholar] [CrossRef]
- Mohr, S.; Wang, J.; Ellem, G.; Ward, J.; Giurco, D. Projection of world fossil fuels by country. Fuel 2015, 141, 120–135. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Wei, Y. Combustion behavior of corncob/bituminous coal and hardwood/bituminous coal. Renew. Energy 2015, 81, 355–365. [Google Scholar] [CrossRef]
- Sharma, P.; Diwan, P.K. Study of thermal decomposition process and the reaction mechanism of the eucalyptus wood. Wood Sci. Technol. 2017, 51, 1081–1094. [Google Scholar] [CrossRef]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. J. Anal. Appl. Pyrolysis 2013, 104, 531–539. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Y.; Zhu, X. Determination of effective moisture diffusivity and drying kinetics for poplar sawdust by thermogravimetric analysis under isothermal condition. Bioresour. Technol. 2012, 107, 451–455. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Y.; Zhu, X. In-depth investigation on the pyrolysis kinetics of raw biomass. Part I: Kinetic analysis for the drying and devolatilization stages. Bioresour. Technol. 2013, 131, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Chakraborty, S. A kinetic study of pyrolysis and combustion of microalgae Chlorella vulgaris using thermo-gravimetric analysis. Bioresour. Technol. 2013, 128, 72–80. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour. Technol. 2013, 127, 298–305. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.S.; Carneiro, A.D.C.O.; Martinez, C.L.M.; Vital, B.R.; Carneiro, A.P.S.; de Assis, M.R. Thermal decomposition fundamentals in large-diameter wooden logs during slow pyrolysis. Wood Sci. Technol. 2019, 53, 1353–1372. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Salvachúa, D.; Martínez, M.; Diaz, L.; Zamorano, M. Analysis of the relation between the cellulose, hemicellulose and lignin content and the thermal behavior of residual biomass from olive trees. Waste Manag. 2013, 33, 2245–2249. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Liu, H.; Evrendilek, F.; Buyukada, M. Pyrolysis of water hyacinth biomass parts: Bioenergy, gas emissions, and by-products using TG-FTIR and Py-GC/MS analyses. Energy Convers. Manag. 2020, 207, 112552. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Wei, Y. Kinetics based on two-stage scheme for co-combustion of herbaceous biomass and bituminous coal. Fuel 2015, 143, 577–585. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nyakuma, B.B.; Ahmad, A.; Johari, A.; Abdullah, T.T.; Oladokun, O.; Aminu, D.Y. Non-isothermal kinetic analysis of oil palm empty fruit bunch pellets by thermogravimetric analysis. Chem. Eng. Trans. 2015, 45, 1327–1332. [Google Scholar]
- Ceylan, S.; Goldfarb, J.L. Green tide to green fuels: TG–FTIR analysis and kinetic study of Ulva prolifera pyrolysis. Energy Convers. Manag. 2015, 111, 263–270. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Physicochemical characterization and pyrolysis kinetics of wood sawdust. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 2536–2544. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Cui, Y.Z.; Qu, Y.X.; Tang, M.T.; Gao, P.; Zhang, G.K. Hot Air Ignition Characteristics of Cotton and Corn Straw Pellet Based on TG-DTG. Appl. Mech. Mater. 2014, 672–674, 164–167. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Dong, A.; Guo, X.; Chen, C.; Xiong, S. Co-pyrolysis of biomass and pingshuo coal. In Proceedings of the International Conference on Materials for Renewable Energy & Environment, Chengdu, China, 19–21 August 2013. [Google Scholar]
- Harun, N.Y.; Afzal, M.T. Chemical and mechanical properties of pellets made from agricultural and woody biomass blends. Trans. ASABE 2015, 58, 921–930. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Ceylan, S.; Topçu, Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour. Technol. 2014, 156, 182–188. [Google Scholar] [CrossRef]
- Maryandyshev, P.A.; Popova, E.I.; Chernov, A.A.; Popov, M.S.; Lyubov, V.K.; Trouve, G.; Kehrli, D.; Brillard, A.; Brilhac, J.-F. Thermal decomposition and combustion of biofuels. Solid Fuel Chem. 2017, 51, 370–378. [Google Scholar] [CrossRef]
- Kocabaş-Ataklı, Z.Ö.; Okyay-Öner, F.; Yürüm, Y. Combustion characteristics of Turkish hazelnut shell biomass, lignite coal, and their respective blends via thermogravimetric analysis. J. Therm. Anal. Calorim. 2015, 119, 1723–1729. [Google Scholar] [CrossRef]
- Liu, H.; Gong, S.; Jia, C.; Wang, Q. TG-FTIR analysis of co-combustion characteristics of oil shale semi-coke and corn straw. J. Therm. Anal. Calorim. 2017, 127, 2531–2544. [Google Scholar] [CrossRef]
- Striūgas, N.; Skvorčinskienė, R.; Paulauskas, R.; Zakarauskas, K.; Vorotinskienė, L. Evaluation of straw with absorbed glycerol thermal degradation during pyrolysis and combustion by TG-FTIR and TG-GC/MS. Fuel 2017, 204, 227–235. [Google Scholar] [CrossRef]
- Sun, C.; Li, C.; Tan, H.; Zhang, Y. Synergistic effects of wood fiber and polylactic acid during co-pyrolysis using TG-FTIR-MS and Py-GC/MS. Energy Convers. Manag. 2019, 202, 112212. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.; Liu, H.; Jia, C.; Li, S. Interactions and kinetic analysis of oil shale semi-coke with cornstalk during co-combustion. Appl. Energy 2011, 88, 2080–2087. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. Multi-Gaussian-DAEM-reaction model for thermal decompositions of cellulose, hemicellulose and lignin: Comparison of N-2 and CO2 atmosphere. Bioresour. Technol. 2014, 166C, 87–95. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).