Anthropogenic Modifications to Estuaries Facilitate the Invasion of Non-Native Species
Abstract
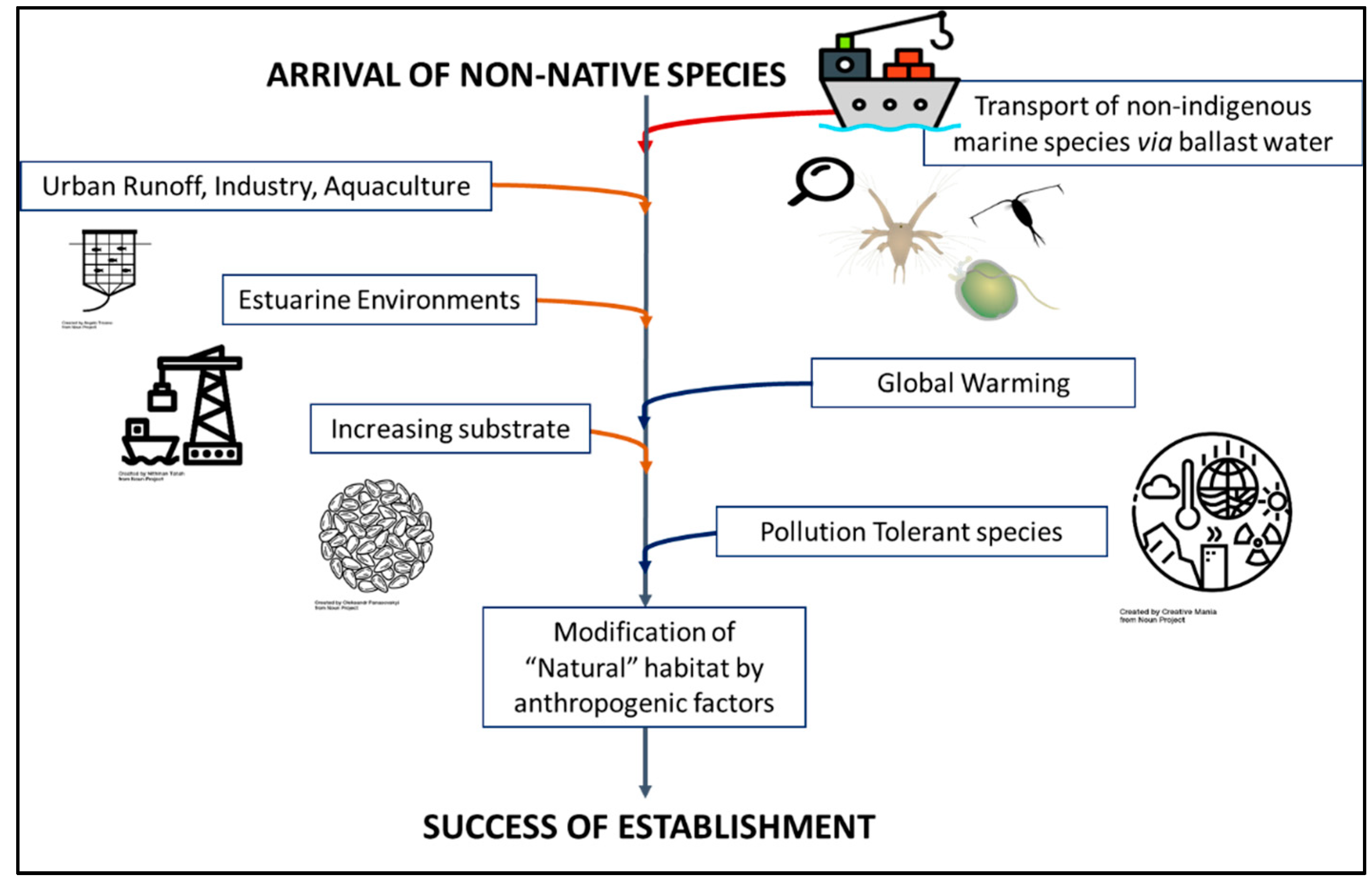
1. Introduction: An Increase in the Arrival of Species
2. The Suitability of an Estuarine Environment to Host Non-Native Species
3. Pollution-Tolerant Species: Species Likely to Be Invasive
4. Modifications to the Coastal Habitat: Increasing the Substrate of Non-Native Species
5. Climate-Related Invasion of Non-Native Species
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Ortegón, E.; Jenkins, S.; Galil, B.S.; Drake, P.; Cuesta, J.A. Accelerated invasion of decapod crustaceans in the southernmost point of the Atlantic coast of Europe: A non-natives’ hot spot? Biol. Invasions 2020, 22, 3487–3492. [Google Scholar] [CrossRef]
- Johnston, E.L.; Dafforn, K.A.; Clark, G.F.; Rius, M.; Floerl, O. Anthropogenic activities promoting the establishment and spread of marine non-indigenous species post-arrival. In Oceanography and Marine Biology: An Annual Reviewl; Hawkins, S.J., Evans, A.J., Dale, A.C., Firth, L.B., Hughes, D.J., Smith, I.P., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 389–419. [Google Scholar]
- Ricciardi, A. Facilitative interactions among aquatic invaders: Is an “invasional meltdown” occurring in the Great Lakes? Can J. Fish Aquat. Sci. 2001, 58, 2513–2525. [Google Scholar] [CrossRef]
- Carlton, J.T.; Ruiz, G.M. The magnitude and consequences of bioinvasions in marine ecosystems: Implications for conservation biology. In Marine Conservation Biology: The Science of Maintaining the Sea’s Biodiversity; Norse, E.A., Crowder, L.B., Eds.; Island Press: Washington, DC, USA, 2003; pp. 123–148. [Google Scholar]
- Minchin, D.; Gollasch, S.; Cohen, A.N.; Hewitt, C.L.; Olenin, S. Characterizing vectors of marine invasion. In Biological Invasions in Marine Ecosystem; Springer: Berlin/Heidelberg, Germany, 2009; pp. 109–116. [Google Scholar]
- Hewitt, C.L.; Gollasch, S.; Minchin, D. The vessel as a vector–biofouling, ballast water and sediments. In Biological Invasions in Marine Ecosystems; Springer: Berlin/Heidelberg, Germany, 2009; pp. 117–131. [Google Scholar]
- Piola, R.F.; Johnston, E.L. Pollution reduces native diversity and increases invader dominance in marine hard-substrate communities. Diversity Distrib. 2008, 14, 329–342. [Google Scholar] [CrossRef]
- Floerl, O.; Pool, T.K.; Inglis, G.J. Positive interactions between nonindigenous species facilitate transport by human vectors. Ecol. Appl. 2004, 14, 1724–1736. [Google Scholar] [CrossRef]
- Hess-Erga, O.-K.; Moreno-Andrés, J.; Enger, Ø.; Vadstein, O. Microorganisms in ballast water: Disinfection, community dynamics, and implications for management. Sci. Total Environ. 2019, 657, 704–716. [Google Scholar] [CrossRef]
- Gollasch, S.; Hewitt, C.L.; Bailey, S.; David, M. Introductions and transfers of species by ballast water in the Adriatic Sea. Mar. Pollut. Bull. 2019, 147, 8–15. [Google Scholar] [CrossRef] [PubMed]
- DiBacco, C.; Humphrey, D.B.; Nasmith, L.E.; Levings, C.D. Ballast water transport of non-indigenous zooplankton to Canadian ports. ICES J. Mar. Sci. 2012, 69, 483–491. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T. Episodic global dispersal in shallow water marine organisms: The case history of the European shore crabs Carcinus maenas and C. aestuarii. J. Biogeogr. 2003, 30, 1809–1820. [Google Scholar]
- Behrens Yamada, S.B.; Dumbauld, A.; Kalin, C.E.; Hunt, R.; Figlar-Barnes, R.; Randall, A. Growth and persistence of a recent invader Carcinus maenas in estuaries of the northeastern Pacific. Biol. Invasions 2005, 7, 309–321. [Google Scholar] [CrossRef]
- Rey, A.; Basurko, O.C.; Rodríguez-Ezpeleta, N. The challenges and promises of genetic approaches for ballast water management. J. Sea Res. 2018, 133, 134–145. [Google Scholar] [CrossRef]
- Darling, J.A.; Martinson, J.; Pagenkopp Lohan, K.M.; Carney, K.J.; Pilgrim, E.; Banerji, A.; Holzer, K.K.; Ruiz, G.M. Metabarcoding quantifies differences in accumulation of ballast water borne biodiversity among three port systems in the United States. Sci. Total Environ. 2020, 749, 141456. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A.; Savini, D. Biological invasions as a component of global change in stressed marine ecosystems. Mar. Pollut. Bull. 2003, 46, 542–551. [Google Scholar] [CrossRef]
- Forrest, B.M.; Gardner, J.P.A.; Taylor, M.D. Internal borders for managing invasive marine species. J. Appl. Ecol. 2009, 46, 46–54. [Google Scholar] [CrossRef]
- Gollasch, S.; David, M. Abiotic and biological differences in ballast water uptake and discharge samples. Mar. Pollut. Bull. 2021, 164, 112046. [Google Scholar] [CrossRef] [PubMed]
- Galil, B.S.; Nehring, S.; Panov, V. Waterways as invasion highways–Impact of climate change and globalization. In Biological Invasions; Nentwig W, Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 59–74. [Google Scholar] [CrossRef]
- Graham, W.M.; Bayha, K.M. Biological invasions by marine jellyfish. In Biological Invasions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 239–255. [Google Scholar]
- Anger, K. Salinity tolerance of the larvae and first juveniles of a semiterrestrial grapsid crab, Armases miersii (Rathbun). J. Exp. Mar. Biol. Ecol. 1996, 202, 205–223. [Google Scholar] [CrossRef]
- González-Ortegón, E.; Pascual, E.; Cuesta, J.A.; Drake, P. Field distribution and osmoregulatory capacity of shrimps in a temperate European estuary (SW Spain). Estuar. Coast. Shelf Sci. 2006, 67, 293–302. [Google Scholar] [CrossRef]
- Butrón, A.; Orive, E.; Madariaga, I. Potential risk of harmful algae transport by ballast waters: The case of Bilbao Harbour. Mar. Pollut. Bull. 2011, 62, 747–757. [Google Scholar] [CrossRef]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; West, J.J.; Klemm, K.; Kobos, J.; Lehtinen, S.; et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 2021, 101989. [Google Scholar] [CrossRef] [PubMed]
- Kennish, M.J. Environmental threats and environmental future of estuaries. Environ. Conserve. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Jackson, J.B. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Boyes, S.; Elliott, M. Organic matter and nutrient inputs to the Humber Estuary, England. Mar Pollut. Bull. 2006, 53, 136–143. [Google Scholar] [CrossRef]
- Kromkamp, J.; Peene, J.; Rijswijk, P.V.; Sandee, A.; Goosen, N. Nutrients, light and primary production by phytoplankton and microphytobenthos in the eutrophic, turbid Westerschelde estuary (The Netherlands). Hydrobiologia 1995, 311, 9–19. [Google Scholar] [CrossRef]
- González-Ortegón, E.; Drake, P. Effects of freshwater inputs on the lower trophic levels of a temperate estuary: Physical, physiological or trophic forcing? Aquat. Sci. 2012, 74, 455–469. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Braungardt, C.; Achterberg, E.; Morley, N.; Cossa, D.; Beckers, J.-M.; Nomérange, P.; Cruzado, A.; Leblanc, M. Metal biogeochemistry in the Tinto–Odiel rivers (Southern Spain) and in the Gulf of Cadiz: A synthesis of the results of TOROS Project. Cont. Shelf Res. 2001, 21, 1961–1973. [Google Scholar] [CrossRef]
- Clarke Murray, C.; Pakhomov, E.A.; Therriault, T.W. Recreational boating: A large unregulated vector transporting marine invasive species. Divers. Distrib. 2011, 17, 1161–1172. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Nosrati-Ghods, N.; Ghadiri, M.; Früh, W.G. Management and environmental risk study of the physicochemical parameters of ballast water. Mar. Pollut. Bull. 2017, 114, 428–438. [Google Scholar] [CrossRef]
- Valković, V.; Obhođaš, J. Sediments in the ship’s ballast water tank: A problem to be solved. J. Soils Sedim. 2020, 1–7. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Soleimani, F.; Nabipour, I.; Saeedi, R.; Mohammadi, M.J. Heavy metal levels of ballast waters in commercial ships entering Bushehr port along the Persian Gulf. Mar. Pollut. Bull. 2018, 126, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.A.; Duggan, I.C.; Jenkins, P.T.; MacIsaac, H.J. Invertebrate resting stages in residual ballast sediment of transoceanic ships. Can. J. Fish. Aquat. Sci. 2005, 62, 1090–1103. [Google Scholar] [CrossRef]
- Tamburri, M.N.; Ruiz, G.M.; Apple, R.; Altshuller, D.; Fellbeck, H.; Hurley, W.L. Evaluations of a ballast water treatment to stop invasive species and tank corrosion. Discussion. Trans.-Soc. Naval Archit. Mar. Eng. 2005, 113, 558–568. [Google Scholar]
- Fernandes, J.A.; Santos, L.; Vance, T.; Fileman, T.; Smith, D.; Bishop, J.D.D.; Viard, F.; Queirós, A.M.; Merino, G.; Buisman, E.; et al. Costs and benefits to European shipping of ballast-water and hull-fouling treatment: Impacts of native and non-indigenous species. Mar. Policy 2016, 64, 148–155. [Google Scholar] [CrossRef]
- Piola, R.F.; Dafforn, K.A.; Johnston, E.L. The influence of antifouling practices on marine invasions: A mini-review. Biofouling 2009, 2009 25, 633–644. [Google Scholar] [CrossRef]
- Gerhard, W.A.; Gunsch, C.K. Higher normalized concentrations of tetracycline resistance found in ballast and harbor water compared to ocean water. Mar. Pollut. Bull. 2020, 151, 110796. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Cui, Y.; Tian, W.; Wei, H.; Chen, Q.; Liu, B.; Zhang, D.; Xie, B. Vessel transport of antibiotic resistance genes across oceans and its implications for ballast water management. Chemosphere 2020, 126697. [Google Scholar] [CrossRef] [PubMed]
- González-Ortegón, E.; Blasco, J.; Le Vay, L.; Giménez, L. A multiple stressor approach to study the toxicity and sub-lethal effects of pharmaceutical compounds on the larval development of a marine invertebrate. J. Hazard. Mater. 2013, 263, 233–238. [Google Scholar] [CrossRef] [PubMed]
- González-Ortegón, E.; Blasco, J.; Nieto, E.; Hampel, M.; Le Vay, L.; Giménez, L. Individual and mixture effects of selected pharmaceuticals on larval development of the estuarine shrimp Palaemon longirostris. Sci. Total Environ. 2016, 540, 260–266. [Google Scholar] [CrossRef]
- Emblidge, J.P.; DeLorenzo, M.E. Preliminary risk assessment of the lipidregulating pharmaceutical clofibric acid, for three estuarine species. Environ. Res. 2006, 100, 216–226. [Google Scholar] [CrossRef]
- Weigel, W.; Kuhlmann, J.; Hühnerfuss, H. Drugs and personal care products as ubiquitous pollutants: Occurrence and distribution of clofibric acid, caffeine and DEET in the North Sea. Sci. Total Environ. 2002, 295, 131–141. [Google Scholar] [CrossRef]
- Pechenik, J.A. Environmental influences on larval survival and development. In Reproduction of Marine Invertebrates vol IX; Giese, A.C., Pearse, J.S., Pearse, V.B., Eds.; Blackwell Scientific Publications and Boxwood Press: Pacific Grove, CA, USA, 1987; pp. 551–668. [Google Scholar]
- Kalčíková, G.; Englert, D.; Rosenfeldt, R.R.; Seitz, F.; Schulz, R.; Bundschuh, M. Combined effect of UV-irradiation and TiO2-nanoparticles on the predator–prey interaction of gammarids and mayfly nymphs. Environ. Pollut. 2014, 186, 136–140. [Google Scholar] [CrossRef]
- Jager, T.; Posthuma, L.; der Zwart, D.; van de Meent, D. Novel view on predicting acute toxicity, decomposing toxicity data in species vulnerability and chemical potency. Ecotoxicol. Environ. Saf. 2007, 67, 311–322. [Google Scholar] [CrossRef]
- León, V.M.; Moreno-González, R.; González, E.; Martínez, F.; García, V.; Campillo, J.A. Interspecific comparison of polycyclic aromatic hydrocarbons and persistent organochlorines bioaccumulation in bivalves from a Mediterranean coastal lagoon. Sci. Total Environ. 2013, 463, 9075–9987. [Google Scholar] [CrossRef]
- González-Ortegón, E.; Giménez, L.; Blasco, J.; Le Vay, L. Effects of food limitation and pharmaceutical compounds on the larval development and morphology of Palaemon serratus. Sci. Total Environ. 2015, 503, 171–178. [Google Scholar] [CrossRef]
- Elliott, M.; Hemingway, K.L.; Costello, M.J.; Duhamel, S.; Hostens, K.; Lapropoulou, M.; Marshall, S.; Winkler, H. Links between fish and other trophic levels. In Fishes in Estuaries; Elliott, M., Hemingway, K.L., Eds.; Blackwell Science Ltd.: Oxford, UK, 2002; pp. 124–216. [Google Scholar]
- Hall, L.W.; Scott, M.C.; Killen, W.D. Ecological risk assessment of copper and cadmium in Surface waters of Chesapeake Bay watershed. Environ. Toxicol. Chem. 1998, 17, 1172–1189. [Google Scholar] [CrossRef]
- Johnston, E.L.; Marzinelli, E.M.; Wood, C.A.; Speranza, D.; Bishop, J.D.D. Bearing the burden of boat harbours: Heavy contaminant and fouling loads in a native habitat-forming alga. Mar. Pollut. Bull. 2011, 62, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Toh, K.B.; Ng, C.S.L.; Wu, B.; Toh, T.C.; Cheo, P.R.; Tun, K.; Chou, L.M. Spatial variability of epibiotic assemblages on marina pontoons in Singapore. Urban Ecosyst. 2016. [Google Scholar] [CrossRef]
- Knights, A.M.; Firth, L.B.; Thompson, R.C.; Yunnie, A.L.E.; Hiscock, K.; Hawkins, S.J. Plymouth–A World Harbour through the ages. Reg. Stud. Mar. Sci. 2016, 8, 297–307. [Google Scholar] [CrossRef]
- Airoldi, L.; Turon, X.; Perkol-Finkel, S.; Rius, M. Corridors for aliens but not for natives: Effects of marine urban sprawl at a regional scale. Div. Distrib. 2015, 21, 755–768. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Sempere-Valverde, J.; González, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From exotic to invasive in record time: The extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef]
- Dugan, J.E.; Airoldi, L.; Chapman, M.G.; Walker, S.J.; Schlacher, T. Estuarine and coastal structures: Environmental effects, a focus on shore and nearshore structures. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D., Eds.; Academic Press: Waltham, MA, USA, 2011; Volume 8, pp. 17–41. [Google Scholar]
- Connell, S.D. Urban structures as marine habitats: An experimental comparison of the composition and abundance of subtidal epibiota among pilings, pontoons and rocky reefs. Mar. Environ. Res. 2001, 52, 115–125. [Google Scholar] [CrossRef]
- Drillet, G. Food security: Protect aquaculture from ship pathogens. Nature 2016, 539, 31. [Google Scholar] [CrossRef] [PubMed]
- Drillet, G.; Juhel, G.; Trottet, A.; Eikaas, H.; Saunders, J. Aquaculture biosecurity challenges in the light of the Ballast Water Management Convention. Asian Fish. Sci. 2018, 31, 168–181. [Google Scholar] [CrossRef]
- Tan, C.K.F.; Nowak, B.F.; Hodson, S.L. Biofouling as a reservoir of Neoparamoeba permaquidensis (Page 1970), the causative agent of amoebic gill disease in Atlantic salmon. Aquaculture 2002, 210, 49–58. [Google Scholar] [CrossRef]
- Culloty, S.C.; Mulcahy, M.F. Bonamia ostreae in the Native Oyster, Ostrea Edulis: A Review; Marine Institute: San Pedro, CA, USA, 2007. [Google Scholar]
- González-Ortegón, E.; Baldó, F.; Arias, A.; Cuesta, J.A.; Fernández-Delgado, C.; Vilas, C.; Drake, P. Freshwater scarcity effects on the aquatic macrofauna of a European Mediterranean-climate estuary. Sci. Total Environ. 2015, 503, 213–221. [Google Scholar] [CrossRef]
- Fernández-Delgado, C.; Baldó, F.; Vilas, C.; García-González, D.; Cuesta, J.A.; González-Ortegón, E.; Drake, P. Effects of the river discharge management on the nursery function of the Guadalquivir river estuary (SW Spain). Hydrobiologia 2007, 587, 125–136. [Google Scholar] [CrossRef]
- Morais, P.; Chícharo, M.A.; Chícharo, L. Changes in a temperate estuary during the filling of the biggest European dam. Sci. Total Environ. 2009, 407, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Galil, B.; Boero, F.; Campbell, M.; Carlton, J.; Cook, E.; Fraschetti, S.; Gollasch, S.; Hewitt, C.; Jelmert, A.; Macpherson, E.; et al. ‘Double trouble’: The expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biol. Invasions 2015, 17, 973–976. [Google Scholar] [CrossRef]
- Frisch, D.; Moreno-Ostos, E.; Green, A.J. Species richness and distribution of copepods and cladocerans and their relation to hydroperiod and other environmental variables in Doñana, south-west Spain. Hydrobiologia 2006, 556, 327–340. [Google Scholar] [CrossRef]
- Winder, M.; Jassby, A.D.; Mac Nally, R. Synergies between climate anomalies and hydrological modifications facilitate estuarine biotic invasions. Ecol. Lett. 2011, 14, 749–757. [Google Scholar] [CrossRef]
- Trottet, A.; George, C.; Drillet, G.; Lauro, F.M. Aquaculture in coastal urbanized areas: A comparative review of the challenges posed by Harmful Algal Blooms. Crit. Rev. Environ. Sci. Technol. 2021, 1–42. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- ICES. Interim Report of the ICES-IOC Working Group on Harmful Algal Bloom Dynamics (WGHABD), 24–28 April 2018, Tarragona, Spain; ICES CM 2018/EPDSG:11; ICES: Tarragona, Spain, 2018; p. 45. [Google Scholar]
- Jang, P.-G.; Hyun, B.; Shin, K. Ballast Water Treatment Performance Evaluation under Real Changing Conditions. J. Mar. Sci. Eng. 2020, 8, 817. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ortegón, E.; Moreno-Andrés, J. Anthropogenic Modifications to Estuaries Facilitate the Invasion of Non-Native Species. Processes 2021, 9, 740. https://doi.org/10.3390/pr9050740
González-Ortegón E, Moreno-Andrés J. Anthropogenic Modifications to Estuaries Facilitate the Invasion of Non-Native Species. Processes. 2021; 9(5):740. https://doi.org/10.3390/pr9050740
Chicago/Turabian StyleGonzález-Ortegón, Enrique, and Javier Moreno-Andrés. 2021. "Anthropogenic Modifications to Estuaries Facilitate the Invasion of Non-Native Species" Processes 9, no. 5: 740. https://doi.org/10.3390/pr9050740
APA StyleGonzález-Ortegón, E., & Moreno-Andrés, J. (2021). Anthropogenic Modifications to Estuaries Facilitate the Invasion of Non-Native Species. Processes, 9(5), 740. https://doi.org/10.3390/pr9050740






