Abstract
Discharge from sewage treatment plants (STPs) is a significant pathway of entry for microplastics (MPs) to the environment. Therefore, STPs should be considered as an important barrier to the distribution and circulation of MPs in the aquatic environment. In this study, the fate and material-specific properties of MPs were investigated in an STP-equipped and granule-activated carbon (GAC) tower with a thermal regeneration system. This system functioned with a tertiary treatment unit. The GAC with thermal regeneration removed 92.8% of MPs and was useful for removing MPs with a specific gravity less than that of water and with a size of 20–50 µm, which had negligible removal in the conventional STP process. In addition, a lab-scale electric-coagulation experiment was conducted to examine its potential utility as a pretreatment process for further enhancing the removal efficiency of MPs by GAC. After 30 min of electro-coagulation using aluminum electrodes, 90% of MPs were converted into separable flocs by centrifugation. These flocs may be effectively removed by GAC or other tertiary treatment steps. This study demonstrates that GAC with thermal regeneration is a tertiary process that can efficiently prohibit the release of MPs from STPs and circulation of MPs in the natural environment.
1. Introduction
Microplastics (MPs) in the aquatic environment are of increasing concern because they pose a threat to the aquatic ecosystem and human population [1]. Depending on the requirements of the final product, polymers can be mixed with different additives to enhance their performance. These include plasticizers, antioxidants, flame retardants, ultraviolet stabilizers, lubricants, and colorants, to customize the characteristics of plastics (e.g., flexibility, strength, resistance to heat, electrical isolation, etc.) [2]. The most common additives used in the fabrication process and found in the microplastic debris collected in environmental surveys are phthalates, bisphenol A, polybrominated diphenyl ethers, and nonylphenols [3]. For wildlife and human beings, ingested plastics can cause internal damage and reduce feeding, disturb the digestive enzyme system and hormone balance, and have an impact on reproduction [4]. MPs may also exhibit a considerable potential to transfer water pollutants to aquatic ecosystems and human health.
Several studies have reported that sewage treatment plants (STPs) are a significant source of MPs found in the environment [5,6]. Although an STP may be the entrance to the water environment for MPs, it can also act as a barrier to MPs entering the water environment. Previous studies reported that 72–98% of MPs in raw wastewater can be removed from STPs by using primary and secondary treatment processes [7,8]. However, an STP serving approximately 650,000 people may release 65,000,000 particles MPs of debris daily [7]. These large quantities of MPs can be challenging to remove by conventional STP processes.
For the past decades, wastewater treatment technologies have been continuously researched to increase the stability of water quality and energy reduction during the operation and maintenance of facilities. However, wastewater treatment technologies have not been specially designed to control micro-pollutants such as MPs [9]. Few studies have indicated that some tertiary technologies, such as microfiltration techniques including membrane bioreactors, micro-screen filtration, and sand filtration, can enhance the MP removal rates compared with conventional STP processes [10]. Bing et al. (2020) reported that the sludge produced from pyrolysis showed significantly decreased MP concentrations. In addition, pyrolysis resulted in a rough surface morphology of the MPs, making it easier to adsorb contaminants [11].
This study proposes an approach to retrofit the existing STPs to enhance MP removal and thereby prevent the circulation of MPs in nature. Here, MP coagulation and granule-activated carbon (GAC) adsorption with thermal regeneration based on pyrolysis is proposed as a tertiary treatment step after primary and biological treatment. Material-specific MP particles in the sewage water were quantified at different treatment stages in an STP, including the influent of the primary settling tank, the final effluent of the secondary settling tank, and the effluent of the GAC tower with thermal regeneration. In addition, electrocoagulation performance was examined as a potential pretreatment process for enhancing MP removal by GAC. To the best of our knowledge, this is the first study to monitor the fate and material-specific properties of MPs in an STP equipped with a GAC tower with thermal regeneration. This unique dataset can provide an improved means to retrofit an existing STP to manage or eliminate the emerging pollutants of concern such as MPs.
2. Materials and Methods
2.1. Sewage Sample Collection
Sewage samples were collected from a domestic sewage treatment plant (STP) located in Inchun, Korea. The untreated sewage sample contained 258.8 ± 27.2 mg L−1 of biochemical oxygen demand (BOD), 134.2 ± 28.1 mg L−1 of suspended solids (SS), 54.4 ± 4.2 mg L−1 of total nitrogen (TN), 53.1 ± 4.2 mg L−1 of total Kjeldahl nitrogen (TKN), 35.3 ± 3.2 mg L−1 of ammonium nitrogen (NH4+-N), 0.21 ± 0.2 mg L−1 of NOx−-N (sum of nitrite and nitrate nitrogen), 6.5 ± 0.4 mg L−1 of total phosphorous (TP), and 203.3 ± 6.0 mg L−1 of alkalinity as CaCO3. It was equipped with pre-, primary, secondary, and tertiary treatment, articulated in screening, grit removal stages, biological treatment (anaerobic–anoxic–aerobic or A2O process), and sedimentation. The annual average inflow rate of the STP was approximately 30,000 m3/day. To assess the characteristics and removal efficiencies of MPs in the plant, water samples were collected at the inlet and outlet of the plant. The collected samples were immediately transported to the laboratory at the Suwon University campus and were filtered using stainless steel sieves (ISO 3310 and ASTM 11, DIN 3310/2) with a mesh size of 5 mm.
2.2. Analytical Methods for Microplastics
Density separation for the MPs in the filtered samples was carried out to optimize MP measurement using FTIR (Figure 1). Each filtered sample (1 L) was mixed in a glass bottle containing 15% hydrogen peroxide (H2O2, 1 L) for 3 d in a laminar-flow hood at room temperature to digest organic matter and to avoid atmospheric contamination of MPs [12]. Subsequently, 1 L of sodium chloride solution (NaCl, 30%) was added to a glass bottle containing the mixed sample with the aim of separating MPs from other particulate matter, such as sediments [13]. The inorganic materials in the samples are usually removed based on density separation using a salt solution. Saturated sodium chloride (NaCl) solution (density: 1.2 kg/L) is commonly used because of its low cost and non-toxicity [14]. The mixture was stirred overnight at 4 °C. The supernatant was then filtered through a 20 μm pore-sized stainless membrane filter (Sungin mesh co, Sus 316 L, pore size, 20 μm, 25 mm in diameter) using a vacuum pump. To remove salt crystals, we put 1 L of MilliQ® ultrapure water into the same filtration apparatus with a vacuum pump. Subsequent filtering was performed using the obtained cellulose membrane filter with the collected MP debris. Following filtration, each membrane filter containing MPs was placed into a glass Petri dish under a laminar-flow hood at room temperature to remove moisture and to avoid atmospheric contamination of the MPs for 7 d. Then, the glass Petri dishes containing membrane filters with MP were wrapped in aluminum foil and stored in a desiccator containing silica gel at 25 °C. From the obtained filters, MPs > 20 μm were quantified and characterized using an FTIR spectrometer Nicolet 6700 (Thermo Fisher Scientific, Waltham, MA, USA) complemented by a Continuum microscope (MCT detector, beam splitter KBr). The band from 4000 to 715 cm−1 was collected, with an applied resolution of 16 cm−1. Each spectrum was an average of 128 scans. Collected data were processed using Omnic software (Thermo Fisher Scientific, USA), and the composition of MP particles was determined by comparing the obtained spectra to a database (Restaurator library, UCT Prague, Praha, Czech Republic), while the match factor threshold was set to 0.80 [14]. For the blank sample analysis to confirm any additional plastic contamination during sample preparation and filtration, blank filters using HPLC-grade water as a sample were prepared and analyzed as a control.
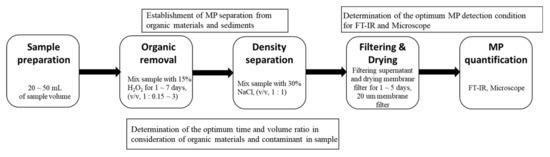
Figure 1.
Procedure of microplastic (MP) analysis in wastewater.
2.3. Pilot Scale Experiments of Microplastics Removal
A pilot-scale facility for the MP removal experiment was constructed and consisted of a GAC tower with a thermal regeneration system that could treat approximately 10 m3/day. The facility was operated with an approximate total mass of 800 kg of GAC in the contact tower. This facility, shown in Figure 2A, was comprised of a GAC tower unit and overheating steam unit to remove MPs and to regenerate GAC. This GAC tower system was set up at the STP where the sewage samples were collected for MP analysis. The 10 m3/day STP effluent was treated in the GAC tower to remove MPs. As needed, back washing was carried out once a month. Thermal regeneration of GAC using an overheating system was performed with the aim of removing MPs adsorbed onto the GAC. As shown in Figure 2A, saturated steam above 100 °C was generated in the steam boiler and converted into overheated steam of 500–700 °C through the line heater. The converted overheated steam was sprayed onto the GAC within the effective radius through the irradiation tube nozzle inside the tower and raised the temperature of the GAC. The moisture of the GAC pore was evaporated by spraying superheated steam at 500–700 °C, and the adsorbed organic matter and MPs were decomposed into water, carbon monoxide or carbon dioxide, and low molecular weight compounds through an oxygen-free low-temperature pyrolysis process by overheated steam. At this time, the undissolved low-molecular-weight organic compounds that had been undecomposed were mixed with condensate water and returned to the STP influent. The steam discharged from the lower part of the GAC regeneration device was cooled by a heat exchanger and then introduced into the condensate water storage tank and returned to the STP influent or reprocessed. For the analysis of MP removal efficiency in the GAC tower with thermal regeneration, samples were collected from the influent and effluent of the GAC tower (GAC inf. and GAC eff.) and condensate water, all of which were filtered using stainless steel sieves (ISO 3310 and ASTM 11, DIN 3310/2) with a mesh of 5 mm and applied to pretreatment for MP analysis as described in Section 2.1 and Section 2.2.
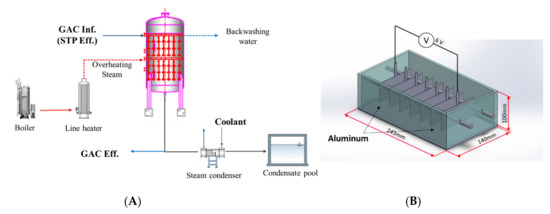
Figure 2.
Experimental setup: (A) pilot-scale granule-activated carbon (GAC) unit; (B) lab-scale device for electro-coagulation.
2.4. Lab Experiments of Microplastics Electro-Coagulation
The experimental electrocoagulation was performed in a lab-scale stirred-tank batch reactor. A schematic diagram of electrocoagulation is presented in Figure 2B. The electrocoagulation reactor consisted of a rectangular reactor (243 mm × 140 mm × 100 mm, 3 L) containing the STP effluent mixed with fluorescent green, spherical microbeads of 38–45 μm. Seven aluminum electrodes (100 mm × 50 mm × 2 mm) were placed parallel along the reactor. The two outermost aluminum electrodes were connected to DC power (6V), which controlled the voltage and electric current. The outermost electrodes were connected to a wire made of copper and DC power. Here, both outermost electrodes were operated as the anode and cathode, respectively. Five unconnected aluminum electrodes located between the outermost electrodes were operated as sacrificial anodes, constructing a bipolar electrode setup. The bipolar configuration was selected because several electrocoagulation investigations reported that aluminum electrodes arranged in bipolar mode appeared to be more efficient in removing solutes [15,16]. To evenly distribute the formed flocs, the reactor was mixed using magnetic stirrers set at 70 rpm. The connections between the powered electrodes were reversed after each experiment to prevent oxide (passive) layers forming on one electrode [17]. The sample of STP eff. (2 L) containing fluorescent green, spherical microbeads of 38–45 μm (2.2 g/L, 1.305 g/cm3) and surfactant (0.3 g/L) were used for the analysis of MP coagulation. Surfactants have been known to evenly form a suspension of microbeads. Their average concentration in domestic sewage is 300 mg/L [18]. After sampling 50 mL from inside the center of the reactor every 10 min for 1 h, the collected samples were centrifuged at 160× g for 5 min to separate settleable polymeric flocs consisting of microbeads and Al(OH)3 from the solution and to wash out microbubbles [19]. This is because anything with a density greater than water sinks faster and anything with a density less than water rises faster when in a centrifuge. The concentration of microbeads remaining in the water after centrifugation was determined by measuring the absorbance at 600 nm. The solutions of microbeads (38–45 μm) with known concentrations were used for calibration.
2.5. Statistical Analysis
To evaluate the significant differences (* p < 0.05, ** p < 0.01) in the MPs between different treatment stages (STP inf., STP eff., and GAC eff.), one-way analysis of variance (one-way ANOVA) was performed with the Fisher’s least significant difference post hoc test. The ANOVA test was performed on the log-transformed MP size data after the log-normality of the MP size data was confirmed using the Kolmogorov–Smirnov normality test. For all statistical analyses, we used SYSTAT 12 statistics software (Chicago, IL, USA).
3. Results and Discussion
3.1. Contamination Control
Blank sample analysis indicated that no MP particles were detected. This infers that the experimental equipment, surrounding air, and HPLC grade water during sample preparation were free of MP contamination. Few fibers were detected from the blank samples: a total of three fibers were found in blank sample 2, but not in blank 1 or 3. It was assumed that the fibers probably originated from the surrounding air or a lab coat during sample preparation. These contamination levels of MPs are lower than or comparable with the results of Talvitie et al. and K. Enders et al. [20,21,22]. As all the fibers detected in the blank sample were non-plastic, assuming lab clothing during laboratory work, the blank samples were thought to be free of MP contamination. For the real samples of the STP, therefore, no corrections on the characterization or quantification of MPs were required.
3.2. Characteristics of Microplastics in the Sewage Treatment Plant
The number concentrations and size distributions of MP particles observed in the influent and effluent of the STP (STP inf. and STP eff.) are displayed in Figure 3A,B, respectively. Seven different MP particles were observed in the untreated sewage sample, including polyethylene (PE), polypropylene (PP), acrylate (AC), polystyrene (PS), polyester (PES), alkyd (AKD), and polytetrafluoroethylene (PTFE). PE was the most abundant MP material class, occupying 72% of the total MP particles, followed by PP (13%), AC (6%), and other polymers with relatively small number fractions (1–4%). The order of the quantity of the MP material class from the sample was assumed to be related to the specific gravity and market share (% of market) of MP raw material. MP particles with a lower specific gravity might be more efficiently delivered with less gravity settling from the source location to the STP through sewer pipes. The specific gravity of each MP particle type were as follows: PE (0.89~0.98 g/cm3), PP (0.83~0.92 g/cm3), AC (1.16~1.20 g/cm3), PS (1.04~1.10), PES (1.24~2.30 g/cm3), AKD (1.24~2.10 g/cm3), and PTFE (2.10~2.30 g/cm3) (Table 1). Here, AKD resins are a segment of a large class of polymers known as polyesters. The market shares of MP classes were as follows: PE (29.5%), PP (18.8%), AC (<1%), PS (7.4%), PES (<1%), AKD (<1%), and PTFE (<1%) [23]. Therefore, specific gravity and market share might be important factors determining the relative abundance of different MP classes in untreated sewage water entering an STP. The materials of the MP particles observed in sewage indicate that MPs were largely originated from daily living supplies, such as personal care products, cosmetics, synthetic cloth washing, packaging films, water bottles and caps, ropes, strapping, paints, utensils, and containers [6,24,25,26,27] The MP particles in the untreated sewage largely varied in their size, ranging from 30.2 to 39,592 µm, with mean and median values of 1051.2 µm and 430.8 µm, respectively (Figure 4B and Table 1).
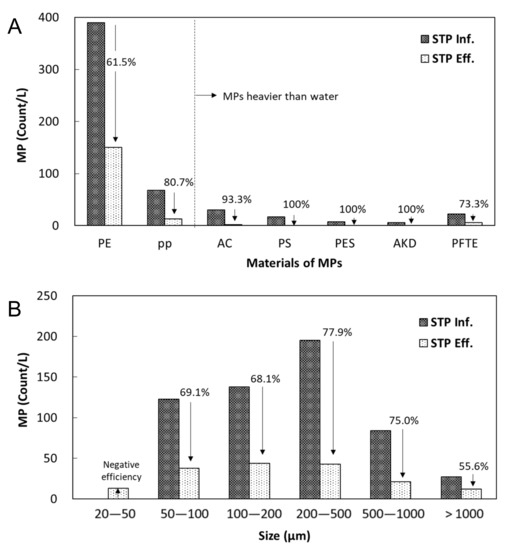
Figure 3.
Number distributions of MP particles in the influent and effluent of the conventional anaerobic–anoxic–aerobic (A2O) system for (A) different materials and (B) different size ranges. PE: Polyethylene, PP: Polypropylene, AC: Acrylate, PS: Polystyrene, PES: Polyester, AKD: Alkyd, PTFE: Polytetrafluoroethylene.

Table 1.
Summary statistics of microplastic sizes at different treatment stages.
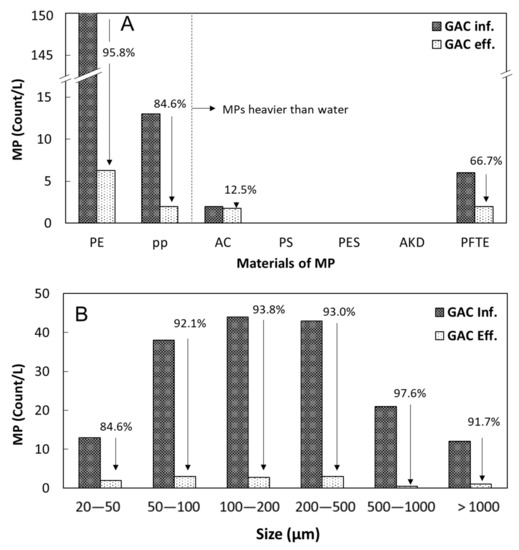
Figure 4.
Removal efficiencies of MP particles by the GAC unit for (A) different materials and (B) different size ranges. PE: Polyethylene, PP: Polypropylene, AC: Acrylate, PS: Polystyrene, PES: Polyester, AKD: Alkyd, PTFE: Polytetrafluoroethylene.
By the primary settling and subsequent biological treatment steps, 68.3% of the total influent MP particles were removed with different removal efficiencies for different MP material classes. MP particles heavier than water, such as AC, PS, PES, and AKD, showed greater than 90% removal rate. However, PE particles showed the least efficient removal (61.5%), which is likely due to their relatively low specific gravity and abundance of small particles, which reduces the efficiency of physical skimming and primary settling, which are important removal mechanisms of MPs in an STP [6,7]. Unlike the behavior of MPs heavier than water, PTFE displayed a relatively low removal efficiency (73.3%) despite its having the highest specific gravity of 2.2 g/cm3. This result is probably related to the PTFE size of less than 70 µm, which was more than 50% of the total MP particles. The 200–500 µm sized MP particles were higher in their removal efficiency (77.9%) than the ones in a larger or smaller size range (Figure 3B). The comparatively low removal of MP particles larger than 1000 µm (55.6%) could be attributed to the low specific surface area that agglomerates with the activated sludge flocs. Lee and Kim (2018) reported that MP particles smaller than 300 µm consistently showed a greater removal efficiency than larger MP particles from three different biological sewage treatment processes, suggesting an easier adhesion of smaller MPs to activated sludge flocs [28]. The 20–50 µm sized MP particles were hardly removed (negative removal efficiency), probably because of the zeta potential induced on the small MP particles, stabilizing the particles in water [29]. Over 30% of the total MP particles were in the 200–500 µm size range, and the highest removal efficiency in this size range resulted in the decreased mean and median sizes of the MPs after the treatment (Table 1).
3.3. Performance of GAC
3.3.1. Removal Efficiencies of MPs
The number concentrations and size distributions of MP particles observed in the influent and effluent of the pilot-scale GAC unit are displayed in Figure 4A,B, respectively. By GAC treatment, significant additional MP removal was achieved (92.9%). Particularly, GAC treatment was found to be advantageous in removing MPs lighter than water, such as PE and PP as well as MPs in all size ranges, including the 20–50 µm sized MPs, which were rarely removed by the primary and biological treatment. With regard to MP removal efficiency, similar results were obtained for the DWTP (drinking water treatment plant) equipped with the GAC filtration system (88% cumulative removal) [30]. Considering the size distribution and material classes of 20–50 µm sized MPs in oceans and effluent of STPs, the majority (64%) of MP particles detected from the Atlantic Ocean were under 40 μm and 70% of MP particles in the STP effluent were in the range of 20–100 μm. Moreover, the most abundant MPs in both ocean and STP eff. were PE and PP [9,20,22]. However, the removal efficiency of the MPs in the smallest size range (20–50 µm; 84.6%) was slightly lower than those of the MPs in the larger size ranges (91.7–97.6%). As shown in Table 1, the mean and median MP sizes decreased after GAC treatment because the MP removal increased as the MP size increased up to 1000 µm. The ANOVA test (Figure 5) showed a significant decrease in the average size across all MP classes after GAC treatment as compared with the MPs in the untreated sewage (p < 0.01 between STP influent and GAC effluent), although the differences in the MP size between two consecutive treatment stages (e.g., STP influent vs. STP effluent and GAC influent vs. GAC effluent) were statistically less significant. Interestingly, the mean and median sizes of PE and PP particles increased after GAC treatment because of the smaller removal efficiency of the larger PE or PP particles in the GAC unit. The lower removal efficiency of larger MPs lighter than water might be attributed to the buoyant force of the particles, which can probably reduce the bulk and internal diffusion into the GAC. Pivokonský et al. (2020) and Wang et al. (year) reported that 73.7–98.5% of MPs removed by GAC filtration had particles ranging from 1 to 5 μm in size, and GAC filtration exhibited a high removal efficiency for MPs with different compositions; the removal efficiency of PE was 72.9–86.2%, followed by PP (around 59.4–66.8%) and polyacrylamide (PAM, around 49.0–54.6%), all of which were similar to the results of lower removal efficiency of the larger MPs [30,31]. Their results indicated that the reduction of small-size MPs was mainly responsible for the removal of MPs in GAC filtration.
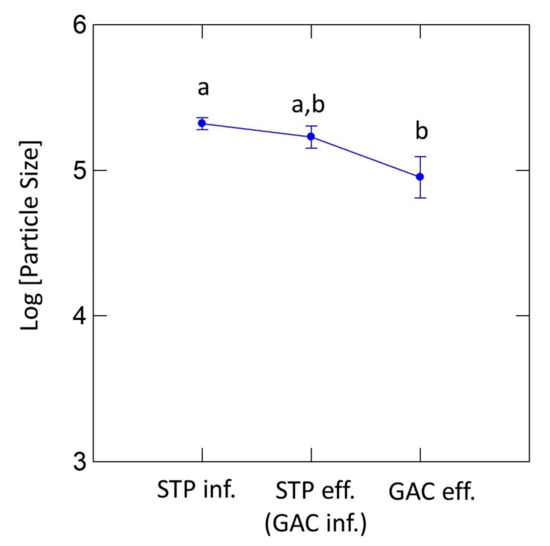
Figure 5.
Size distributions of MP particles at different stages. Different letters indicate significant differences in MP sizes (one-way ANOVA with a least significant difference (LSD) post hoc test). Error bars indicate 1 standard deviation.
3.3.2. Effect of Thermal Regeneration with Steam
Figure 6 compares the MP removal performance of the virgin and regenerated GACs. The performance of the regenerated GAC was comparable with or even better than that of the virgin GAC. The removal efficiencies of MP particles were 92.9% and 93.8% for the virgin and regenerated GACs, respectively. Thermal regeneration is known to be effective in gasifying the organics adsorbed on GAC, recovering the adsorption capacity [32]. In addition, thermal regeneration with steam was reported to be more effective at regenerating micropore volume compared with that of nitrogen, and therefore adsorbing larger molecular size compounds [33]. Thus, the thermal regeneration with steam used in this study presumably had a negligible effect in deteriorating the GAC adsorption capacity for MP particles that were greater than 20 µm. The number concentrations of MPs in GAC inf., GAC, eff., and condensate are compared in Figure 6. The number concentration was 185 MP particles/L, 13 MP particles/L, and 0.023 MP particles/L for GAC inf., GAC, eff., and condensate, respectively, which indicates that the MP particles captured by GAC were effectively decomposed by the pyrolysis process containing steam (600 °C) during the GAC regeneration step. Here, the condensate was water discharged from the GAC after thermal regeneration, such as pyrolysis. Previous studies on the pyrolysis of plastics contained in STP sludge reported that many polymers, such as PE, PP, PS, and PA, started to decompose at 375–500 °C [11,34]. However, it should be noted that there is no other study in the literature using GAC thermal regeneration for the removal of MPs. The results of the current study suggest the effectiveness of the GAC with thermal generation as a plausible method for permanently removing MPs from STPs, which otherwise can enter the geo-environmental cycle. However, an investigation of the performance evolution with repeated operation–regeneration cycles and of the lifespan and long-term performance of the GAC remains for future studies to estimate.
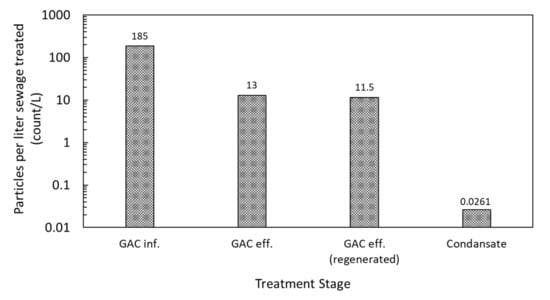
Figure 6.
Number of MP particles in the wastewater with different stages of GAC treatment. Values are averages of two independent experiments.
3.4. Electro-Coagulation of Microbeads
The significant size reduction in the GAC-treated MPs suggests the need to enhance the removal of smaller MPs that are more stable in water [29]. In addition, GAC adsorption of MPs lighter than water was less efficient than that of MPs heavier than water. Thus, a preliminary electrocoagulation experiment was conducted to examine its potential use as an effective pretreatment method for enhancing the removal of smaller and lighter MPs in the subsequent GAC unit. From the electrocoagulation experiment, coagulation of the microbeads gradually developed and was visually observed as the time elapsed (light green color in Figure 7A). With regard to Al-based salts in the presence of MPs, the smaller the MP particle size, the higher the removal efficiency, ionic strength, and concentration of natural organic matter (NOM), while the turbidity level had little influence on the removal efficiency of MP particles [35]. The water separability of coagulated MPs was tested using mild centrifugation at 160× g for 5 min, and the results are shown in Figure 7B. After 30 min of electrocoagulation, inseparable MPs decreased by 90% with little change thereafter, indicating that the flocculation mechanism predominates between 0 and 30 min. These results suggest that electrocoagulation can be effective in increasing the size and density of MPs, which can be easily removed in the following GAC adsorption process.
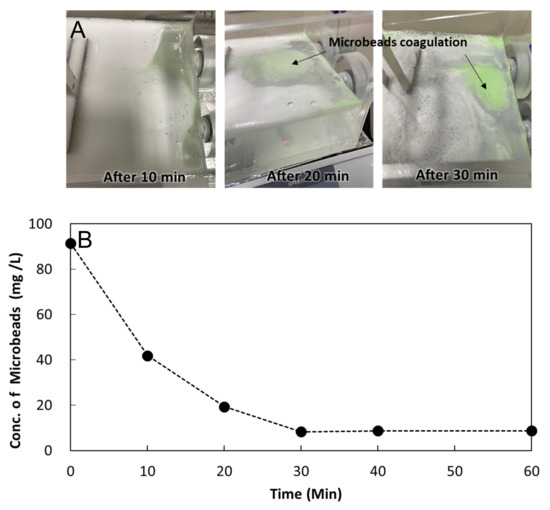
Figure 7.
Results of the electro-coagulation experiment: (A) progress of microbeads aggregation; (B) concentration of the inseparable microbeads by centrifugation with time elapsed. Each data point is the mean value from the duplicate experiments.
3.5. MP Balance in the Suggested Treatment Process
In an STP, MP particles may be resistant to the degradation process that occurs in biological treatment systems and could be part of the chemical that is returned to the environment. For the suggested sewage treatment system in this study, MPs may be discharged as a component of waste sludge from biological processes and volatile components of pyrolysis from GAC thermal generation. Based on the experimental results of the GAC adsorption, the mass balances of MPs in the suggested STP system consisting of primary settling, biological treatment, and GAC adsorption with thermal regeneration were calculated using the MP particles of STP inf., GAC inf., GAC eff., condensate effluent, and corresponding flow data (Figure 8, Table 2). For an STP treating 30,000 m3/d, the total number of MP particles in the STP inf., GAC inf., GAC eff., and condensate effluent were estimated to be 16,200 MP particles/d, 5130 MP particles/d, 369 MP particles/d, and 61 MP particles/d, respectively. Considering the MP particles removed by pyrolysis of GAC thermal regeneration with a removal efficiency of 92.8%, it was estimated that 4700 MP particles/d could be volatilized or converted into other sizes and materials of MPs. Moreover, MP particles generated from the condensate water during the regeneration process, a total of 61 MP particles/d, are predicted to enter the regeneration GAC process, which is a substantial reduction compared with the one from the conventional primary and biological treatment (5130 MP particles/d). In contrast, 11,070 MP particles/d were expected to be discharged to the environment through sludge disposal. The results indicate that the GAC thermal regeneration system could efficiently reduce MPs from the STP retrofitted with the GAC treatment process. Although existing studies on the removal of MPs from the GAC thermal regeneration system are rare, pyrolysis similar to GAC thermal regeneration systems is recognized as a promising sludge treatment technology for removing MPs in an STP [11]. For the removal of MPs in an STP, the major advantages of the GAC thermal regeneration system include the mechanism of filtering and pyrolysis.
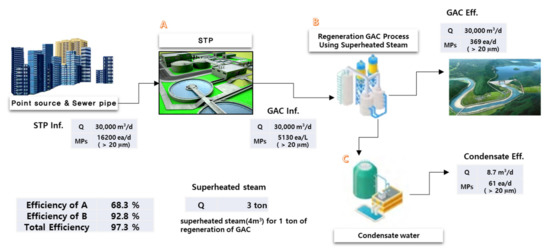
Figure 8.
Estimated mass flow of MPs in the suggested sewage treatment system (based on Q = 30,000 m3/d).

Table 2.
Mass balance equations for MPs at suggested sewage treatment system.
4. Conclusions
Discharge from an STP is an important pathway for microplastics to enter water supply systems and the environment. Hence, with a better understanding of MP characteristics, appropriate treatment technologies should be developed to prohibit the introduction and circulation of microplastics in the environment. Currently, membrane biological reactor (MBR)-based methods are the most well-known methods for MP removal, although these methods have the disadvantages of high costs for installation and operation as well as short life spans. In this regard, this study provides the first insight into the effectiveness of microplastic control in an STP that uses a tertiary treatment of GAC, which can remove more than 92% of the total MP particles entering the system. Moreover, the results show that GAC treatment with thermal regeneration can be more effective in removing microplastics in sizes ranging from 20 to 50 µm than a conventional STP, which is not efficient in removing microplastics smaller than 200 µm. On the other hand, electric coagulation could enhance the removal efficiency within 30 min of coagulation. Electrocoagulation was found to be a good choice to enhance the removal efficiency of microplastic particles by increasing the size and density of microplastics. Therefore, a combination of conventional biological treatment, electrocoagulation, and GAC with thermal regeneration is suggested as a key candidate system for coping with emerging environmental issues related to micro-hazardous materials such as microplastics.
Author Contributions
Investigation K.T.K. and S.P.; writing—original draft, K.T.K.; writing—review and editing, K.T.K. and S.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Environment Industry & Technology Institute (KEITI) through the Intelligent Management Program for Urban Water Resources Project, funded by the Korea Ministry of Environment (MOE) (grant number 2019002950002).
Data Availability Statement
Due to confidentiality agreements, supporting data can only be made available to bona fide researchers subject to a non-disclosure agreement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Roma, Italy, 2017. [Google Scholar]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.-P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution–Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Mintenig, S.; Int-Veen, I.; Löder, M.G.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Ni, B.-J.; Zhu, Z.-R.; Li, W.-H.; Yan, X.; Wei, W.; Xu, Q.; Xia, Z.; Dai, X.; Sun, J. Microplastics Mitigation in Sewage Sludge through Pyrolysis: The Role of Pyrolysis Temperature. Environ. Sci. Technol. Lett. 2020. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Marine Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Palahouane, B.; Drouiche, N.; Aoudj, S.; Bensadok, K. Cost-effective electrocoagulation process for the remediation of fluoride from pretreated photovoltaic wastewater. J. Ind. Eng. Chem. 2015, 22, 127–131. [Google Scholar] [CrossRef]
- Kobya, M.; Ulu, F.; Gebologlu, U.; Demirbas, E.; Oncel, M.S. Treatment of potable water containing low concentration of arsenic with electrocoagulation: Different connection modes and Fe–Al electrodes. Sep. Purif. Technol. 2011, 77, 283–293. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef]
- Tomczak-Wandzel, R.; Dereszewska, A.; Cytawa, S.; Medrzycka, K.; Swarzewo, W.T.P. The effect of surfactants on activated sludge process. In Proceedings of the Polish-Swedish-Ukrainian Seminar, Stockholm, Sweden, 23–25 September 2009; pp. 73–80. [Google Scholar]
- Ferrante, E.A.; Pickard, J.E.; Rychak, J.; Klibanov, A.; Ley, K. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J. Control. Release 2009, 140, 100–107. [Google Scholar] [CrossRef]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater? A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef]
- Sundt, P.; Schulze, P.-E.; Syversen, F. Sources of Microplastic-Pollution to the Marine Environment; Mepex: Asker, Norway, 2014. [Google Scholar]
- Mark, A.; Phillip, C.; Stewart, J. Accumulations of Micro Plastic on Shorelines Worldwide: Sources and Winks. Computer Aided Optimum Design of Structures VIII. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar]
- Kershaw, P. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; International Maritime Organization: London, UK, 2015; pp. 1020–4873. [Google Scholar]
- Lassen, C.; Hansen, S.F.; Magnusson, K.; Hartmann, N.B.; Jensen, P.R.; Nielsen, T.G.; Brinch, A. Microplastics: Occurrence, Effects and Sources of Releases to the Environment in Denmark; Danish Environmental Protection Agency: Copenhagen, Denmark, 2015.
- Prata, J.C. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar. Pollut. Bull. 2018, 129, 262–265. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Kleinberg, K.A. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem. Eng. J. 2020, 386, 123807. [Google Scholar] [CrossRef]
- Pivokonský, M.; Pivokonská, L.; Novotná, K.; Čermáková, L.; Klimtová, M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef]
- Lambert, S.D.; Miguel, G.S.; Graham, N.J. Deleterious effects of inorganic compounds during thermal regeneration of GAC: A review. J. Am. Water Work. Assoc. 2002, 94, 109–119. [Google Scholar] [CrossRef]
- San Miguel, G.; Lambert, S.; Graham, N. Thermal regeneration of granular activated carbons using inert atmospheric conditions. Environ. Technol. 2002, 23, 1337–1346. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.-K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).