Therapeutic Effect of Cinnamomum osmophloeum Leaf Extract on Oral Mucositis Model Rats Induced by 5-Fluororacil via Influencing IL-1β and IL-6 Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Methods
2.2.1. Animal Preparation
2.2.2. 5-FU and Sodium Pentobarbital Injections
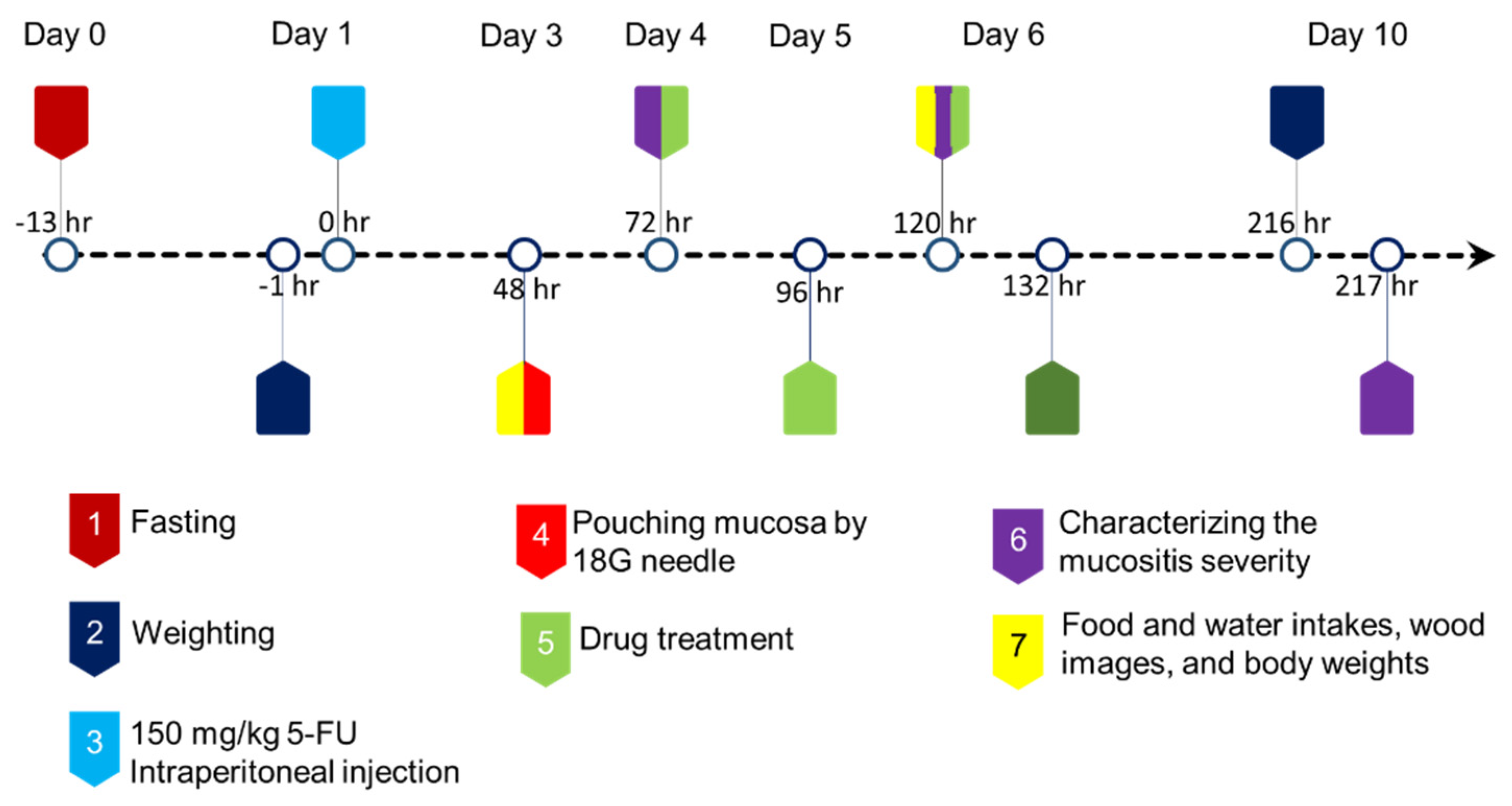
2.2.3. Animal Protocol
2.2.4. Rat Body Weight Changes and Water and Food Intakes
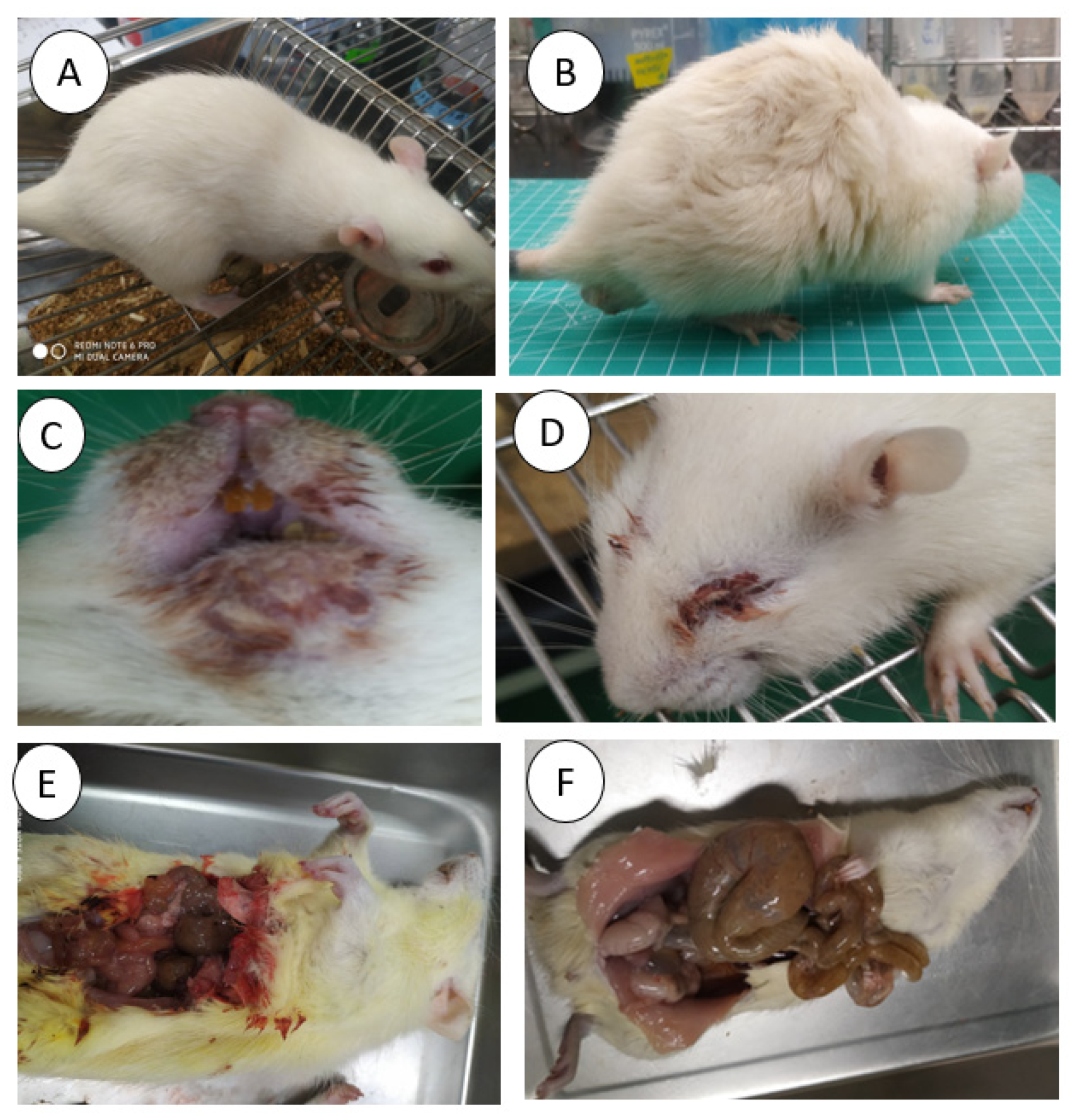
2.2.5. Clinical Evaluation
2.2.6. Histopathologic Examination
2.2.7. Serum Proinflammatory Cytokine Levels by ELISA
2.3. Statistical Analysis
3. Results
3.1. Body Weight Changes of Rats
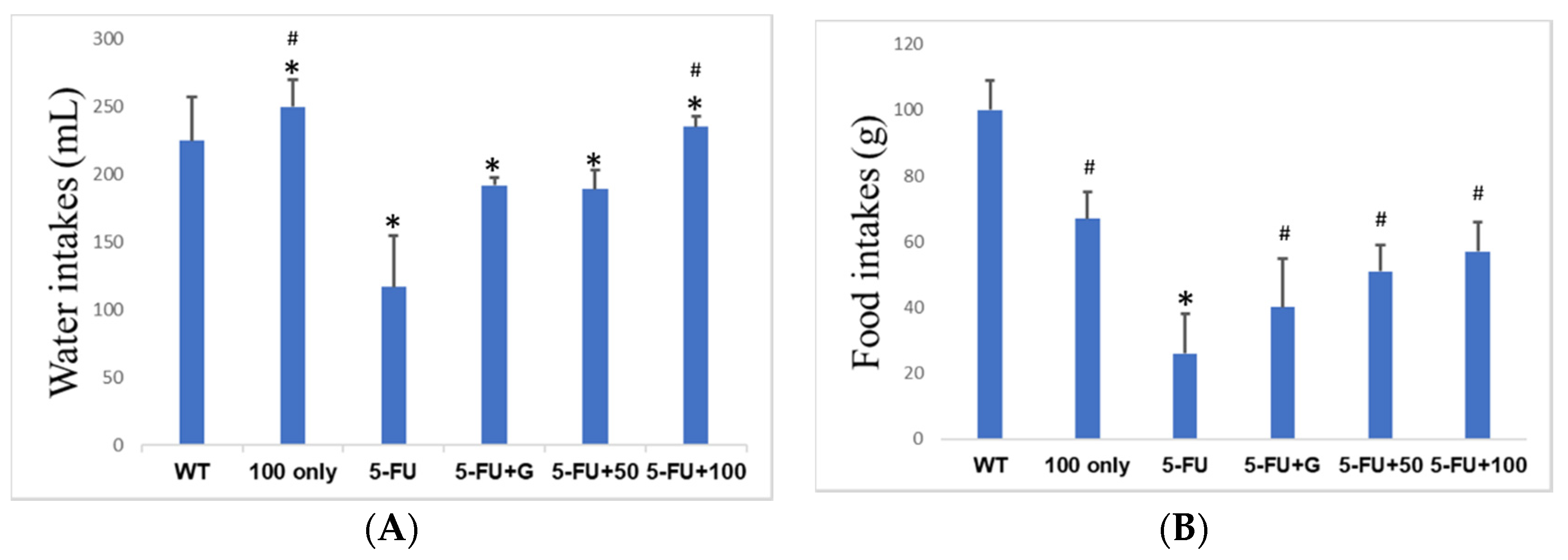
3.2. Food and Water Intakes
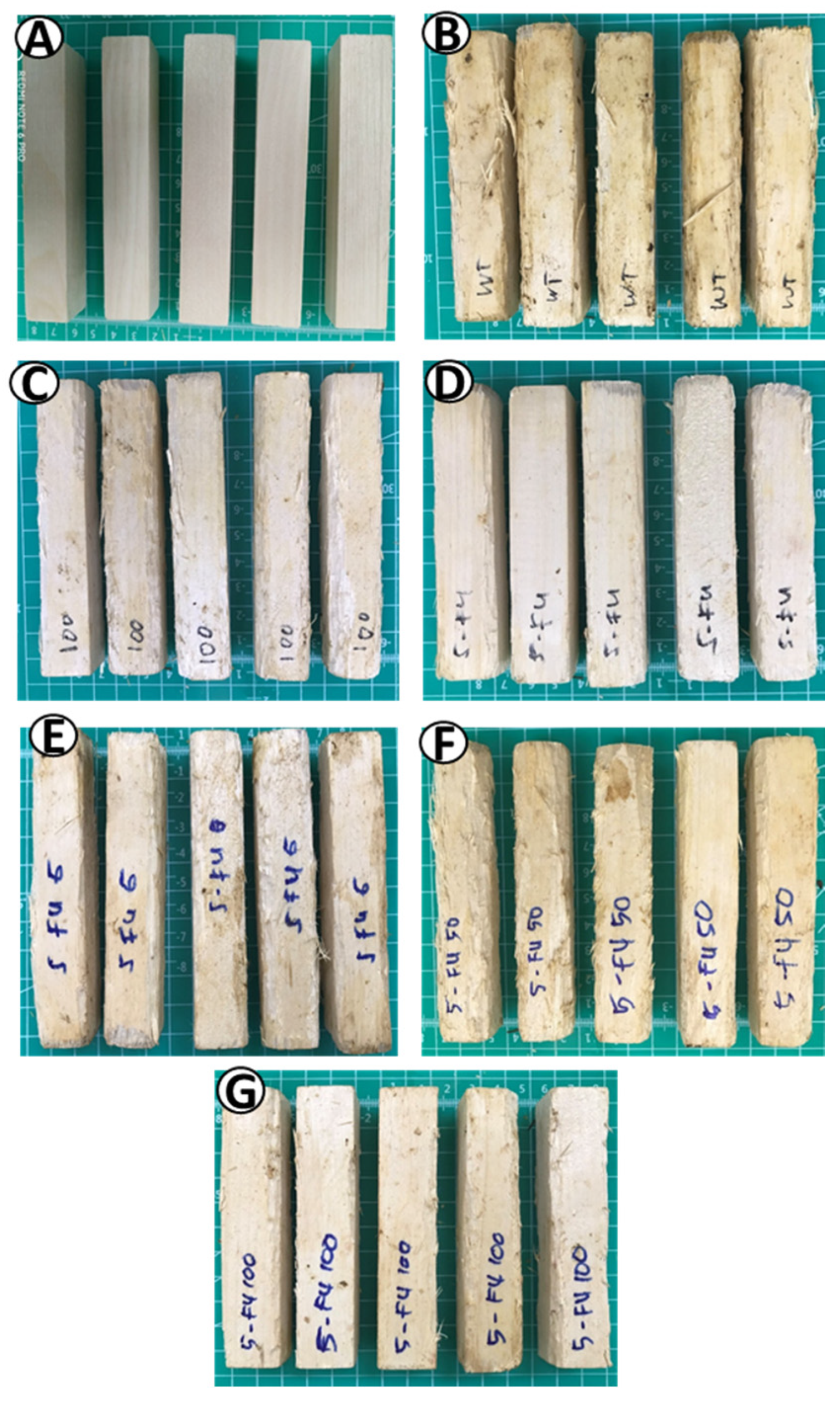
3.3. Wood Appearances
3.4. OM Clinical Appearances
3.5. Histopathology of Oral Mucosa
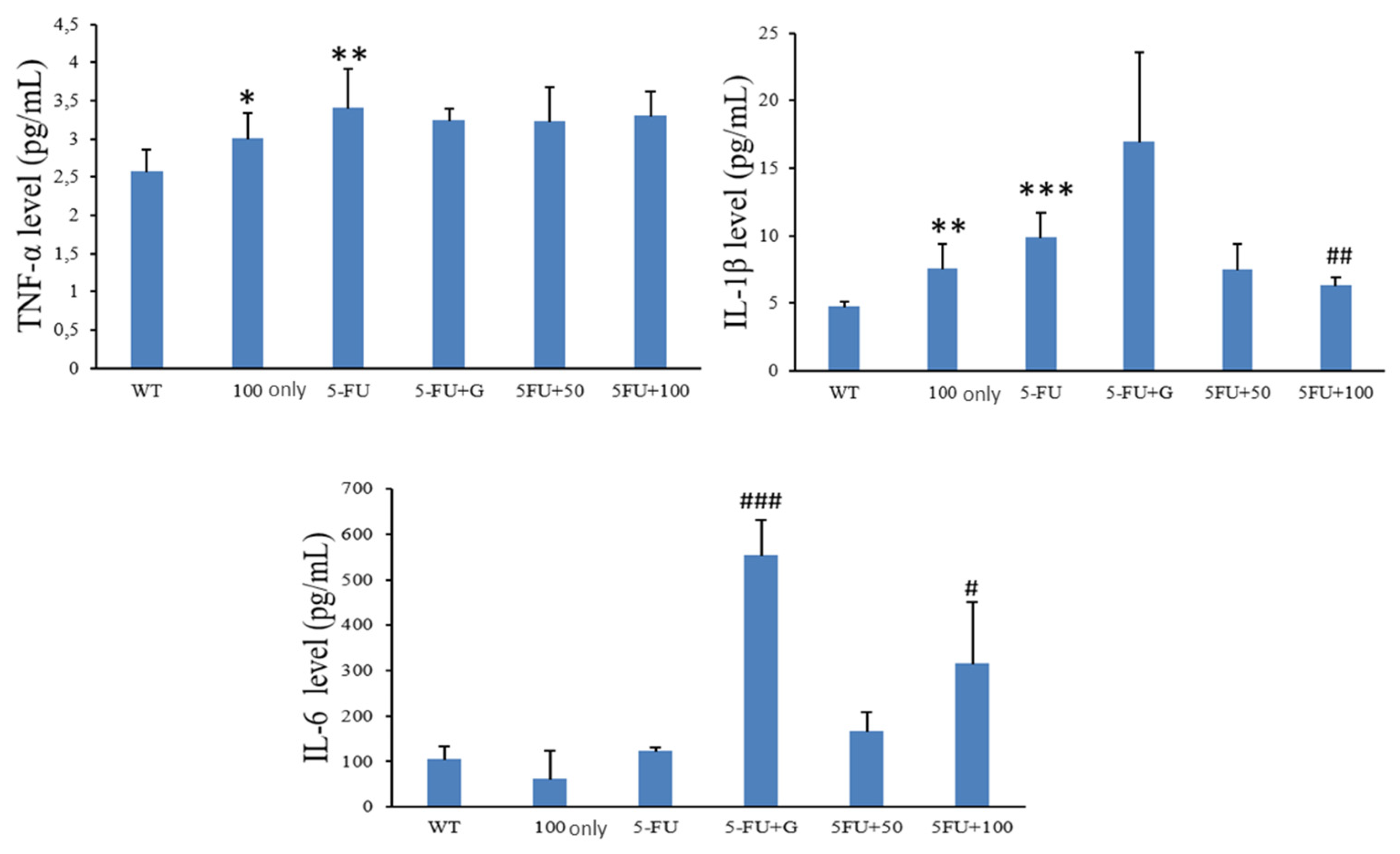
3.6. Serum Proinflammatory Cytokines
3.7. Mortality Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral mucositis. Oral Oncol. 2010, 46, 452–456. [Google Scholar] [CrossRef]
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.L.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, Outcomes, and Costs of Radiation-Induced Oral Mucositis among Patients with Head-and-Neck Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef]
- Murphy, B.A.; Beaumont, J.L.; Isitt, J.; Garden, A.S.; Gwede, C.K.; Trotti, A.M.; Meredith, R.F.; Epstein, J.B.; Le, Q.T.; Brizel, D.M.; et al. Mucositis-Related Morbidity and Resource Utilization in Head and Neck Cancer Patients Receiving Radiation Therapy With or Without Chemotherapy. J. Pain Symptom Manag. 2009, 38, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Sonis, S.T.; Hospital, W. Pathobiology of Oral Mucositis: Novel Insights and Opportunities. J. Supportive Oncol. 2007, 5, 3–11. [Google Scholar]
- Bakar, A.; Yao, P.-C.; Ningrum, V.; Liu, C.-T.; Lee, S.-C. Beneficial Biological Activities of Cinnamomum osmophloeum and Its Potential Use in the Alleviation of Oral Mucositis: A Systematic Review. Biomedicines 2020, 8, 3. [Google Scholar] [CrossRef]
- Lee, S.C.; Xu, W.X.; Lin, L.Y.; Yang, J.J.; Liu, C.T. Chemical composition and hypoglycemic and pancreas-protective effect of leaf essential oil from indigenous cinnamon (Cinnamomum osmophloeum Kanehira). J. Agric. Food Chem. 2013, 61, 4905–4913. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Huang, C.C.; Ho, S.T.; Kuo, Y.H.; Lin, C.C.; Lin, C.T.; Wu, J.H. Bioactive phytochemicals of leaf essential oils of Cinnamomum osmophloeum prevent lipopolysaccharide/d -galactosamine (LPS/d -GalN)-induced acute hepatitis in mice. J. Agric. Food Chem. 2011, 59, 8117–8123. [Google Scholar] [CrossRef]
- Molania, T.; Moghadamnia, A.A.; Pouramir, M.; Aghel, S.; Moslemi, D.; Ghassemi, L.; Motallebnejad, M. The effect of Cinnamaldehyde on mucositis and salivary antioxidant capacity in gamma-irradiated rats (a preliminary study). DARU J. Pharm. Sci. 2012, 20, 1–5. [Google Scholar] [CrossRef]
- Veilleux, M.P.; Grenier, D. Determination of the effects of cinnamon bark fractions on Candida albicans and oral epithelial cells. BMC Complement. Altern. Med. 2019, 19, 303. [Google Scholar] [CrossRef]
- Calapai, G.; Miroddi, M.; Mannucci, C.; Minciullo, P.L.; Gangemi, S. Oral adverse reactions due to cinnamon-flavoured chewing gums consumption. Oral Dis. 2014, 20, 637–643. [Google Scholar] [CrossRef]
- Lee, M.G.; Kuo, S.Y.; Yen, S.Y.; Hsu, H.F.; Leung, C.H.; Ma, D.L.; Wen, Z.H.; Wang, H.M.D. Evaluation of Cinnamomum osmophloeum kanehira extracts on tyrosinase suppressor, wound repair promoter, and antioxidant. Sci. World J. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Chao, L.K.; Hua, K.F.; Hsu, H.Y.; Cheng, S.S.; Lin, I.F.; Chen, C.J.; Chen, S.T.; Chang, S.T. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 2008, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Calderín, S.; García-Núñez, J.A.; Gómez, C. Short-term clinical and osteoimmunological effects of scaling and root planing complemented by simple or repeated laser phototherapy in chronic periodontitis. Lasers Med. Sci. 2013, 28, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Streetz, K.L.; Luedde, T.; Manns, M.P.; Trautwein, C. Interleukin 6 and liver regeneration. Gut 2000, 47, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, F.; Ghalayani, P.; Emami, H.; Isfahani, M.; Noorshargh, P. A novel formulation for radiotherapy-induced oral mucositis: Triamcinolone acetonide mucoadhesive film. J. Res. Med. Sci. 2019, 24, 63. [Google Scholar] [PubMed]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.L.; Burlage, F.R.; Coppes, R.P. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef]
- Patel, A.; Biswas, S.; Shoja, M.H.; Ramalingayya, G.V.; Nandakumar, K. Protective effects of aqueous extract of Solanum nigrum Linn. leaves in rat models of oral mucositis. Sci. World J. 2014, 2014, 345939. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E.R. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef]
- Vizel, M.; Oster, M.W. Ocular Side Effects of Cancer Chemotherapy. Cancer 1982, 49, 1999–2002. [Google Scholar] [CrossRef]
- Esmaeli, B.; Diba, R.; Ahmadi, M.A.; Saadati, H.G.; Faustina, M.M.; Shepler, T.R.; Talpaz, M.; Fraunfelder, R.; Rios, M.B.; Kantarjian, H. Periorbital oedema and epiphora as ocular side effects of imatinib mesylate (Gleevac). Eye 2004, 18, 760–762. [Google Scholar] [CrossRef]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar] [PubMed]
- Hawley, P.; Hovan, A.; Mcgahan, C.E. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Supportive Care Cancer 2014, 22, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Miranzadeh, S.; Adib-hajbaghery, M.; Soleymanpoor, L.; Ehsani, M. A New mouthwash for Chemotherapy Induced Stomatitis. Nurs. Midwifery Stud. 2014, 3, e20249. [Google Scholar] [CrossRef]
- Tiemann, P.; Toelg, M.; Ramos, F.M.H. Administration of Ratanhia-based herbal oral care products for the prophylaxis of oral mucositis in cancer chemotherapy patients: A clinical trial. Evid Based Complement. Altern. Med. 2007, 4, 361–366. [Google Scholar] [CrossRef]
- Aoyama, T.; Nishikawa, K.; Takiguchi, N.; Tanabe, K. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharm. 2014, 73, 1047–1054. [Google Scholar] [CrossRef]
- Rashad, U.M.; Al Gezawy, S.M.; El Gezawy, E.; Azzaz, A.N. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer Main Article Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J. Laryngol. Otol. 2009, 123, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, M.; Jafari, S.; Mortazavi, H. Herbs in oral mucositis. J. Clin. Diagn. Res. 2017, 11, ZE05–ZE11. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakar, A.; Ningrum, V.; Lee, S.-C.; Li, C.-T.; Hsieh, C.-W.; Wang, S.-H.; Tsai, M.-S. Therapeutic Effect of Cinnamomum osmophloeum Leaf Extract on Oral Mucositis Model Rats Induced by 5-Fluororacil via Influencing IL-1β and IL-6 Levels. Processes 2021, 9, 615. https://doi.org/10.3390/pr9040615
Bakar A, Ningrum V, Lee S-C, Li C-T, Hsieh C-W, Wang S-H, Tsai M-S. Therapeutic Effect of Cinnamomum osmophloeum Leaf Extract on Oral Mucositis Model Rats Induced by 5-Fluororacil via Influencing IL-1β and IL-6 Levels. Processes. 2021; 9(4):615. https://doi.org/10.3390/pr9040615
Chicago/Turabian StyleBakar, Abu, Valendriyani Ningrum, Shih-Chieh Lee, Chi-Ting Li, Chang-Wei Hsieh, Sue-Hong Wang, and Ming-Shun Tsai. 2021. "Therapeutic Effect of Cinnamomum osmophloeum Leaf Extract on Oral Mucositis Model Rats Induced by 5-Fluororacil via Influencing IL-1β and IL-6 Levels" Processes 9, no. 4: 615. https://doi.org/10.3390/pr9040615
APA StyleBakar, A., Ningrum, V., Lee, S.-C., Li, C.-T., Hsieh, C.-W., Wang, S.-H., & Tsai, M.-S. (2021). Therapeutic Effect of Cinnamomum osmophloeum Leaf Extract on Oral Mucositis Model Rats Induced by 5-Fluororacil via Influencing IL-1β and IL-6 Levels. Processes, 9(4), 615. https://doi.org/10.3390/pr9040615







