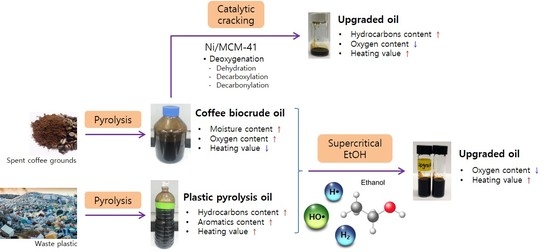
Upgrading of Coffee Biocrude Oil Produced by Pyrolysis of Spent Coffee Grounds: Behavior of Fatty Acids in Supercritical Ethanol Reaction and Catalytic Cracking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Catalysts
2.3. Supercritical Ethanol Reaction and Catalytic Cracking Procedures
2.4. Analyses
3. Results and Discussion
3.1. Characterization of Spent Coffee Grounds, Coffee Biocrude Oil, and Plastic Pyrolysis Oil
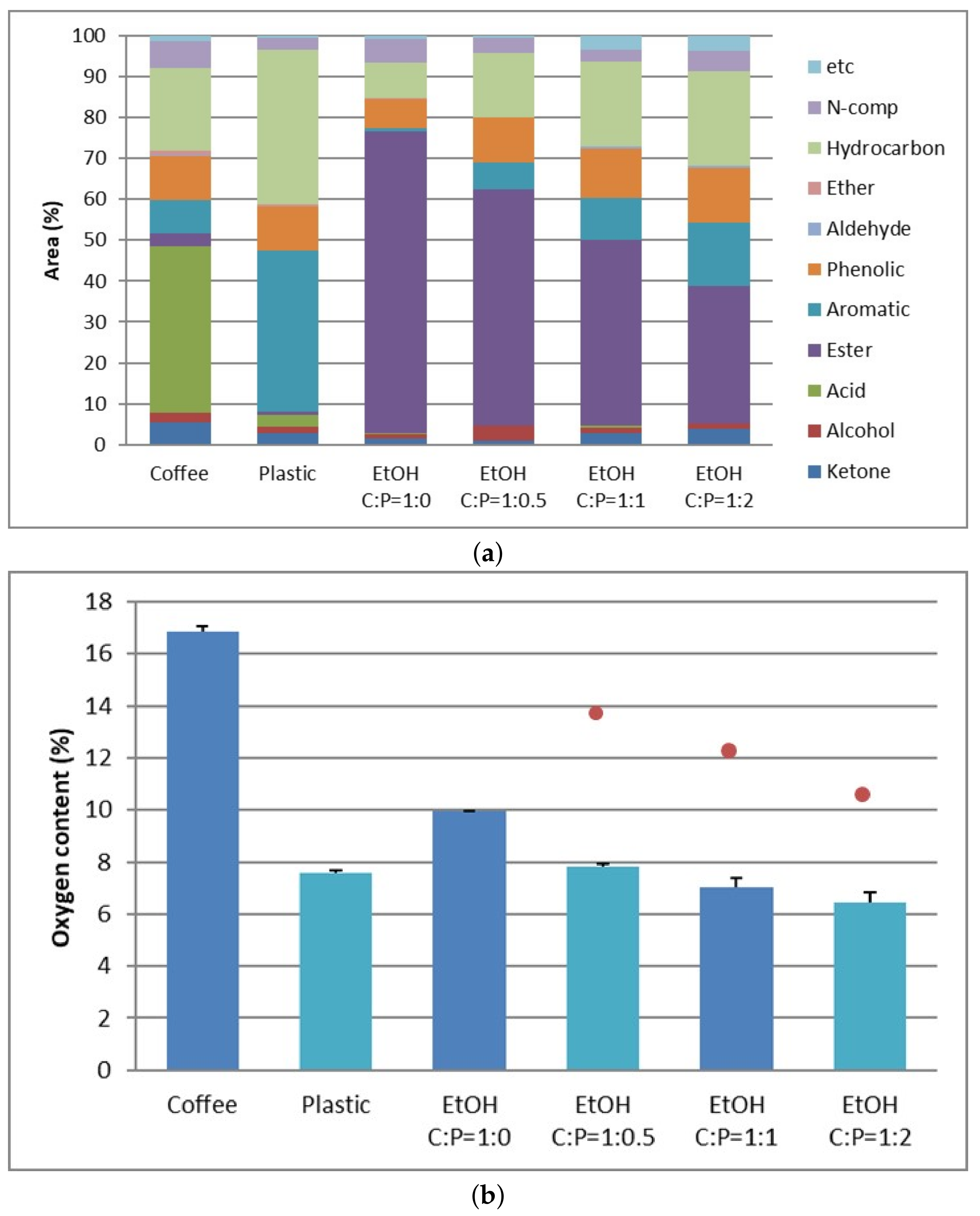
3.2. Upgrading Coffee Biocrude Oil by Supercritical Ethanol Reaction
3.3. Upgrading Coffee Biocrude Oil by Supercritical Ethanol Reaction with Plastic Pyrolysis Oil
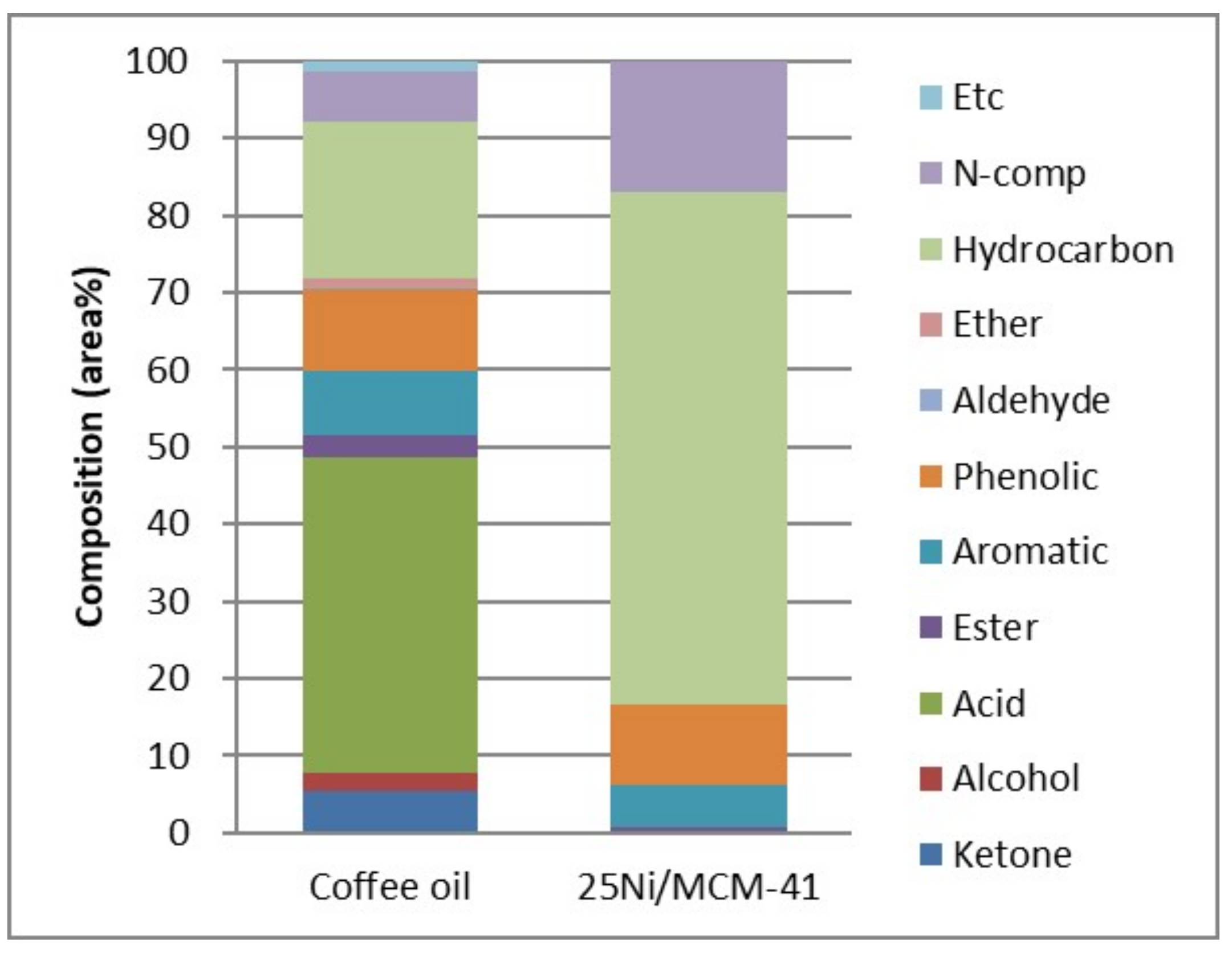
3.4. Catalytic Cracking of Coffee Crude Oil by Ni/MCM-41 Catalyst without Supercritical Ethanol
3.5. Comparison of Methods for Upgrading Coffee Biocrude Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muigai, H.H.; Choudhury, B.J.; Kalita, P.; Moholkar, V.S. Physico-chemical characterization and pyrolysis kinetics of Eichhornia Crassipes, Thevetia Peruviana, and Saccharum Officinarum. Fuel 2021, 289, 119949. [Google Scholar] [CrossRef]
- Magalhaes, D.; Gurel, K.; Matsakas, L.; Christakopoulos, P.; Pisano, I.; Leahy, J.J.; Kazanc, F.; Trubetskaya, A. Prediction of yields and composition of char from fast pyrolysis of commercial lignocellulosic materials, organosolv fractionated and torrefied olive stones. Fuel 2021, 289, 119862. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Anto, S.; Rene, E.R.; Sekar, M.; Mathimani, T.; Chi, N.T.L.; Pugazhendhi, A. Effect of reaction temperature on the conversion of algal biomass to bio-oil and biochar through pyrolysis and hydrothermal liquefaction. Fuel 2021, 285, 119106. [Google Scholar] [CrossRef]
- Choi, Y.S.; Choi, S.K.; Kim, S.J.; Jeong, Y.W.; Soysa, R.; Rahman, T. Fast pyrolysis of coffee ground in a tilted-slide reactor and characteristics of biocrude oil. Environ. Prog. Sustain. Energy 2017, 36, 655–661. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Wang, T.; Li, B.; Xu, Y.; Ma, L. Efficient upgrading process for production of low quality fuel from bio-oil. Fuel 2016, 179, 312–321. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Y.; Li, Y.; Wang, T.; Zhang, Q.; Ma, L.; He, M.; Li, K. Investigation on the esterification by using supercritical ethanol for bio-oil upgrading. Appl. Energy 2015, 160, 633–640. [Google Scholar] [CrossRef]
- Prajitno, H.; Insyani, R.; Park, J.; Ryu, C.; Kim, J. Non-catalytic upgrading of fast pyrolysis bio-oil in supercritical ethanol and combustion behavior of the upgraded oil. Appl. Energy 2016, 172, 12–22. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sust. Energ. Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Hai, A.; Banat, F.; Taher, H.; Schmidt, J.E.; Show, P.L. Catalytic hydrodeoxygenation of biomass-derived pyrolysis oil over alloyed bimetallic Ni3Fe nanocatalyst for high-grade biofuel production. Energy Convers. Manag. 2020, 213, 112859. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, I.G.; Jeon, W.; Ha, J.H.; Lee, K.Y. Catalytic upgrading of bio-tar over a MgNiMo/activated charcoal catalyst under supercritical ethanol conditions. Catal. Today 2018, 316, 237–243. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yuan, Z.; Rohani, S.; Xu, C. Effects of nano-structured CoMo Catalysts on hydrodeoxygenation of fast pyrolysis oil in supercritical ethanol. Catal. Today 2016, 269, 182–194. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Muthukumarappan, K.; Kharel, P.R. Upgrading pyrolysis bio-oil to biofuel over bifunctional Co-Zn/HZSM-5 catalyst in supercritical methanol. Energy Convers. Manag. 2017, 147, 19–28. [Google Scholar] [CrossRef]
- Isa, K.M.; Snape, C.E.; Uguna, C.; Meredith, W.; Deng, H. Pyrolysis oil upgrading in high conversions using sub- and supercritical water above 400 ∘C. J. Anal. Appl. Pyrol. 2016, 119, 180–188. [Google Scholar] [CrossRef]
- Li, W.; Xie, X.A.; Tang, C.Z.; Li, Y.; Li, L.; Wang, Y.L.; Fan, D.; Wei, X. The distribution of bio-oil components with the effects of sub/supercritical ethanol and free radicals during cellulose liquefaction. BioResources 2016, 11, 9771–9778. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Xie, X.A.; Tang, C.Z.; Li, Y.; Li, L.; Wang, Y.L.; Wei, X.; Fan, D. Effects of hydroxyl and hydrogen free radicals on the liquefaction of cellulose in sub/supercritical ethanol. J. Fuel Chem. Technol. 2016, 44, 415–421. [Google Scholar] [CrossRef]
- Primaz, C.T.; Schena, T.; Lazzari, E.; Caramao, E.B.; Jacques, R.A. Influence of the temperature in the yield and composition of the bio-oil from the pyrolysis of spent coffee grounds: Characterization by comprehensive two dimensional gas chromatography. Fuel 2018, 232, 572–580. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. Two-step sequential pretreatment for the enhanced enzymatic hydrolysis of coffee spent waste. Bioresour. Technol. 2017, 239, 276–284. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Chong, C.T.; Chen, W.H.; Leong, K.Y.; Tan, S.X.; Lee, X.J. Ultrasonic assisted oil extraction and biodiesel synthesis of spent coffee ground. Fuel 2020, 261, 116121. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Active biocatalyst for biodiesel production from spent coffee ground. Bioresour. Technol. 2018, 266, 431–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Ma, D.; Chen, C.; Wang, Y.; Wang, W.; Li, A. 2020. Exergy and energy analysis of pyrolysis of plastic wastes in rotary kiln with heat carrier. Process Saf. Environ. Protect. 2020, 142, 203–211. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Yerrayya, A.; Nagababu, G.; Guduru, R.K.; Kumar, T.H. Recovery of renewable aromatic and aliphatic hydrocarbon resources from microwave pyrolysis/co-pyrolysis of agro-residues and plastic wastes. Bioresour. Technol. 2020, 318, 124277. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Nam, H.; Wang, S.; Kim, H.; Kim, J.H.; Won, Y.; Hwang, B.W.; Kim, Y.D.; Nam, H.; Lee, K.H.; et al. Characteristics of fractionated drop-in liquid fuel of plastic wastes from a commercial pyrolysis plant. Waste Manag. 2021, 126, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jeon, W.J.; Lee, J.H.; Nam, B.; Lee, I.G. Effects of supercritical fluids in catalytic upgrading of biomass pyrolysis oil. Chem. Eng. J. 2019, 377, 120312. [Google Scholar] [CrossRef]
- Somnuk, K.; Eawlex, P.; Prateepchaikul, G. Optimization of coffee oi extraction from spent coffee grounds using four solvents and prototype-scale extraction using circulation process. Agric. Nat. Resour. 2017, 51, 181–189. [Google Scholar]
- Fischer, A.; Du, S.; Valla, J.A.; Bollas, G.M. The effect of temperature, heating rate, and ZSM-5 catalyst on the product selectivity of the fast pyrolysis of spent coffee grounds. RSC Adv. 2015, 5, 29252–29261. [Google Scholar] [CrossRef]
- Bok, J.P.; Choi, H.S.; Choi, Y.S.; Park, H.C.; Kim, S.J. Fast pyrolysis of coffee grounds: Characteristics of product yields and biocrude oil quality. Energy 2012, 47, 17–24. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y. Biodiesel production in supercritical methanol using a novel spiral reactor. Procedia Environ. Sci. 2015, 28, 204–213. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Kaewchada, A.; Jaree, A. Production of biodiesel from palm oil under supercritical ethanol in the presence of ethyl acetate. Energy Fuels 2019, 33, 5322–5331. [Google Scholar] [CrossRef]
- Iisa, K.; Robichaud, D.J.; Watson, M.J.; Dam, J.T.; Dutta, A.; Mukarakate, C.; Kim, S.; Nimlos, M.R.; Baldwin, R.M. Improving biomass pyrolysis economics by integrating vapor and liquid phase upgrading. Green Chem. 2018, 20, 567–582. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; Abomohra, A.E.F.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jae, J.; Kim, B.S.; Hong, Y.; Jung, S.C.; Park, Y.K. Catalytic co-pyrolysis of torrefied yellow poplar and high-density polyethylene using microporous HZSM-5 and mesoporous Al-MCM-41 catalysts. Energy Convers. Manag. 2017, 149, 966–973. [Google Scholar] [CrossRef]
- Lin, X.; Lei, H.; Huo, E.; Qian, M.; Mateo, W.; Zhang, Q.; Zhao, Y.; Wang, C.; Villota, E. Enhancing jet fuel range hydrocarbons production from catalystic co-pyrolysis of Douglas fir and low-density polyethylene over bifunctional activated carbon catalysts. Energy Convers. Manag. 2020, 211, 112757. [Google Scholar] [CrossRef]
- Jeon, M.J.; Choi, S.J.; Yoo, K.S.; Ryu, C.; Park, S.H.; Lee, J.M.; Jeon, J.K.; Park, Y.K.; Kim, S. Copyrolysis of block polypropylene with waste wood chip. Korean J. Chem. Eng. 2011, 28, 497–501. [Google Scholar] [CrossRef]
- Chen, W.; Shi, S.; Chen, M.; Zhou, Z. Fast co-pyrolysis of waster newspaper with high-density polyethylene for high yields of alcohols and hydrocarbons. Waste Manag. 2017, 67, 155–162. [Google Scholar] [CrossRef]
- Zanella, E.; Zassa, M.D.; Navarini, L.; Canu, P. Low-temperature co-pyrolysis of polypropylene and coffee wastes to fuels. Energy Fuels 2013, 27, 1357–1364. [Google Scholar] [CrossRef]
- Hu, M.; Laghari, M.; Cui, B.; Xiao, B.; Zhang, B.; Guo, D. Catalytic cracking of biomass tar over char supported nickel catalyst. Energy 2018, 145, 228–237. [Google Scholar] [CrossRef]
- Kanak, M.A.A.; Park, J.Y.; Lee, I.G. Catalytic cracking of oleic acid over zeolites. Key Eng. Mater. 2019, 814, 517–521. [Google Scholar] [CrossRef]
- Twaiq, F.A.; Mohamed, A.R.; Bhatia, S. Liquid hydrocarbon fuels from palm oil by catalytic cracking over aluminosilicate mesoporous catalysts with various Si/Al ratios. Microporous Mesoporous Mat. 2003, 64, 95–107. [Google Scholar] [CrossRef]
- Hu, W.; Wang, H.; Lin, H.; Zheng, Y.; Ng, S.; Shi, M.; Zhao, Y.; Xu, R. Catalytic decomposition of oleic acid to fuels and chemicals: Roles of catalyst acidity and basicity on product distribution and reaction pathways. Catalysts 2019, 9, 1063. [Google Scholar] [CrossRef]
- Mancio, A.A.; da Costa, K.M.B.; Ferreira, C.C.; Santos, M.C.; Lhamas, D.E.L.; da Mota, S.A.P.; Leao, R.A.C.; de Souza, R.O.M.A.; Araujo, M.E.; Borges, L.E.P.; et al. Thermal catalytic cracking of crude palm oil at pilot scale: Effect of the percentage of Na2CO3 on the quality of biofuels. Ind. Crop. Prod. 2016, 91, 32–43. [Google Scholar] [CrossRef]
- Twaiq, F.A.; Zabidi, N.A.M.; Bhatia, S. Catalytic conversion of palm oil to hydrocarbons: Performance of various zeolite catalysts. Ind. Eng. Chem. Res. 1999, 38, 3230–3237. [Google Scholar] [CrossRef]




| Spent Coffee Grounds | Coffee Biocrude Oil | |||
|---|---|---|---|---|
| Fatty Acids | Content (mg/g) | Composition (wt%) | Content (mg/g) | Composition (wt%) |
| Palmitic acid (C16:0) | 61.1 | 35.2 | 99.7 | 59.4 |
| Stearic acid (C18:0) | 12.2 | 7.0 | 19.3 | 11.5 |
| Oleic acid (C18:1) | 13.8 | 8.0 | 17.5 | 10.4 |
| Linoleic acid (C18:2) | 76.3 | 44.0 | 17.8 | 10.6 |
| Linolenic acid (C18:3) | 3.5 | 2.0 | 1.2 | 0.7 |
| Arachidic acid (C20:0) | 4.9 | 2.8 | 7.6 | 4.5 |
| Eicosenoic acid (C20:1) | 0.5 | 0.3 | 3.0 | 1.8 |
| Behenic acid (C22:0) | 1.2 | 0.7 | 1.8 | 1.1 |
| Total | 173.5 | 100.0 | 167.9 | 100.0 |
| Spent Coffee Grounds | Coffee Biocrude Oil | Plastic Pyrolysis Oil | |
|---|---|---|---|
| C (wt%) | 55.3 | 70.0 | 80.5 |
| H (wt%) | 7.4 | 9.0 | 11.3 |
| N (wt%) | 34.8 | 3.8 | 0.6 |
| O (wt%) | 24.4 | 16.9 | 7.6 |
| HHV (MJ/kg) | 24.4 | 33.3 | 40.6 |
| BET Surface Area (m/g) | Pore Volume (cm/g) | Avg. Pore Diameter (nm) | |
|---|---|---|---|
| AC | 687.9 | 0.56 | 3.25 |
| MgNiMo/AC | 518.4 | 0.45 | 3.51 |
| MCM-41 | 1010.2 | 0.92 | 3.17 |
| Ni/MCM-41 | 465.8 | 0.32 | 3.06 |
| EtOH | EtOH-AC | EtOH-MgNiMo/AC | |
|---|---|---|---|
| C16:0 ethyl ester (area%) | 30.3 | 31.9 | 31.2 |
| C18:0 ethyl ester (area%) | 9.2 | 10.1 | 9.1 |
| C18:1 ethyl ester (area%) | 10.9 | 11.7 | 17.0 |
| C18:2 ethyl ester (area%) | 14.2 | 13.7 | 8.9 |
| Total | 64.6 | 67.4 | 66.2 |
| Coffee Biocrude Oil | Plastic Pyrolysis Oil | Upgraded Coffee Biocrude Oils (Supercritical EtOH) | ||||
|---|---|---|---|---|---|---|
| C:P = 1:0 | C:P = 1:0.5 | C:P = 1:1 | C:P = 1:2 | |||
| HHV (MJ/kg) | 33.3 | 40.6 | 37.0 | 38.8 | 39.6 | 40.1 |
| Upgrading Methods | O (wt%) | HHV (MJ/kg) | Liquid Yield (wt%) |
|---|---|---|---|
| Coffee biocrude oil | 16.9 | 33.3 | 100.0 |
| Supercritical EtOH | 9.9 | 37.0 | 79.7 |
| Supercritical EtOH with MgNiMo/AC | 8.5 | 38.0 | 84.7 |
| Supercritical EtOH with plastic pyrolysis oil | 6.4 | 40.1 | 65.2 |
| Catalytic cracking with Ni/MCM-41 | 2.8 | 41.9 | 37.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Kanak, M.A.A.; Lee, I.-G. Upgrading of Coffee Biocrude Oil Produced by Pyrolysis of Spent Coffee Grounds: Behavior of Fatty Acids in Supercritical Ethanol Reaction and Catalytic Cracking. Processes 2021, 9, 835. https://doi.org/10.3390/pr9050835
Park J-Y, Kanak MAA, Lee I-G. Upgrading of Coffee Biocrude Oil Produced by Pyrolysis of Spent Coffee Grounds: Behavior of Fatty Acids in Supercritical Ethanol Reaction and Catalytic Cracking. Processes. 2021; 9(5):835. https://doi.org/10.3390/pr9050835
Chicago/Turabian StylePark, Ji-Yeon, Md Amirul Alam Kanak, and In-Gu Lee. 2021. "Upgrading of Coffee Biocrude Oil Produced by Pyrolysis of Spent Coffee Grounds: Behavior of Fatty Acids in Supercritical Ethanol Reaction and Catalytic Cracking" Processes 9, no. 5: 835. https://doi.org/10.3390/pr9050835
APA StylePark, J.-Y., Kanak, M. A. A., & Lee, I.-G. (2021). Upgrading of Coffee Biocrude Oil Produced by Pyrolysis of Spent Coffee Grounds: Behavior of Fatty Acids in Supercritical Ethanol Reaction and Catalytic Cracking. Processes, 9(5), 835. https://doi.org/10.3390/pr9050835