Preparation of Ophthalmic Microemulsions Containing Lithuanian Royal Jelly and Their Biopharmaceutical Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantitative Determination of 10-HDA
2.3. Moisture Content of RJ
2.4. Determination of pH Values
2.5. Antioxidant Activity of RJ
2.6. Amino Acid Content Determination Using GC-MS
2.7. Formulation of Microemulsions Containing RJ
2.8. Physical Characterization of Microemulsions
2.9. In Vitro Release Test Determining the Amount of 10-HDA
2.10. SIRC Cell Viability by MTT Assay
2.11. The Short-Time Exposure Test
2.12. Measurement of Incracellular ROS Generation
2.13. 3D Model—Dry Eye Model and Cell Viability
2.14. Statistical Analysis
3. Results
3.1. Quality Evaluation of Lithuanian Royal Jelly
3.2. Amino Acid Content in RJ Determined with GC-MS (Gas Chromatography–Mass Spectrometry)
3.3. Components of Microemulsion Formulations
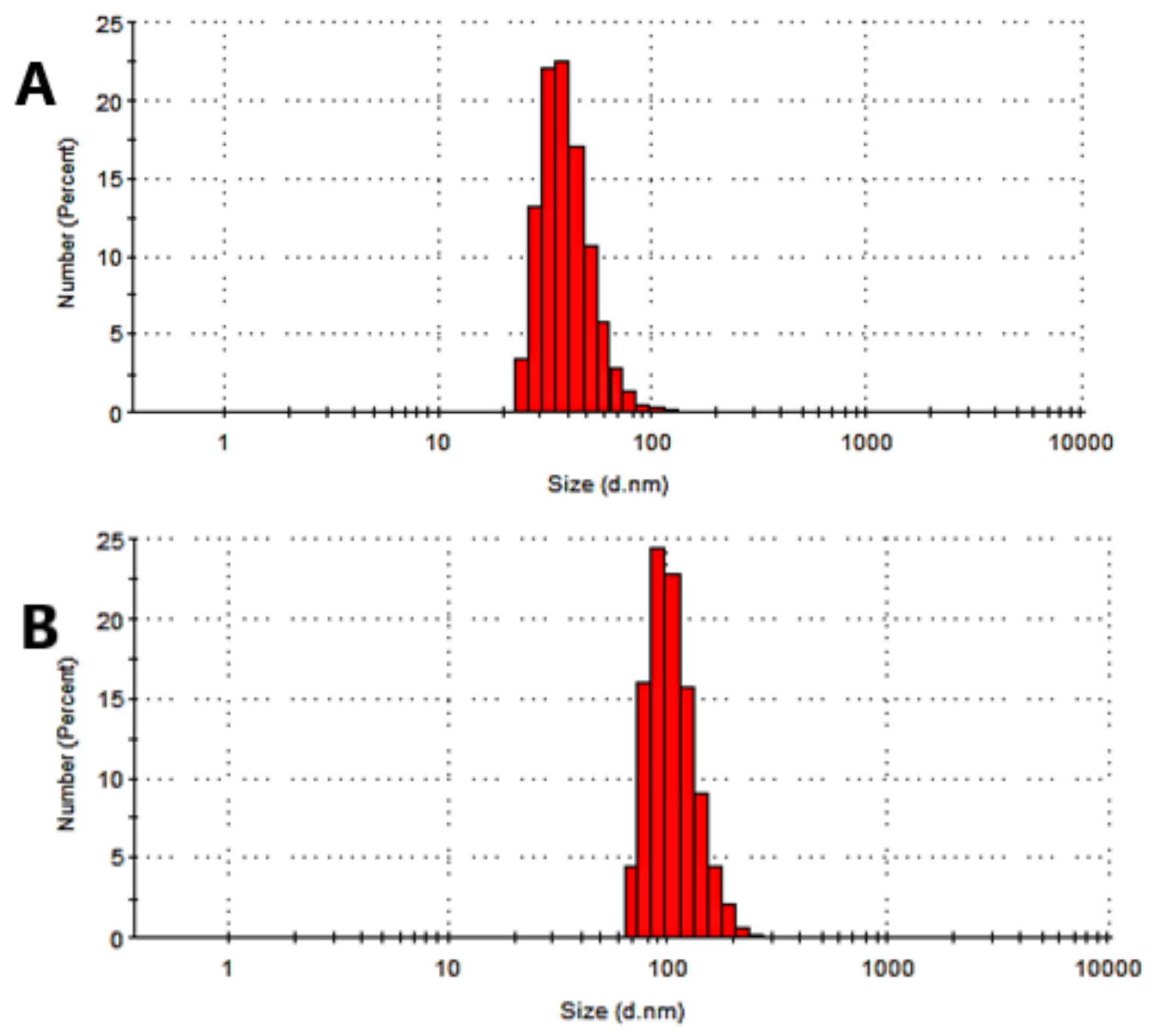
3.4. Physical Characterization of the Microemulsions
3.5. Determination of Physiochemical Parameters of Microemulsions
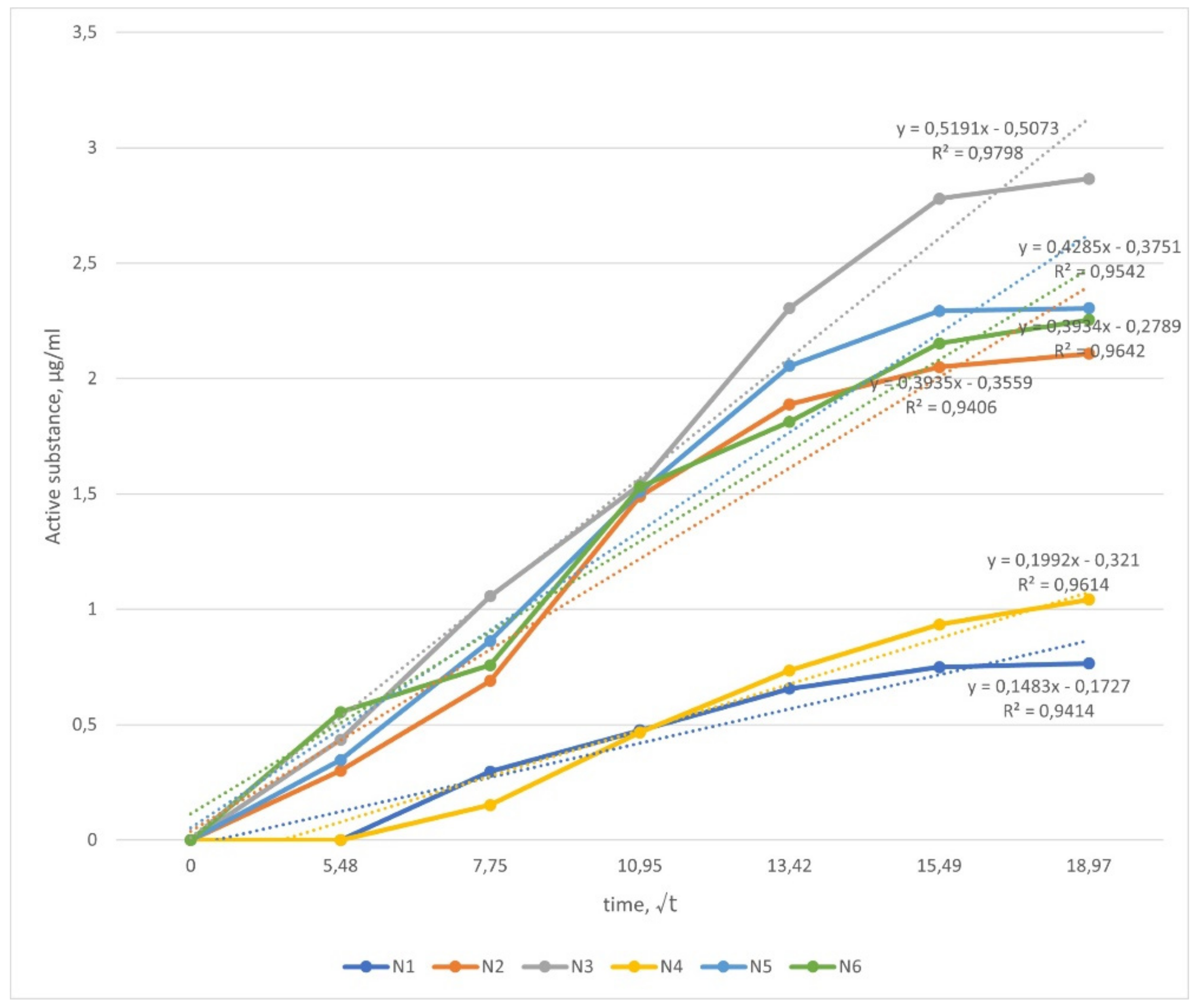
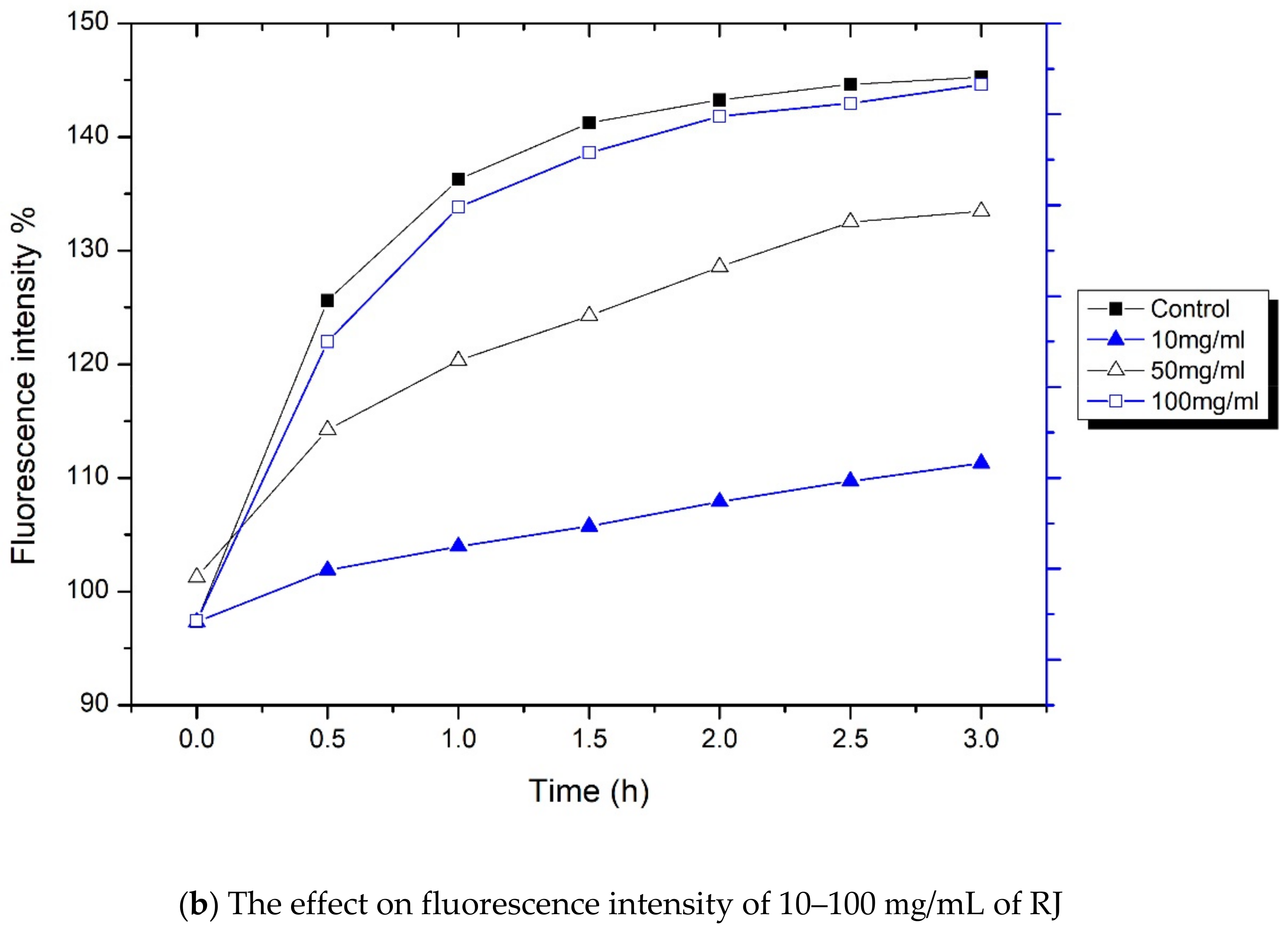
3.6. In Vitro Drug Release Study
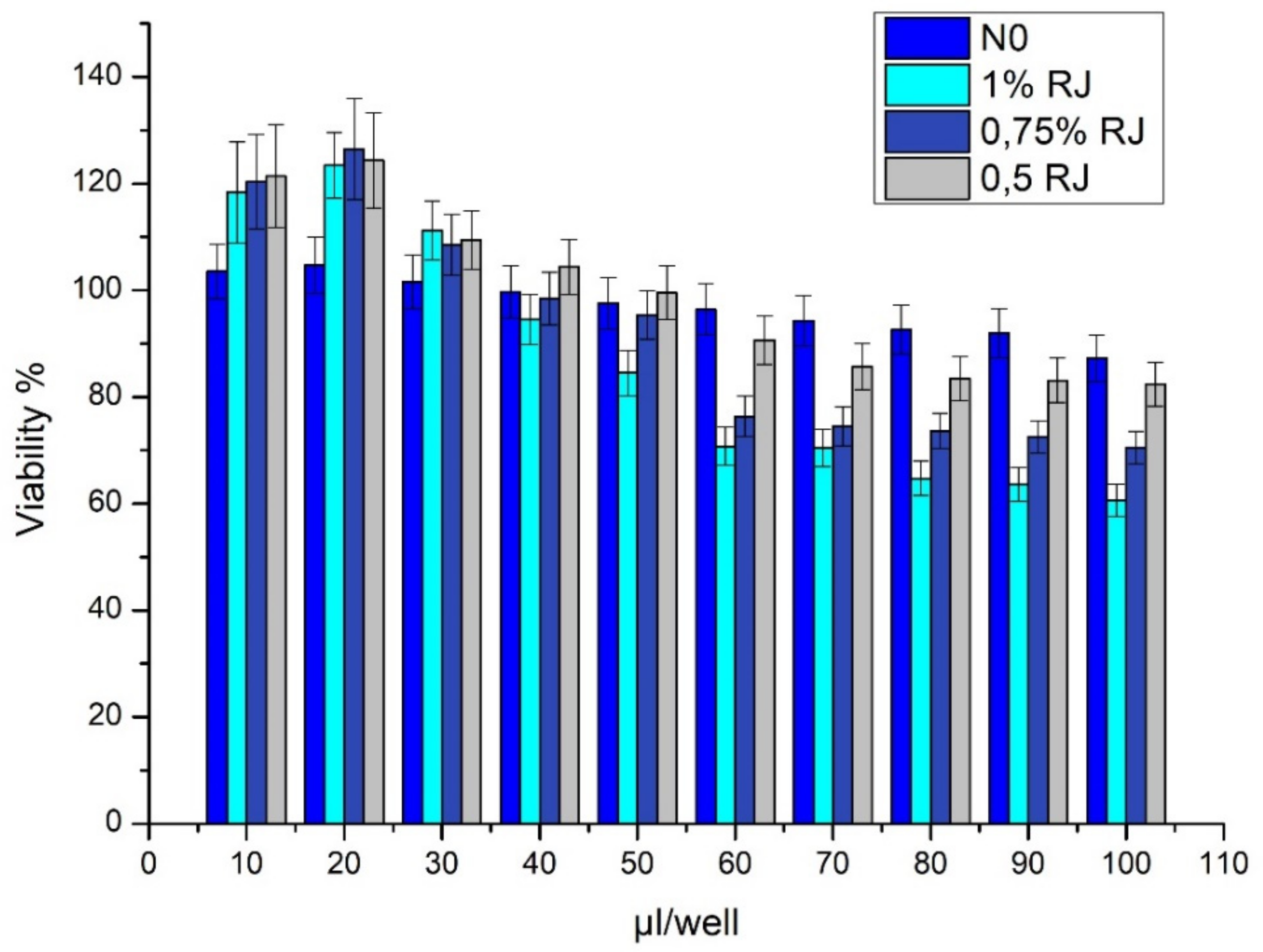
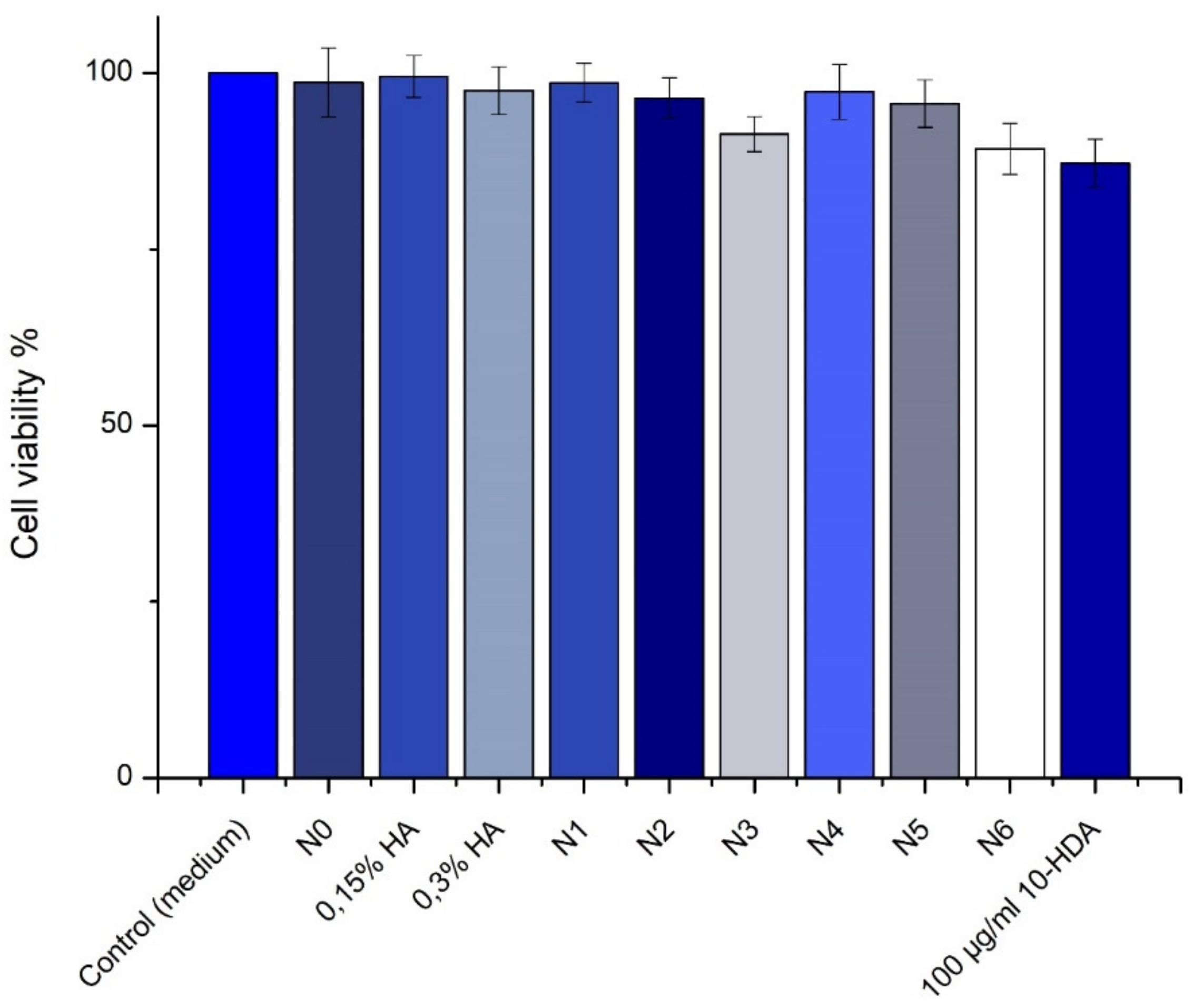
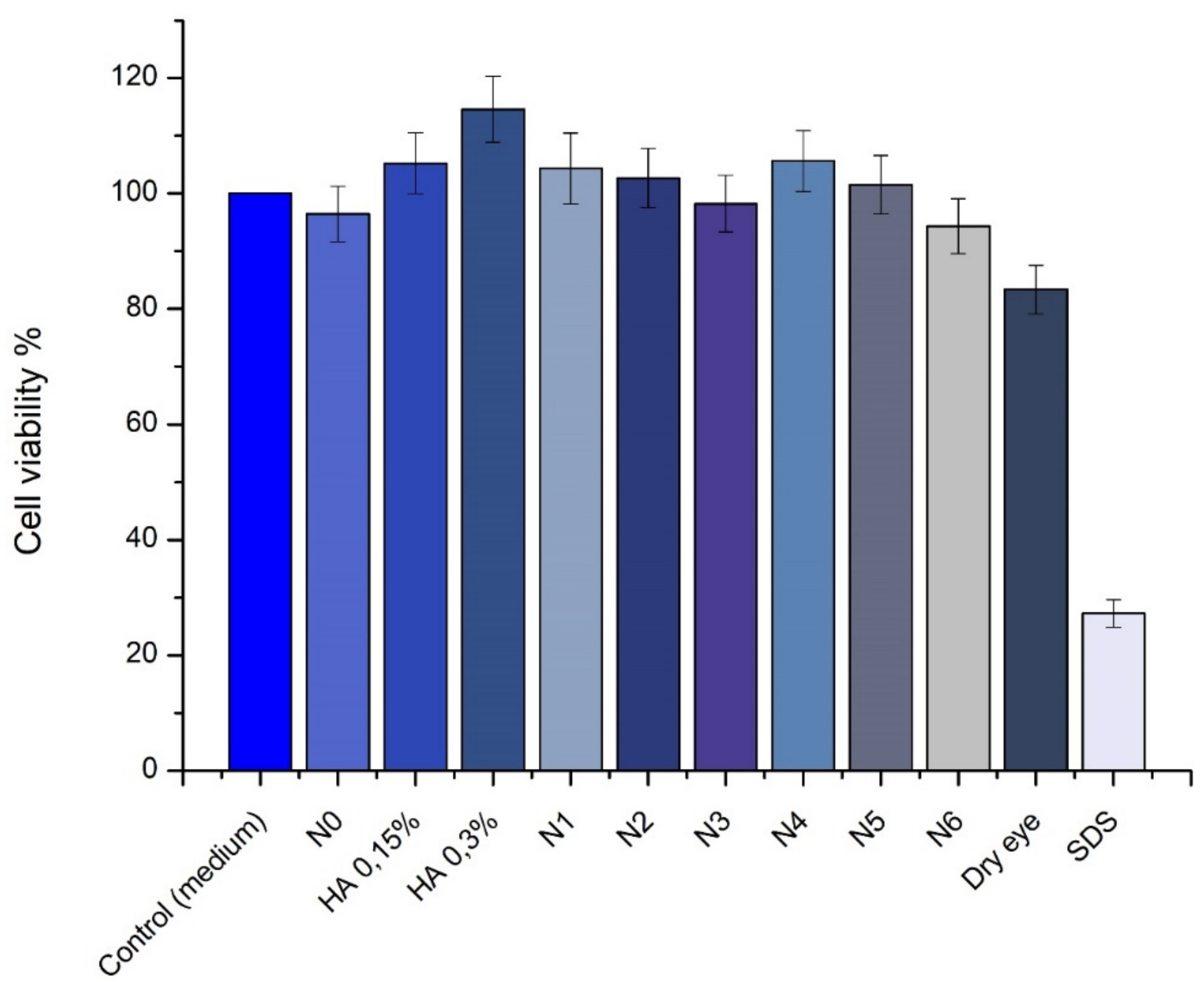
3.7. SIRC Cell Viability Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nebbioso, M.; Del Regno, P.; Gharbiya, M.; Sacchetti, M.; Plateroti, R.; Lambiase, A. Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders. Int. J. Mol. Sci. 2017, 18, 1764. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Rania, L.; Roszkowska, A.M.; Spinella, R.; Postorino, E.; Puzzolo, D.; Micali, A. Effects of amino acids enriched tears substitutes on the cornea of patients with dysfunctional tear syndrome. Acta Ophthalmol. 2013, 91, e437–e444. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Molina-Leyva, I.; Bueno-Cavanillas, A. Efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids in dry eye syndrome: A systematic review of randomized clinical trials. Acta Ophthalmol. 2017, 95, e677–e685. [Google Scholar] [CrossRef]
- Atalay, K.; Cabuk, K.S.; Kirgiz, A.; Caglar, A.K. Treatment of corneal alkali burn with chestnut honey, royal jelly, and chestnut honey-royal jelly combination. Beyoglu Eye J. 2019, 4, 196–201. [Google Scholar] [CrossRef]
- Gagliano, C.; Papa, V.; Amato, R.; Malaguarnera, G.; Avitabile, T. Measurement of the Retention Time of Different Ophthalmic Formulations with Ultrahigh-Resolution Optical Coherence Tomography. Curr. Eye Res. 2018, 43, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Stepanski, M.; Alzhrani, R.M.; Jung, R.; Boddu, S.H. Development and Evaluation of a Novel Microemulsion of Dexamethasone and Tobramycin for Topical Ocular Administration. J. Ocul. Pharmacol. Ther. 2018, 34, 312–324. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Almasi, L.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of antimicrobial biopolymer films containing essential oil-loaded microemulsions or nanoemulsions. Food Hydrocoll. 2021, 117, 106733. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, L.; Zhang, W.; Xu, Y.; Suolangzhaxi; Zhi, X.; Cui, E.; Song, X. Microemulsion can improve the immune-enhancing activity of propolis flavonoid on immunosuppression and immune response. Int. J. Biol. Macromol. 2014, 63, 126–132. [Google Scholar] [CrossRef]
- Vandamme, T. Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog. Retin. Eye Res. 2002, 21, 15–34. [Google Scholar] [CrossRef]
- Ashraf, O.; Nasr, M.; Nebsen, M.; Said, A.M.A.; Sammour, O. In vitro stabilization and in vivo improvement of ocular pharmacokinetics of the multi-therapeutic agent baicalin: Delineating the most suitable vesicular systems. Int. J. Pharm. 2018, 539, 83–94. [Google Scholar] [CrossRef]
- Prokopenko, Y.; Jakštas, V.; Žvikas, V.; Georgiyants, V.; Ivanauskas, L. Hilic MS/MS determination of amino acids in herbs of Fumaria schleicheri L., Ocimum basilicum L., and leaves of Corylus avellana L. Nat. Prod. Res. 2018, 33, 1961–1963. [Google Scholar] [CrossRef]
- Okur, N. Üstündağ; Çağlar, E. Şefik; Siafaka, P.I. Novel Ocular Drug Delivery Systems: An Update on Microemulsions. J. Ocul. Pharmacol. Ther. 2020, 36, 342–354. [Google Scholar] [CrossRef]
- Cadogan, S.P.; Hahn, C.J.; Rausch, M.H.; Fröba, A.P. Study on the applicability of dynamic light scattering (DLS) to microemulsions including supercritical carbon dioxide-swollen micelles. J. Colloid Interface Sci. 2017, 499, 202–208. [Google Scholar] [CrossRef]
- Perminaitė, K.; Marksa, M.; Ivanauskas, L.; Inkėnienė, A.M.; Grigonis, A.; Ramanauskienė, K. Lithuanian Royal jelly: Quality assessment, biological activity and quantitative determination of trans-10-hydroxy-2-decenoic acid in various solvents. Chemija 2020, 31. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hayashi, T.; Koike, M.; Sakaguchi, H.; Kuwahara, H.; Nishiyama, N. An interlaboratory study of the short time exposure (STE) test using SIRC cells for predicting eye irritation potential. Cutan. Ocul. Toxicol. 2010, 29, 77–90. [Google Scholar] [CrossRef]
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Pan, X.; Wu, C.-Y.; Wu, C. Ocular Cubosome Drug Delivery System for Timolol Maleate: Preparation, Characterization, Cytotoxicity, Ex Vivo, and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2919–2926. [Google Scholar] [CrossRef]
- Kubilienė, L.; Jekabsone, A.; Žilius, M.; Trumbeckaitė, S.; Simanaviciute, D.; Gerbutaviciene, R.; Majiene, D. Comparison of aqueous, polyethylene glycol-aqueous and ethanolic propolis extracts: Antioxidant and mitochondria modulating properties. BMC Complement. Altern. Med. 2018, 18, 165. [Google Scholar] [CrossRef]
- Carter, K.; Lee, H.J.; Na, K.-S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef]
- Abdelkader, H.; Longman, M.R.; Alany, R.G.; Pierscionek, B. Phytosome-hyaluronic acid systems for ocular delivery of L-carnosine. Int. J. Nanomed. 2016, 11, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Naz, T.; Abbas, M.; Nazir, S.; Younas, N.; Majeed, S.; Qureshi, N.; Akhtar, M.N. Chloramphenicol Loaded Microemulsions: Development, Characterization and Stability. Colloid Interface Sci. Commun. 2019, 28, 41–48. [Google Scholar] [CrossRef]
- Lv, F.-F.; Zheng, L.-Q.; Tung, C.-H. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int. J. Pharm. 2005, 301, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Chou, W.-M.; Widowati, D.A.; Lin, I.-P.; Peng, C.-C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Jihong, W.; Xinghuai, S.; Shenghai, Z. Royal jelly Peptide Promotes Retinal Ganglion Cell Survival in Experimental Model of Glaucoma Through Up-regulating BDNF and GDNF. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6581. [Google Scholar]
- Inoue, S.; Kawashima, M.; Hisamura, R.; Imada, T.; Izuta, Y.; Nakamura, S.; Ito, M.; Tsubota, K. Clinical Evaluation of a Royal Jelly Supplementation for the Restoration of Dry Eye: A Prospective Randomized Double Blind Placebo Controlled Study and an Experimental Mouse Model. PLoS ONE 2017, 12, e0169069. [Google Scholar] [CrossRef] [PubMed]
- Antinelli, J.-F.; Zeggane, S.; Davico, R.; Rognone, C.; Faucon, J.-P.; Lizzani, L. Evaluation of (E)-10-hydroxydec-2-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Ferioli, F.; Marcazzan, G.L.; Caboni, M.F. Determination of (E)-10-hydroxy-2-decenoic acid content in pure royal jelly: A comparison between a new CZE method and HPLC. J. Sep. Sci. 2007, 30, 1061–1069. [Google Scholar] [CrossRef]
- Balkanska, R.; Zhelyazkova, I.; Ignatova, M. Physico-chemical quality characteristics of royal jelly from three regions of Bulgaria. Agric. Sci. Technol. 2012, 4, 302–305. [Google Scholar]
- Kurth, T.; Kretschmar, S.; Buttstedt, A. Royal jelly in focus. Insectes Soc. 2018, 66, 81–89. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; Almeida-Muradian, L.B. Quality and standardisation of royal jelly. J. Api.Prod. ApiMed. Sci. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Dash, P.; Rath, G.; Ghosh, G. In Vivo Antioxidant Potential of Protein Hydrolysates of some Cucurbitaceae Seed. J. Drug Deliv. Ther. 2020, 10, 128–132. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar] [CrossRef]
- Boselli, E.; Caboni, M.F.; Sabatini, A.G.; Marcazzan, G.L.; Lercker, G. Determination and changes of free amino acids in royal jelly during storage. Apidologie 2003, 34, 129–137. [Google Scholar] [CrossRef]
- Liming, W.; Jinhui, Z.; Xiaofeng, X.; Yi, L.; Jing, Z. Fast determination of 26 amino acids and their content changes in royal jelly during storage using ultra-performance liquid chromatography. J. Food Compos. Anal. 2009, 22, 242–249. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Amino acid composition, antioxidant and functional properties of protein hydrolysates from Cucurbitaceae seeds. J. Food Sci. Technol. 2017, 54, 4162–4172. [Google Scholar] [CrossRef]
- Peyman, G. Ocular Solutions. U.S. Patent No. US7087237B2, 7,087, 8 August 2006. [Google Scholar]
- Kanamoto, T.; Tachibana, T.; Kitaoka, Y.; Hisatomi, T.; Ikeda, Y.; Murakami, Y.; Tobiume, K.; Asaoka, R.; Kiuchi, Y. Effect of Ocular Hypertension on D-β-Aspartic Acid-Containing Proteins in the Retinas of Rats. J. Ophthalmol. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Shunmugaperumal, T.; Sudalaimuthu Ramachandran, S.; Raj, B.; Thenrajan, R.S. Manufacturing techniques and excipients used during the formulation of oil-in-water type nanosized emulsions for medical applications. J. Excip. Food Chem. 2010, 1, 11–29. [Google Scholar]
- Rusciano, D.; Roszkowska, A.M.; Gagliano, C.; Pezzino, S. Free amino acids: An innovative treatment for ocular surface disease. Eur. J. Pharmacol. 2016, 787, 9–19. [Google Scholar] [CrossRef]
- Vinciguerra, P.; Camesasca, F.I.; Ponzin, D. Use of amino acids in refractive surgery. J. Refract. Surg. 2002, 18, S374–S377. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf. B Biointerfaces 2014, 117, 82–88. [Google Scholar] [CrossRef]
- Kamel, O.A.; Youshia, J.; El Shamy, A.; Mansour, S. Design of cationic nanostructured heterolipid matrices for ocular delivery of methazolamide. Int. J. Nanomed. 2012, 7, 2483–2496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hegde, R.R.; Bhattacharya, S.S.; Verma, A.; Ghosh, A. Physicochemical and Pharmacological Investigation of Water/Oil Microemulsion of Non-Selective Beta Blocker for Treatment of Glaucoma. Curr. Eye Res. 2013, 39, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mathis, G.A. Clinical Ophthalmic Pharmacology and Therapeutics: Ocular Drug Delivery. Veterinary Ophthalmology; Lippincott Williams & Wilkins: Orlando, FL, USA, 1999; pp. 291–297. [Google Scholar]
- Radomska-Soukharev, A.; Wojciechowska, J. Microemulsions as potential ocular drug delivery systems: Phase diagrams and physical properties depending on ingredients. Acta Pol. Pharm. Drug Res. 2006, 62, 465–471. [Google Scholar]
- La Gatta, A.; Corsuto, L.; Salzillo, R.; D’Agostino, A.; De Rosa, M.; Bracco, A.; Schiraldi, C. In Vitro Evaluation of Hybrid Cooperative Complexes of Hyaluronic Acid as a Potential New Ophthalmic Treatment. J. Ocul. Pharmacol. Ther. 2018, 34, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Moulik, S.P.; Rakshit, A.K. Physicochemisty and applications of microemulsions. J. Surf. Sci. Technol. 2006, 22, 159. [Google Scholar]
- Zhu, H.; Chauhan, A. Effect of Viscosity on Tear Drainage and Ocular Residence Time. Optom. Vis. Sci. 2008, 85, E715–E725. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, K.; Kant, S.; Singh, P.N.; Pandit, J.K. Mucoadhesive Chitosan-Coated Cationic Microemulsion of Dexamethasone for Ocular Delivery: In Vitro and In Vivo Evaluation. Curr. Eye Res. 2012, 38, 342–352. [Google Scholar] [CrossRef]
- Okur, N. Üstündağ; Yavaşoğlu, A.; Karasulu, H.Y. Preparation and Evaluation of Microemulsion Formulations of Naproxen for Dermal Delivery. Chem. Pharm. Bull. 2014, 62, 135–143. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Subongkot, T. Development and mechanistic study of a microemulsion containing vitamin E TPGS for the enhancement of oral absorption of celecoxib. Int. J. Nanomed. 2019, 14, 3087–3102. [Google Scholar] [CrossRef]
- Kundu, B.; Sanyal, D.; Basu, D. Physiological and elastic properties of highly porous hydroxyapatite potential for integrated eye implants: Effects of SIRC and L-929 cell lines. Ceram. Int. 2013, 39, 2651–2664. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Siejak, P.; Rezler, R.; Pawlicz, J.; Trzeciak, T.; Jarzębska, M.; Majchrzak, O.; Kaczorek, E.; Kazemian, P.; et al. Aesculus hippocastanum L. as a Stabilizer in Hemp Seed Oil Nanoemulsions for Potential Biomedical and Food Applications. Int. J. Mol. Sci. 2021, 22, 887. [Google Scholar] [CrossRef]
- Soliman, O.A.E.-A.; Mohamed, E.A.; Khatera, N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro–in vivo evaluation. Pharm. Dev. Technol. 2017, 24, 48–62. [Google Scholar] [CrossRef]
- Takahashi, Y.; Koike, M.; Honda, H.; Ito, Y.; Sakaguchi, H.; Suzuki, H.; Nishiyama, N. Development of the short time exposure (STE) test: An in vitro eye irritation test using SIRC cells. Toxicol. Vitr. 2008, 22, 760–770. [Google Scholar] [CrossRef]
- Iwasawa, A.; Ayaki, M.; Niwano, Y. Cell viability score (CVS) as a good indicator of critical concentration of benzalkonium chloride for toxicity in cultured ocular surface cell lines. Regul. Toxicol. Pharmacol. 2013, 66, 177–183. [Google Scholar] [CrossRef]
- Bucolo, C.; Fidilio, A.; Platania, C.B.M.; Geraci, F.; Lazzara, F.; Drago, F. Antioxidant and Osmoprotecting Activity of Taurine in Dry Eye Models. J. Ocul. Pharmacol. Ther. 2018, 34, 188–194. [Google Scholar] [CrossRef]
- Than, A.; Liu, C.; Chang, H.; Duong, P.K.; Cheung, C.M.G.; Xu, C.; Wang, X.; Chen, P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems. Pharmaceutics 2018, 10, 110. [Google Scholar] [CrossRef]
- Aldebasi, Y.H.; Nouh, W.G.; Abdel Atti, N.M.; Bekhit, M.S.; Qureshi, M.A.; Aly, S.M. Comparative pathological studies on the healing effect of natural (Terfezia claveryi) and synthetic (Vigamox) antimicrobials on corneal ulcers in rabbits. J. Pharm. Biomed. Sci. 2012, 2, 66–77. [Google Scholar]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New Findings on Biological Actions and Clinical Applications of Royal Jelly: A Review. J. Diet. Suppl. 2017, 15, 757–775. [Google Scholar] [CrossRef]
- Pandeya, P.R.; Lamichhane, R.; Lee, K.-H.; Kim, S.-G.; Lee, D.-H.; Lee, H.-K.; Jung, H.-J. Bioassay-guided isolation of active anti-adipogenic compound from royal jelly and the study of possible mechanisms. BMC Complement. Altern. Med. 2019, 19, 1–14. [Google Scholar] [CrossRef]



| Sample | pH | Water Content (%) (w/w) | 10-HDA (%) (w/w) | Antiradical Activity (DPPH) (%) |
|---|---|---|---|---|
| RJ | 3.52 ± 0.04 | 60.8 ± 0.31 | 2.67 ± 0.02 | 47.3 ± 3.4 |
| Microemulsion Formulation | RJ (%) | Isopropyl Myristate (Oil Phase), % | Tween 80/Tween 20 (S/CoS), % | Distilled Water (Aqueous Phase), % |
|---|---|---|---|---|
| N0 | - | 5 | 3:1 (28%) | 67 |
| N1 | 0.5 | 5 | 3:1 (28%) | 66.5 |
| N2 | 0.75 | 5 | 3:1 (24%) | 70.25 |
| N3 | 1 | 5 | 3:1(21.5%) | 72.5 |
| N4 | 0.5 | 5 | 2.5:1 (28%) | 66.5 |
| N5 | 0.75 | 5 | 2.5:1 (24%) | 70.25 |
| N6 | 1 | 5 | 2.5:1 (21.5%) | 72.5 |
| ME Formulation | Droplet Size, nm | PDI |
|---|---|---|
| N0 | 57.25 ± 0.463 | 0.106 ± 0.032 |
| N1 | 124.2 ± 0.710 | 0.154 ± 0.033 |
| N2 | 81.35 ± 0.478 | 0.116 ± 0.004 |
| N3 | 81.35 ± 0.824 | 0.154 ± 0.034 |
| N4 | 86.50 ± 0.779 | 0.180 ± 0.020 |
| N5 | 88.54 ± 0.865 | 0.175 ± 0.033 |
| N6 | 67.88 ± 0.777 | 0.160 ± 0.031 |
| Microemulsion Formulation | pH | Viscosity (mPas) | Conductivity (µs/Cm) | Refractive Index |
|---|---|---|---|---|
| N0 N1 | 7.42 ± 0.25 6.80 ± 0.20 | 27.6 ± 0.23 21.2 ± 0.12 | 126.3 ± 0.81 164.8 ± 0.85 | 1.367 ± 0.003 1.332 ± 0.002 |
| N2 | 5.68 ± 0.15 | 20.2 ± 0.17 | 147.5 ± 0.92 | 1.373 ± 0.004 |
| N3 | 6.77 ± 0.24 | 14.7 ± 0.22 | 141.3 ± 0.73 | 1.371 ± 0.003 |
| N4 | 6.66 ± 0.17 | 29.0 ± 0.19 | 147.7 ± 0.82 | 1.377 ± 0.002 |
| N5 | 6.74 ± 0.11 | 21.4 ± 0.21 | 144.5 ± 0.69 | 1.376 ± 0.003 |
| N6 | 6.89 ± 0.23 | 17.5 ± 0.18 | 140.9 ± 0.87 | 1.371 ± 0.005 |
| Formulation | The Amount of 10-HDA in Sample (µg/mL) |
|---|---|
| N1 | 13.753 ± 2.345 |
| N2 | 34.926 ± 3.526 |
| N3 | 44.486 ± 5.423 |
| N4 | 13.852 ± 1.675 |
| N5 | 31.829 ± 2.956 |
| N6 | 42.660 ± 4.635 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perminaite, K.; Marksa, M.; Ivanauskas, L.; Ramanauskiene, K. Preparation of Ophthalmic Microemulsions Containing Lithuanian Royal Jelly and Their Biopharmaceutical Evaluation. Processes 2021, 9, 616. https://doi.org/10.3390/pr9040616
Perminaite K, Marksa M, Ivanauskas L, Ramanauskiene K. Preparation of Ophthalmic Microemulsions Containing Lithuanian Royal Jelly and Their Biopharmaceutical Evaluation. Processes. 2021; 9(4):616. https://doi.org/10.3390/pr9040616
Chicago/Turabian StylePerminaite, Kristina, Mindaugas Marksa, Liudas Ivanauskas, and Kristina Ramanauskiene. 2021. "Preparation of Ophthalmic Microemulsions Containing Lithuanian Royal Jelly and Their Biopharmaceutical Evaluation" Processes 9, no. 4: 616. https://doi.org/10.3390/pr9040616
APA StylePerminaite, K., Marksa, M., Ivanauskas, L., & Ramanauskiene, K. (2021). Preparation of Ophthalmic Microemulsions Containing Lithuanian Royal Jelly and Their Biopharmaceutical Evaluation. Processes, 9(4), 616. https://doi.org/10.3390/pr9040616






