Abstract
The high moisture content present in sewage sludge hinders the use of sewage sludge in incineration or energy application. This limitation of moisture present in sewage sludge can be obviated by using the hydrothermal carbonization (HTC) process. In sewage sludge management, the HTC process requires less energy compared to other conventional thermo–chemical management processes. The HTC process produces energy-rich hydrochar products and simultaneously enables phosphorus recovery. This study investigates the influence of organic acids, inorganic acid, and alkali as additives on phosphorus transformation, yield, proximate analysis and the heating value of subsequently produced hydrochar. The analysis includes various process temperatures (200 °C, 220 °C, and 240 °C) in the presence of deionized water, acids (0.1 M and 0.25 M; H2SO4, HCOOH, CH3COOH), and alkali (0.1 M and 0.25 M; NaOH) solutions as feed water. The results show that phosphorus leaching into the process-water, hydrochar yield, proximate analysis, and the heating value of produced hydrochar is pH- and temperature-dependent, and particularly significant in the presence of H2SO4. In contrast, utilization of H2SO4 and NaOH as an additive has a negative influence on the heating value of produced hydrochar.
1. Introduction
The management of sewage sludge produced from wastewater treatment plants is an important global issue due to the presence of high moisture content, harmful pathogens, and poor dewaterability. Conventional sewage sludge management involves the direct application on farmland as fertilizer. However, sewage sludge has attracted greater attention as a feedstock for nutrient recovery and renewable biofuels production [1,2]. In the year 2018, about 23% of sewage sludge produced in Germany was managed by applying directly on farmland, and about 65% of the produced sewage sludge was incinerated [3]. Since 2017, new regulation was placed by the German sewage sludge ordinance (AbfKlärV) based on enabling principles of the Circular Economy Act [4] on sewage sludge management. This new regulation is not only making it mandatory to recover phosphorus from sewage sludge in Germany but also prohibits the direct use of sewage sludge on farmland [5]. According to AbfKlärV, sewage sludge must undergo mandatory phosphorus recovery if the phosphorus content is ≥20 g/kg total dry matter (DM) or ≥2% DM. The thermal pretreatment of sewage sludge is still possible; however, the subsequent recovery of phosphorus in the produced incinerated ash or the carbonaceous residue has to be guaranteed. This new obligation applies from January 2029 for the wastewater treatment plants with size >100,000 populations equivalent (PE). The treatment facilities with >50,000 PE must also comply with the new regulation to recover phosphorus from January 2032. After these dates, soil-related disposal of sewage sludge is no longer permitted. Only the smaller wastewater treatment plants (≤50,000 PE) can use their sewage sludge as a soil amender when the phosphorus content in sludge is <20 g/kg total DM.
The option for managing sewage sludge is getting progressively limited as the result of strict environmental legislation placed by Germany over the last decade. The actual problem exists in states of Germany such as Mecklenburg–Western Pomerania, Lower Saxony, and Rhineland–Palatinate where >50% of the produced sewage sludge is managed by direct application for agriculture and landscaping [3]. In this concern, the current number of thermal treatment facilities might not be able to handle the amount of sludge produced, eventually triggering the new problem associated with the storage of untreated sewage sludge.
Incineration is a widely accepted technique to treat sewage sludge in Germany and can significantly reduce the sludge volume and produce reactively hygiene sludge ash residue with high phosphate content [6]. Yang et al. (2019) studied the effect of chlorine-based additives on phosphorus recovery during sewage sludge incineration. The addition of Magnesium chloride (MgCl2) and Calcium chloride (CaCl2) during incineration increases the fixation rate of total phosphorus (TP) to a maximum of 98.5% in sewage sludge treated with 3% (Magnesium) Mg at 900 °C and 97.8% in sewage sludge treated with 5% Calcium (Ca) at 800 °C. Similar to incineration, pyrolysis can also be an effective alternative process to treat and recover phosphorus from sewage sludge. The study by Atienza–Martínez et al. (2014) indicated phosphorus recovered from pyrolysis is temperature-dependent and that more than 90% of phosphorus can be recovered using pyrolysis followed by char combustion in sulfuric acid.
In recent years, hydrothermal carbonization has gained greater attention for treating sewage sludge as it is greatly regarded as an eco-friendly and promising technology. Hydrothermal carbonization is a technology that demonstrates the high potential to treat the moist/wet biomass without it having to be dewatered. HTC uses moisture present in sludge as the reaction medium to process the sewage sludge without pre-drying. During HTC, higher temperature and pressure will aid the moisture present in sewage sludge to serve as a solvent, reactant, and catalyst for converting sewage sludge into hydrochar. The product hydrochar is hygienic, essentially free of pharmaceuticals, easily dewaterable, and likely to be a coal-like product with high energy density [7,8]. Additionally, the HTC process is proved to save up to 53% thermal energy and 69% electrical energy compared to conventional sludge drying methods [9]. Currently, there are several studies on HTC of sewage sludge, particularly in producing hydrochars and subsequent utilization of hydrochar as effective adsorbents [8,10], soil amendment [11], or as feedstock for energy production [1,12]. Further, there are few studies that explain the effects of additives during HTC of lignocellulosic biomass [13,14,15]. The investigation carried out by Lynam et al. (2012) identifies the benefit of the increased heating value of resulting hydrochar produced using Ca salts (Ca chloride and Ca lactate) as additives during HTC of lignocellulosic biomass.
Previous studies on understanding the effect of feed-water pH during HTC of sewage sludge have mainly focused on investigating phosphorus transformation [6,16,17], risk of heavy metals in hydrochar [18], physicochemical properties of hydrochar [19]. Shi et al. [17] studied the effect of initial pH and HTC reaction temperature on the mobilization of phosphorus. They found that at higher temperatures phosphorus is more likely to be more present in the hydrochar; however, using acid additives in large amounts could shift phosphorus into the liquid phase. Further, the phosphate leached into the process-water can be chemically precipitated by the addition of a coagulant and a mixing of process-water and coagulant. The multivalent metal ions most commonly used are calcium, aluminium, and iron [20,21]. To the authors’ knowledge, very few investigations compare the influence of organic acids, inorganic acids, and alkali additives on the HTC of sewage sludge. Wang et al. (2017) investigated the influence of feed water pH (altered by the addition of acetic acid or sodium hydroxide) on phosphorus transformation during HTC of sewage sludge. The results showed that during the HTC of sewage sludge, metal cations and pH played vital roles in the transformation of phosphorus. The observation made by Ekpo et al. (2016) shows that 94% of the phosphorus in feedstock was recovered into the process-water after hydrothermal treatment of pig manure with a sulfuric acid additive at 170 °C. Reza et al. (2015) [15] studied the influence of using acid and alkali additives on the HTC of wheat straw. However, the behavior of phosphorus transformation greatly differs from the types of biomass, and HTC process conditions and techniques.
The main purpose of this study is to investigate and compare the influence of organic acids (acetic acid and formic acid), inorganic acids (sulfuric acid), and alkali (sodium hydroxide) as additives on the hydrothermal treatment of sewage sludge. Despite the primary objective of this study being to understand the influence of different additives on the P transformation during HTC of sewage sludge, this study also shed light on the effect of additives on dewaterability, yield, and heating value of hydrochar.
2. Material and Methods
2.1. Material
The sewage sludge used in this study was obtained directly from the wastewater treatment plant, Rostock, Germany. The central wastewater treatment plant in Rostock treats both industrial (1/3) and municipal wastewater (2/3) with the capacity to treat wastewater from 320,000 inhabitants [22]. The freshly digested and dewatered sludge was collected in an airtight specimen container and transported immediately to the laboratory. The sewage sludge, after being received in the laboratory, is refrigerated at 4 °C before use. The refrigerated representative samples were directly taken for HTC investigation and respective additives of deionized water, organic acid, inorganic acids, and alkali with known concentration were added and mixed to make a homogeneous slurry. The respective additive solution of 0.1 and 0.25 M concentration was prepared by diluting acetic acid (100%, p.a.), formic acid (≥98.0%, p.a.), sulfuric acid (1 M), and sodium hydroxide (≥97.0% (T), pellets) in the deionized water. The produced additive solution was used on the same day of preparation. The ultimate analysis of the sewage sludge was performed using an organic elemental analyzer by following EN ISO 16948, 2015. Proximate analysis was performed using a LECO Thermogravimetric Analyser (TGA) unit TGA701 to determine moisture content, volatile organic compound, fixed carbon and ash content. The heating value of the sewage sludge and resulting char was determined by Parr 6400 calorimeter (Parr Instruments Inc., Moline, IL, USA) following the method described in EN 14918, 2010. Total phosphate in the obtained sewage sludge was analyzed in an external laboratory following the method described in EN ISO 11885, 2009. All measurements were made in duplicate, and the mean value is reported.
2.2. Hydrothermal Carbonisation Treatment
Hydrothermal carbonization of sewage sludge was carried out in a Parr 4523 reactor (Parr Instrument (Deutschland) GmbH, Zeilweg 15, Frankfurt, Germany) at an autogenic pressure. The processing unit 4523 consists of a reaction vessel of 1-L capacity that can withstand a maximum pressure of 138 bar, a heating jacket equipped with a 2 kW heating coil, a temperature and a pressure sensor, and a stirrer with an attached motor. The reactor temperature and the speed of the stirrer were controlled using a Parr 4848 PID reactor controller. Figure 1 provides an overview of the experimental methodology. The analysis was carried out by charging the reactor with 297.00 g raw sewage sludge (23.5% DM) and it was topped up with 402.00 g of deionized water or additive solution of acetic acid, formic acid, sulfuric acid, or sodium hydroxide in 0.1 M or 0.25 M concentration. The sewage sludge and additives were mixed homogeneously inside the reactor before starting the investigation, and the initial pH of mixed feedstock slurry was noted using WTW pH 3310 m. The defined ratio of sewage sludge to additives was used to produce a homogeneous slurry of 10% DM. The mixture was hydrothermally carbonized at autogenic pressure with a constant heating rate of 4 K/min. The investigation was carried out with the varying temperature of 200 °C, 220 °C, and 240 °C for a retention time of 2 h while keeping the stirrer switched on during the entire process. Later, the reactor was allowed to cool down to room temperature without any additional cooling mechanism.
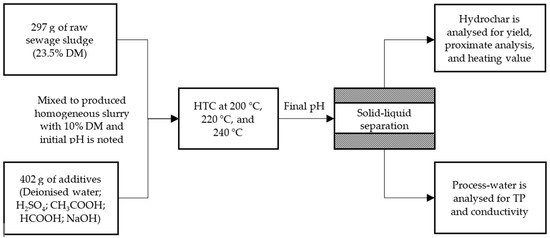
Figure 1.
Schematic representation of experimental methodology.
2.3. Product Recovery and Analysis
The final pH of the HTC-slurry obtained after HTC of sewage sludge was noted, and the resulting hydrochar and process-water were separated by using a vacuum filtration apparatus. Vacuum filtration was carried out at the constant process conditions using a top-feeding procedure in a Büchner funnel. The following rules were kept constant for solid-liquid separation of the HTC-slurry and analyzing the dry matter concentration of hydrochar: (1) entire content HTC-slurry after carbonization was poured into the Büchner funnel, (2) the vacuum pump was switched on to generate the vacuum pressure for solid–liquid separation, (3) the solids (hydrochar) thus obtained using vacuum filtration were oven-dried at 105 °C for 24 h and stored in sealed containers for further analysis or usage. Similarly, process-water produced after filtration was collected and stored in a volumetric flask and refrigerated at 4 °C until it was analyzed for total phosphorus (TP) concentration and conductivity.
The yield of the produced hydrochar is calculated as explained in Equation (3). The lower heating value (LHV) of the hydrochar was determined in a similar way to sewage sludge using a Parr 6400 calorimeter following the method described in EN 15170, 2010. TP in the process-water was analyzed spectrophotometrically after acid hydrolysis and oxidation following EN ISO 6878, 2004. The conductivity of the process-water was measured using a Hach HQ 40 d multifunction meter. By determining the conductivity, it was possible to understand the variations of salt content in the process-water produced at different process parameters. Triplicates of all analyzed results were obtained and the mean value was reported.
2.4. The Fraction Phosphorus Recovered on Hydrochar
The TP recovered on the hydrochar was mathematically calculated using the experimental data obtained on TP concentration in sewage sludge and process-water, and the total yield of the hydrochar after HTC. TP recovered from the hydrochar () can be mathematically defined as follows:
where and is the TP content in process-water and the initial feedstock slurry, respectively, and is the total yield of the process-water after filtration, which is calculated as shown in Equation (2):
where m is the total weight of the feedstock, is the dry weight of the feedstock, is the dry matter percentage of hydrochar after filtration, and is the yield (%) of produced hydrochar and was calculated as the applied formula.
where is the total dry weight of produced hydrochar.
3. Results and Discussion
3.1. Characteristic of Sewage Sludge
The results of the proximate and ultimate analysis of sewage sludge are presented in Table 1. The moisture content of sewage sludge was determined to be 76.53%, leaving behind the total solids content of 23.48%. The analysis also demonstrates noticeably lower ash content of 32.83% DM and higher volatile solids (VS) of 61.46% DM, which was inconsistent with the previous investigation ranges [6,23]. The ultimate analysis of the sewage sludge specified the typical C-H-N-S-O content for sewage sludge in Germany [24] with C: 32.5; H: 5.0; N: 4.98; S: 1.50; and O: 21.4 on a dry basis. The dry sewage sludge is known to contain a higher concentration of phosphorus and a relatively higher heating value. The TP content in the feedstock was determined to be 36.1 g/kg, accounting for 3.6% of total dry sludge, and the heating value was observed to be relatively higher with 13.56 MJ/kg (LHV) in comparison with previous studies [6,23,24]. One possible explanation for increased LHV can be the presence of higher volatile solids and lower ash content. Nevertheless, the overall characteristics of the feedstock have the typical composition of sewage sludge in Germany.

Table 1.
Proximate and ultimate analysis of sewage sludge.
3.2. Effect of Additives and Reaction Temperature on Yield of Hydrochar
Figure 2 compares the total yield (%) of hydrochar produced at different temperatures using various additives. An increase in the process temperature from 200 °C to 240 °C has decreased the hydrochar yield on average by about 10%, which agrees with the earlier investigation results demonstrating a decrease in hydrochar yield with an increase in reaction temperature [25,26]. The maximum hydrochar yield was observed with the carbonization method using inorganic acid as additives in comparison with the carbonization method using organic acid, alkali, and deionized water as additives. The maximum hydrochar yield of 69.09% was achieved using a 0.25 M H2SO4 additive in feedstock (pH 3.78), with the carbonization temperature of 200 °C and 2 h retention time. In contrast, the same reaction temperature and retention time, using 0.25 M CH3COOH (pH 5.44), HCOOH (pH 5.38), NaOH (pH 10.68) and deionized water (pH 7.8) additives has resulted in the hydrochar yield of 62.74%, 63.79%, 55.47%, and 59.66%, respectively. Nevertheless, it is interesting to see that at the lower additive concentration (0.1 M), despite having comparatively similar initial pH range (5.8–6.3) of sewage sludge slurry prepared using CH3COOH (pH 6.3), HCOOH (pH 6.2), and H2SO4 (pH 5.8), hydrochar yield was significantly higher with using H2SO4 as an additive in comparison with other organic acids.
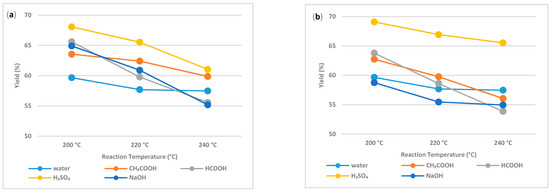
Figure 2.
Influence of additives and temperature on yield of hydrochar; (a) represents the hydrochar yield produced using additives of 0.1 M concentration; (b) represents the hydrochar yield produced using additives of 0.25 M concentration.
The increases in reaction temperature will directly influence eliminating the moisture content in the biomass structure as the effect of hydrolysis reaction and simultaneously foster biomass degradation; this, in turn, decreases hydrochar yield [26,27]. Further, the investigation conducted by Jaruwat et al. (2018) has shown that a longer retention time will increase the yield of the hydrochar as the result of repolymerisation of decomposed biopolymers.
Similar to reaction temperature and retention time, the addition of additives also influences the yield of the hydrochar. Temperature undoubtedly has a greater influence on the mass yield of hydrochar. Nevertheless, similar to temperature, despite having similar pH, retention time, and reaction temperature, using inorganic acid has increased the hydrochar yield in comparison to using organic acids or alkali as additives. An increase in the hydrochar yield can be co-related with the higher molecular mass of H2SO4 and changing pH due to strong acid additive utilization in comparison with the utilization of CH3COOH, HCOOH, NaOH, and deionized water as an additive.
3.3. Effect of Additives and HTC Process Conditions on Solid–Liquid Separation
The dry matter concentration of the various hydrochar residue after filtering the process-water using a vacuum filter at the constant process conditions (top-feeding procedure with a Büchner funnel) is depicted in Figure 3. The HTC treatment was advantageous to sludge dewatering. The dry matter concentration of hydrochar residue after solid–liquid separation increased significantly after the HTC reaction and the use of H2SO4 as an additive, significantly favored dewatering. When 0.25 M H2SO4 solution was used as an additive, the dry matter of hydrochar residue was 27.68–31.75%, which was significantly higher in comparison with using deionized water as an additive (20.70–24.83%). The influence of H2SO4 in enhancing the dewaterability of sewage sludge has also been explained previously [3]. The use of organic acids as an additive did not show any greater difference in the dry matter of hydrochar residue (20.68–26.38%) in comparison with using deionized water as an additive. In contrast, the use of NaOH as an additive had considerably decreased the dry matter of hydrochar residue (1.28–16.51%) at the lower reaction temperature (200 °C); however, at the higher reaction temperatures (220 °C and 220 °C), dry matter of hydrochar residue was higher (27.27–28.82%).
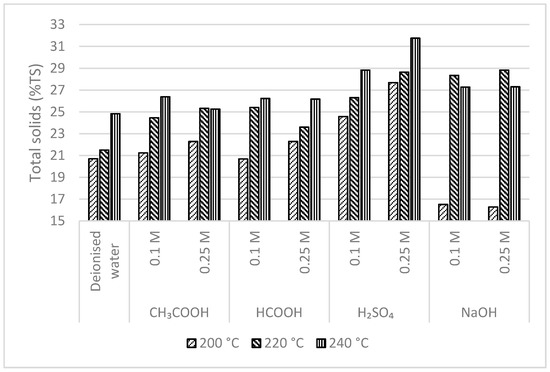
Figure 3.
Dry matter concentration of hydrochar residue after filtering the process-water.
The extracellular polymeric materials in the sewage sludge contain viscous protein material that is extremely hydrophilic [28]. The effective way to enhance the sludge dewatering performance is by breaking the cell wall and destroying the sludge flocs to release and hydrolyze the organic matter present in sewage sludge. This phenomenon can be effectively achieved alongside the higher temperature and pressure that occur in the HTC process. The reduction in the binding force of the sludge particles achieved during the HTC process improves the dewatering performance after HTC and is significantly enhanced using H2SO4 in the reaction medium. In contrast, the use of NaOH additive at lower reaction temperature (200 °C) was not effective in hydrolyzing the organic matter. This could have influence in retaining of the viscous protein material in sewage sludge, making the HTC-slurry hard to dewater. Nevertheless, using NaOH additive at higher reaction temperatures (220 °C and 240 °C) was effective in hydrolyzing the organic matter present in sewage sludge.
3.4. Effect of Additives on Hydrochar Properties: Proximate Analysis and Heating Value
Following HTC, sewage sludge was carbonized into a brownish-grey solid hydrochar with a nutlike smell. The physical appearance of produced hydrochar implied that hydrochar had a uniform composition and could be readily molded into dense pellets. The proximate analysis and LHV were determined to understand the fuel characteristics of the produced hydrochar. Table 2 represents the results comprising volatile matter, ash content, fixed carbon, and LHV of various hydrochar produced at different process conditions. The hydrochar produced using various additives in this investigation had LHV in the range of 14.24–15.63 MJ/kg, which is similar to the results of earlier studies demonstrating fuel characteristics of hydrochar produced using sewage sludge [1].

Table 2.
Proximate analysis and heating value of hydrochar produced using various additives and reaction conditions. (AC-Additive concentration; RT-Reaction temperature; Initial and final pH represents the pH of feedstock slurry before and after HTC. All hydrochars were produced at 2 h retention time.)
The breaking down of biomass at higher temperatures to influence aromatization, polymerization, and condensation to produce hydrochar can be a reason for the increase in fixed carbon content (FC) with increasing reaction temperature [29]. Fixed carbon can be defined as combustible residue present in the char after the volatile matter burned. In general, biomass before carbonization contains high VS content and low FC, but high moisture content [30]. Previously, several studies showed a strong correlation between FC content and calorific value; an increase in the FC content in char can directly increase the heating value of the char [30,31]. The use of H2SO4 and NaOH as an additive has negatively influenced the LHV of the produced hydrochar in comparison with the hydrochar produced using organic acids and deionized water as an additive.
The hydrochar produced using organic acids and deionized water as an additive increased the FC content (7.72–8.94%) in comparison to the FC content of the initial feedstock (5.71%). Here, the increase in the FC content in hydrochar might have influenced increasing the LHV (14.99–15.75 MJ/kg). However, the use of H2SO4 as an additive at higher concentrations (0.25 M) negatively influenced the FC content of produced hydrochar (4.41–5.27%). Similarly, the use of NaOH as an additive at lower reaction temperatures (200 °C and 220 °C) had no noticeable influence on FC% (5.81–6.87%) in comparison with initial feedstock. On other hand, it was also observed that there was a significant decrease in the VS content and increase in the ash content after HTC of sewage sludge. The decrease in VS content can be attributed to the reaction severity and dissolution of organic material into the liquid phase, and an increase in the ash content can be correlated with the decrease in the mass percentage of VS composition of the hydrochar. However, it is interesting to perceive that the ash content in the hydrochar produced using H2SO4 (0.25 M) as an additive is offset more by decreasing FC content than VS content; similar phenomena can also be seen with the hydrochar produced using NaOH as an additive at 200 °C and 220 °C. The decrease in the FC content with the use of H2SO4 at higher concentrations and NaOH at lower reaction temperatures (200 °C and 220 °C) as an additive can explain the lower LHV in the respectively produced hydrochars.
3.5. Conductivity of Process-Water
Figure 4 is the graphical representation of the conductivity of the process-water produced using different acids and alkali as additives. The conductivity measurement, in general, provides a reliable means to understand the ion concentration of a solution. The maximum conductivity of 24.10 µS/cm was observed in the process-water produced using alkali additive. Among acid-based additives, the utilization of inorganic acid (H2SO4) as an additive had process-water with higher conductivity (17.4–20.12 µS/cm) in comparison with organic acid additives (CH3COOH and HCOOH).
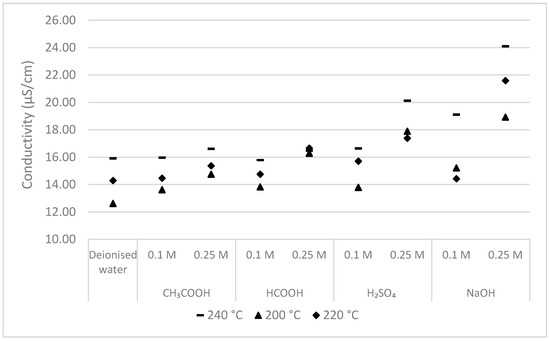
Figure 4.
Conductivity of process-water produced using acids and alkali additive at different concentration.
3.6. Effect of HTC Organic Acids, Inorganic Acids, and Alkali Additive on P-Transformation
3.6.1. The pH of Feedstock Slurry, before and after HTC
Table 2 depicts the pH of the feedstock slurry before and after HTC at various temperatures, additives, and additive concentrations. The HTC process comprises hydrolysis, dehydration, decarboxylation, aromatization, and condensation polymerization [32]. During HTC, the pH of the feedstock slurry decreases as the result of the degradation of macromolecular organic matter into an acidic substance (viz., volatile fatty acids) and subsequent dissolution into the liquid phase. Further, the reaction time and temperature also influence the pH of the sludge hydrolysate. The use of organic acids and inorganic acid as additives resulted in the feedstock initial pH between 3.4 and 6.4. In contrast, the use of NaOH additive resulted in the feedstock initial pH ranging from 9.9–11.0. In the baseline condition, the deionized water additive has an initial pH of 7.8. The final pH represents the pH of the feedstock slurry after HTC. The experimental observation demonstrates that, regardless of variations in the initial pH, the final pH value after HTC always tends to move towards neutral. The obtained results were consistent with an idea that the acids formed during hydrolysis were subsequently decomposed or repolymerized at a higher temperature, which influences the pH of feedstock slurry after HTC [28]. Further, it is also possible that the buffering function of the sewage sludge might have a significant effect on the final pH. The obtained results of the shift in final pH towards neutral agree with several earlier HTC studies carried out on sewage sludge [6], swine manure [33], and wheat straw [15].
3.6.2. Effect of Additives on Phosphorus Transformation
Figure 5 shows the concentration of TP in the process-water produced after the HTC of feedstock slurry at various temperatures. For each experiment, TP in the process-water was analyzed spectrophotometrically after acid hydrolysis and oxidation of the process-water sample. Further, the TP in the hydrochar was calculated mathematically using Equation (1). Figure 6 depicts the influence of additives, additive concentration, and pH of the feedstock slurry on the recovering TP from the raw feedstock into hydrochar after HTC at various temperatures. In brief, the results show that even at a similar pH, higher leaching of TP into process-water is achieved by the utilization of inorganic acid (H2SO4) as an additive in comparison with organic acids.
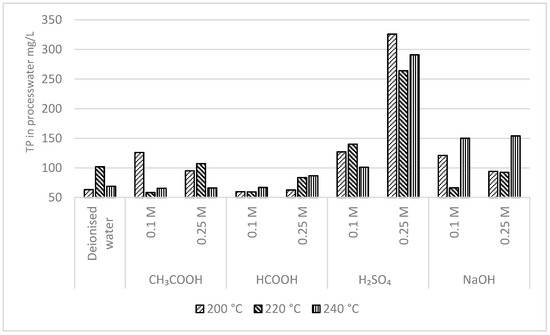
Figure 5.
TP concentration of the process-water.
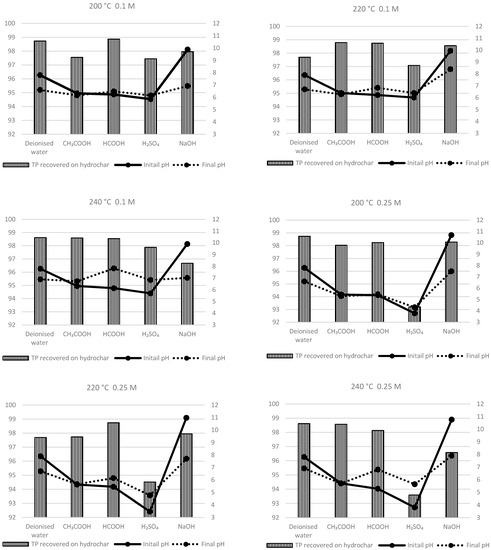
Figure 6.
Influence of additives, additive concentration, temperature, and pH on the recovery percentage of TP into the hydrochar.
Following the HTC of sewage sludge, the highest TP leaching into the process-water (326 mg/L) was observed by using H2SO4 as an additive (pH 3.78), which represents about 93% of TP being recovered from raw feedstock into consequently produced hydrochar. Irrespective of process temperature, using deionised waste as an additive did not have any significant influence on the TP leaching. TP leaching into process-water using deionized water as an additive was observed to be 63–101 mg/L, which represents about 97.7–98.7% of TP being recovered from raw feedstock into consequently produced hydrochar. The TP concentration in the process-water following the treatment at various temperatures and organic acid additives—formic and acetic acid—was observed to be in the range of 58.3–126 mg/L and 59.3–86.6 mg/L, respectively. Likewise, using NaOH as an additive also had comparatively similar TP leaching (66.2–154 mg/L) into the process-water after HTC at various temperatures. The obtained results suggested that organic acids and alkali had a very limited impact on extracting TP from raw feedstock into the process-water, which agrees with the similar results demonstrated by earlier studies [6,33].
During HTC, the extraction of phosphorus into the process-water was generally lower with the utilization of organic acids as additives in comparison with an inorganic acid, regardless of temperature. An increase in H2SO4 additive concentration from 0.1 M to 0.25 M increased the TP leaching into process-water by about 3-fold from 101–127 mg/L to 264–326 mg/L, respectively. However, increasing the concentration of organic acid additives from 0.1 M to 0.25 M obviously decreased the pH of the resulting feedstock slurry, but it did not greatly influence TP leaching into process-water.
The initial pH of feedstock slurry produced using CH3COOH and HCOOH additive was ~6.3 and ~5.6, and ~6.2 and ~5.3, respectively, at 0.1 and 0.25 M concentration. The TP in the process-water was observed to be in the range of 65.5–126 mg/L and 66–105 mg/L when produced using CH3COOH additive at 0.1 and 0.25 M concentration. Similarly, the TP in process-water was in a similar range with 59.3–66.9 mg/L and 62–86.3 mg/L when produced using HCOOH additive at 0.1 and 0.25 M concentration.
Factors influencing the TP immobilization during the HTC process include treatment conditions (temperature, reaction time, and additive properties), and the feedstock itself [28]. The formation of phosphorus salts (calcium phosphate, magnesium ammonium phosphate, and magnesium phosphate) are known to immobilize phosphorus into the hydrochar and this immobilization is influenced by the presence of higher inorganic content of the feedstock (such as the level of Ca, Mg, and others), pH, temperature and additives during HTC.
The element composition of the feedstock, particularly the presence of phosphate precipitating metals (viz., Fe, Al, and Ca) has a higher potential in deciding the phosphate retention in the hydrochar product [34]. During HTC of sewage sludge, the presence of a higher concentration of multivalent metal ions such as Al3+, Ca2+, Fe3+, and Mg2+ are responsible for forming phosphate with low solubility and in turn enabling the phosphate to be retained in subsequently produced hydrochar. However, the previous studies indicated that the treatment using H2SO4 as an additive tends to reduce the level of Ca, Fe, and Mg in hydrochar [33]. Analyzing the conductivity aids in understanding the metal ion concentration in the process-water, and the experimental analysis indicated higher conductivity in the process-water following the use of H2SO4 additives in comparison with other organic acids as additives (see Figure 4). The presence of increasing metal ion concentration can explain the higher level of P immobilization into the process-water, particularly with H2SO4 additives. Nevertheless, despite having relatively higher conductivity following the use of NaOH as an additive, TP concentration in the process-water was comparatively less. One explanation for increased conductivity following the use of alkali additive can be simultaneously induced ionic salts with NaOH additive utilization. The investigated results suggest that HTC of sewage sludge significantly immobilizes phosphorus into hydrochar in all but mineral acid additives. Results are consistent with another study carried out by Ekpo et al. (2016) demonstrating lower TP leaching into the process-water during HTC of swine manure in the presence of CH3COOH, HCOOH, and NaOH as additives.
4. Conclusions
The influence of organic acids, an inorganic acid and alkali as additives on phosphorus mobilization, energy value, yield, and dewaterability by hydrothermally carbonizing sewage sludge was analyzed. Phosphorus extraction into the process-water is pH-dependent and particularly significant in the presence of inorganic acid (H2SO4). The use of H2SO4 and NaOH as additives has decreased the FC content of produced hydrochar, which negatively influences the heating value of the consequently produced hydrochar. A relatively higher reduction in the binding force of the sludge particles was observed during HTC using H2SO4 in the reaction medium; this, in turn, improved the hydrochar dewatering performance in comparison with other additives. In conclusion, if the HTC of sewage sludge is designated to leach the phosphorus into the process water, the use of inorganic acid at a higher concentration is favorable; however, compromises will be made in the fuel characteristic of the hydrochar.
Author Contributions
Conceptualization, V.S.E. and S.N.; investigation, V.S.E.; writing—original draft preparation, V.S.E.; writing—review and editing, V.S.E., S.N., J.S., and M.N.; supervision, S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was funded by the German Research Foundation (DFG) and the Open Access Publication Fund of the University of Rostock.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Ekanthalu, V.S.; Morscheck, G.; Narra, S.; Nelles, M. Hydrothermal Carbonization—A Sustainable Approach to Deal with the Challenges in Sewage Sludge Management. Urban Min. Sustain. Waste Manag. 2020, 293–302. [Google Scholar]
- Statistisches Bundesamt Destatis. Abwasserbehandlung—Klärschlamm. 2020. Available online: https://www.destatis.de (accessed on 15 October 2020).
- Kreislaufwirtschaftsgesetz—KrWG. Gesetz zur Förderung der Kreislaufwirtschaft und Sicherung der Umweltverträglichen Bewirtschaftung von Abfällen; Bundesministeriums der Justiz und für Verbraucherschutz Sowie des Bundesamts für Justiz: Aachen, Germany, 2017. [Google Scholar]
- AbfKlär, V. Verordnung über die Verwertung von Klärschlamm, Klärschlammgemisch und Klärschlammkompost; Bundesministeriums der Justiz und für Verbraucherschutz sowie des Bundesamts für Justiz: Aachen, Germany, 2017. [Google Scholar]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Xu, B.; Li, C.; Zeng, G. Feedwater pH affects phosphorus transformation during hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2017, 245, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Crocker, M. Thermo Chemical Conversion of Biomass to Liquid Fuels and Chemicals; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Saetea, P.; Tippayawong, N. Recovery of Value-Added Products from Hydrothermalc Arbonization of Sewage Sludge; Hindawi Publishing Corporation: London, UK, 2013. [Google Scholar]
- Stucki, M.; Eymann, L.; Gerner, G.; Hartmann, F.; Wanner, R.; Krebs, R. Hydrothermal carbonization of sewage sludge on industrial scale: Energy efficiency, environmental effects and combustion. J. Energy Chall. Mech. 2015, 2, 38–44. [Google Scholar]
- Leng, L.; Yuan, X.; Huang, H.; Shao, J.; Wang, H.; Chen, X.; Zeng, G. Bio-char derived from sewage sludge by liquefaction: Characterization and application for dye adsorption. Appl. Surf. Sci. 2015, 346, 223–231. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Liu, Q. Fate and distribution of nutrients and heavy metals during hydrothermal carbonization of sewage sludge with implication to land application. J. Clean. Prod. 2019, 225, 972–983. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Vasquez, V.R.; Coronella, C.J. Effect of salt addition on hydrothermal carbonization of lignocellulosic biomass. Fuel 2012, 99, 271–273. [Google Scholar] [CrossRef]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Arauzo, P.J.; Becker, G.С.; Kruse, A. Experimental and thermodynamic studies of phosphate behavior during the hydrothermal carbonization of sewage sludge. Sci. Total. Environ. 2019, 692, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Liu, X.; Zhu, Y.; Peng, C.; Wang, T.; Zhu, L.; Li, C.; Zeng, G. Hydrothermal carbonization of sewage sludge: The effect of feed-water pH on fate and risk of heavy metals in hydrochars. Bioresour. Technol. 2016, 218, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhai, Y.; Li, S.; Wang, B.; Wang, T.; Liu, Y.; Qiu, Z.; Li, C. Hydrothermal carbonization of sewage sludge: Effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J. Hazard. Mater. 2020, 388, 122084. [Google Scholar] [CrossRef] [PubMed]
- Lenntech. Phosphorous Removal from Wastewater. Available online: https://www.lenntech.com/phosphorous-removal.htm (accessed on 25 January 2021).
- TerraNova. The TerraNova® Ultra–Process. Available online: https://terranova-energy.com/en/project/process/ (accessed on 27 January 2021).
- UBC Sustainable Cities Commission. Enhanced Nutrient Removal with Bio-Filter at Rostock WWTP, Germany. 20 October 2017. Available online: https://www.balticwaterhub.net/solutions/bio-filter-wwtp-rostock (accessed on 20 December 2020).
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Roskosch, A.; Heidecke, P. Sewage Sludge Disposal in the Federal Republic of Germany; German Environment Agency: Dessau-Roßlau, Germany, 2018. [Google Scholar]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gasco, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef]
- Jaruwat, D.; Udomsap, P.; Chollacoop, N.; Fuji, M.; Eiad-Ua, A. Effects of hydrothermal temperature and time of hydrochar from Cattail lea-ves. In Proceedings of the International Conference on Science and Technology of Emerging Materials, Pattaya, Thailand, 18–20 July 2018. [Google Scholar]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Li, X.; Liu, Y. Distribution and transformation behaviors of heavy metals and phosphorus during hydrothermal carbonization of sewage sludge. Environ. Sci. Pollut. Res. 2020, 27, 17109–17122. [Google Scholar] [CrossRef]
- Mazumder, S.; Saha, P.; Reza, M.T. Co-hydrothermal carbonization of coal waste and food waste: Fuel characteristics. Biomass Convers. Biorefinery 2020, 1–11. [Google Scholar] [CrossRef]
- Putra, H.E.; Damanhuri, E.; Dewi, K.; Pasek, A.D. Hydrothermal carbonization of biomass waste under low temperature condition. In Proceedings of the 2nd International Conference on Engineering and Technology for Sustainable Development, Yogyakarta, Indonesia, 18–20 December 2018. [Google Scholar]
- Imran, A.M.; Widodo, S.; Irvan, U.R. Correlation of fixed carbon content and calorific value of South Sulawesi Coal, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 473, 012106. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Fletcher, L.A. Influence of pH on hydrothermal treatment of swine manure: Impact on extraction of nitrogen and phosphorus in process water. Bioresour. Technol. 2016, 214, 617–644. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fang, C.; Zhang, B.; Tang, Y. Transformations of phosphorus speciation during (hydro) thermal treatments of animal manures. Environ. Sci. Technol. 2018, 52, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).