Chromatographic and Computational Screening of Lipophilicity and Pharmacokinetics of Newly Synthesized Betulin-1,4-quinone Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Betulin-1,4-quinone Hybrids
2.2. Chromatographic Analysis
2.3. Computational Analysis
2.4. Enzymatic Assay
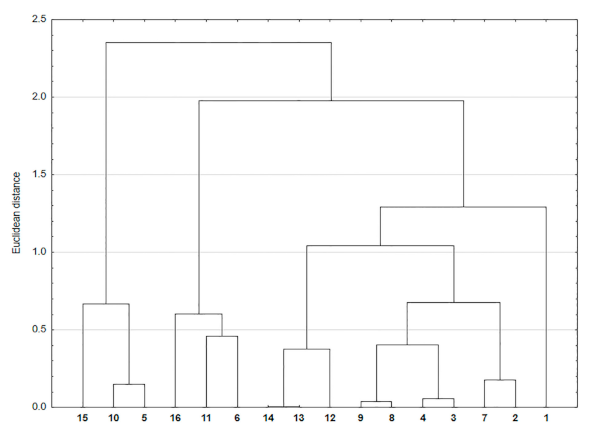
2.5. Correlation and Cluster Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnott, J.; Planey, S. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Bhatt, N.; Chavada, V.D.; Sanyal, M.; Shrivastav, P. Influence of organic modifier and separation modes for lipophilicityassessment of drugs using thin layer chromatography indices. J. Chromatogr. A 2018, 1571, 223–230. [Google Scholar] [CrossRef]
- Rutkowska, E.; Pajak, K.; Jóźwiak, K. Lipophilicity-methods of determination and its role in medicinal chemistry. Acta Pol. Pharm. 2013, 70, 3–18. [Google Scholar]
- Bębenek, E.; Bober-Majnusz, K.; Siudak, S.; Chrobak, E.; Kadela-Tomanek, M.; Wietrzyk, J.; Boryczka, S. Application of TLC to evaluate the lipophilicity of newly synthesized betulin derivatives. J. Chromatogr. Sci. 2020, 15, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Bober, K.; Bębenek, E.; Chrobak, E.; Boryczka, S. Application of thin-layer chromatography to evaluate the lipophilicity of 5,8-quinolinedione compounds. J. Planar Chromatogr.-Mod. TLC 2017, 30, 219–224. [Google Scholar] [CrossRef]
- Bober, K.; Bębenek, E.; Boryczka, S. Application of TLC for evaluation of the lipophilicity of newly synthetized esters: Betulin derivatives. J. Anal. Methods Chem. 2019, 3, 1297659–1297667. [Google Scholar] [CrossRef] [PubMed]
- Pyka, A. Evaluation of the lipophilicity of fat-soluble vitamins. J. Planar Chromatogr.-Med. TLC 2009, 22, 211–215. [Google Scholar] [CrossRef]
- Šegan, S.; Andrić, F.; Radoičić, A.; Opsenica, D.; Šolaja, B.; Zlatović, M.; Milojković-Opsenica, D. Correlation between structure, retention and activity of cholic acid derived cis-trans isomeric bis-steroidal tetraoxanes. J. Sep. Sci. 2011, 34, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 182, 111653–111678. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409–107448. [Google Scholar] [CrossRef]
- Gao, F.; Sun, Z.; Kong, F.; Xiao, J. Artemisinin-derived hybrids and their anticancer activity. Eur. J. Med. Chem. 2020, 188, 112044–112058. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, Z.; Kong, F.; Gao, F. Current scenario of ferrocene-containing hybrids for antimalarial activity. Eur. J. Med. Chem. 2020, 185, 111791–111802. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Jin, L.; Piao, F.; Han, R. Betulin esters with coumarin-3-carboxylic and 3,4,5-trimethoxybenzoic acids. Mendeleev Commun. 2017, 27, 93–94. [Google Scholar] [CrossRef]
- Bori, I.; Hung, H.; Qian, K.; Chen, C.; Morris-Natschke, S.; Lee, K. Anti-AIDS agents 88. Anti-HIV conjugates of betulin and betulinic acid with AZT prepared via click chemistry. Tetrahedron Lett. 2012, 53, 1987–1989. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kashiwada, Y.; Chen, C.; Qian, K.; Morris-Natschke, S.; Lee, K.; Takaishi, Y. Conjugates of betulin derivatives with AZT as potent anti-HIV agents. Bioorg. Med. Chem. 2010, 18, 6451–6469. [Google Scholar] [CrossRef]
- Achrem-Achremowicz, J.; Kepczyńska, E.; Zylewski, M.; Janeczko, Z. Synthesis of betulin derivatives and the determination of their relative lipophilicities using reversed-phase thin-layer chromatography. Biomed. Chromatogr. 2010, 24, 261–267. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Vida, N.; Rárová, L.; Kolář, M.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Polyamine derivatives of betulinic acid and β-sitosterol: A comparative investigation. Steroids 2015, 100, 27–35. [Google Scholar] [CrossRef]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Dufour, P.; Pichette, A. Synthesis and structure-activity relationship study of cytotoxic germanicane- and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorg. Med. Chem. 2007, 15, 6144–6157. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Marciniec, K.; Latocha, M.; Kuśmierz, D.; Jastrzębska, M.; Boryczka, S. Betulin-1,4-quinone hybrids: Synthesis, anticancer activity and molecular docking study with NQO1 enzyme. Eur. J. Med. Chem. 2019, 177, 302–315. [Google Scholar] [CrossRef]
- Virtual Computational Chemistry Laboratory. Available online: http://www.vcclab.org/lab/alogps/ (accessed on 2 January 2021).
- Molsoft, L.L.C. Available online: http://www.molsoft.com/ (accessed on 2 January 2021).
- ACD/I-Lab. Available online: https://ilab.acdlabs.com/iLab2/ (accessed on 2 January 2021).
- Molinspiration Cheminformatics. Available online: https://www.molinspiration.com/ (accessed on 2 January 2021).
- pkCSM. Available online: http://biosig.unimelb.edu.au/pkcsm/prediction_single/ (accessed on 2 January 2021).
- Li, X.; Bian, J.; Wang, N.; Qian, X.; Gu, J.; Mu, T.; Fan, J.; Yang, X.; Li, S.; Yang, T.; et al. Novel naphtho[2,1-d]oxazole-4,5-diones as NQO1 substrates with improved aqueous solubility: Design, synthesis, and in vivo antitumor evaluation. Bioorg. Med. Chem. 2016, 24, 1006–1013. [Google Scholar] [CrossRef]
- Bian, J.; Deng, B.; Xu, L.; Xu, X.; Wang, N.; Hu, T.; Yao, Z.; Du, J.; Yang, L.; Lei, Y.; et al. 2-Substituted 3-methylnaphtho[1,2-b]furan-4,5-diones as novel L-shaped ortho-quinone substrates for NAD(P)H:quinone oxidoreductase (NQO1). Eur. J. Med. Chem. 2014, 82, 56–67. [Google Scholar] [CrossRef]
- Mannhold, R.; Cruciani, G.; Dross, K.; Rekker, R. Multivariate analysis of experimental and computational descriptors of molecular lipophilicity. J. Comput. Aided Mol. Des. 1998, 12, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Bodor, N.; Gabanyi, Z.; Wong, C.K. A new method for the estimation of partition coefficient. J. Am. Chem. Soc. 1989, 111, 3783–3786. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.; Lung, I. Lipophilicity as a central component of drug-like properties of chalchones and flavonoid derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef] [PubMed]
- Mälkiä, A.; Murtomäki, L.; Urtti, A.; Kontturi, K. Drug permeation in biomembranes: In vitro and in silico prediction and influence of physicochemical properties. Eur. J. Pharm. Sci. 2004, 23, 13–47. [Google Scholar] [CrossRef]
- Alavijeh, M.; Chishty, M.; Qaiser, M.; Palmer, A. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx 2005, 5, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Duchowicz, P.; Castro, E. QSPR studies on aqueous solubilities of drug-like compounds. Int. J. Mol. Sci. 2009, 10, 2558–2577. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.; Lombardo, F.; Dominy, B.; Feeney, P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Boryczka, S. Alkoxy and enediyne derivatives containing 1,4-benzoquinone subunits-synthesis and antitumor activity. Molecules 2017, 22, 447. [Google Scholar] [CrossRef]
- Hutter, M.C. Prediction of blood-brain barrier permeation using quantum chemically derived information. J. Comput. Aided Mol. Des. 2003, 17, 415–433. [Google Scholar] [CrossRef] [PubMed]




| Chemical Structure | Chemical Structure | Chemical Structure | |||
|---|---|---|---|---|---|
| 2 | 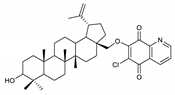 | 7 | 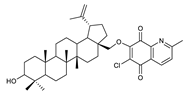 | 12 | 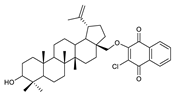 |
| 3 | 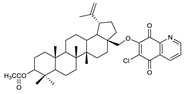 | 8 |  | 13 | 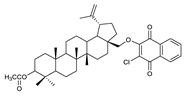 |
| 4 | 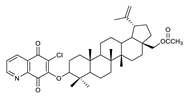 | 9 | 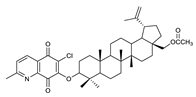 | 14 | 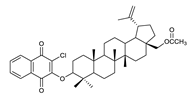 |
| 5 | 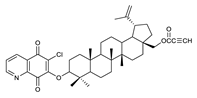 | 10 | 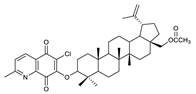 | 15 | 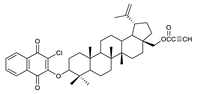 |
| 6 | 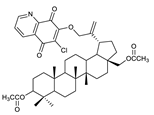 | 11 | 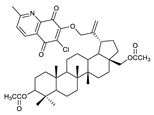 | 16 | 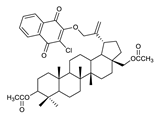 |
| Compound | RM0 | b | r | SD | logPTLC | ϕ0 |
|---|---|---|---|---|---|---|
| 1 | 4.54 | −0.05 | 0.979 | 0.028 | 5.34 | 90.37 |
| 2 | 5.49 | −0.06 | 0.995 | 0.022 | 6.36 | 92.52 |
| 3 | 6.52 | −0.07 | 0.994 | 0.023 | 7.46 | 93.10 |
| 4 | 6.42 | −0.07 | 0.996 | 0.015 | 7.36 | 89.31 |
| 5 | 6.62 | −0.07 | 0.994 | 0.021 | 7.58 | 93.69 |
| 6 | 5.86 | −0.07 | 0.998 | 0.012 | 6.75 | 95.74 |
| 7 | 5.69 | −0.06 | 0.992 | 0.023 | 6.58 | 95.74 |
| 8 | 6.49 | −0.07 | 0.997 | 0.019 | 7.43 | 90.72 |
| 9 | 6.42 | −0.07 | 0.996 | 0.009 | 7.36 | 90.92 |
| 10 | 6.80 | −0.07 | 0.995 | 0.025 | 7.77 | 95.00 |
| 11 | 6.77 | −0.08 | 0.994 | 0.011 | 7.74 | 90.71 |
| 12 | 6.46 | −0.07 | 0.987 | 0.027 | 7.41 | 92.85 |
| 13 | 7.12 | −0.07 | 0.995 | 0.023 | 8.12 | 93.10 |
| 14 | 7.13 | −0.07 | 0.995 | 0.019 | 8.14 | 88.67 |
| 15 | 6.82 | −0.08 | 0.982 | 0.014 | 7.19 | 91.54 |
| 16 | 6.26 | −0.07 | 0.973 | 0.029 | 7.80 | 87.09 |
| Compound | logPlit | RM0 | b | r | SD | logPTLC |
|---|---|---|---|---|---|---|
| Acetanilide | 1.21 | 0.68 | −0.02 | 0.985 | 0.015 | 1.17 |
| 4-Bromoacetophenone | 2.43 | 1.87 | −0.02 | 0.981 | 0.018 | 2.46 |
| Benzophenone | 3.18 | 2.42 | −0.03 | 0.992 | 0.013 | 3.05 |
| Anthracene | 4.45 | 4.08 | −0.05 | 0.970 | 0.022 | 4.84 |
| Dibenzyl | 4.79 | 4.11 | −0.04 | 0.972 | 0.023 | 4.87 |
| 9-Phenylanthracene | 6.01 | 4.87 | −0.06 | 0.985 | 0.019 | 5.69 |
| DDT | 6.38 | 5.50 | −0.06 | 0.975 | 0.019 | 6.37 |
| Compound | ALOGPs | ACLOGP | ALOGP | miLogP | XLOGP2 | XLOGP3 | ACD/logP | MolLogP |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.34 | 5.84 | 6.31 | 7.61 | 7.81 | 8.28 | 9.01 | 7.90 |
| 2 | 6.23 | 7.15 | 7.58 | 8.00 | 9.37 | 10.47 | 10.38 | 9.40 |
| 3 | 6.77 | 7.64 | 7.95 | 8.53 | 10.11 | 11.05 | 11.28 | 9.82 |
| 4 | 6.77 | 7.64 | 7.95 | 8.53 | 10.11 | 11.05 | 11.28 | 9.82 |
| 5 | 6.66 | 7.09 | 9.23 | 8.05 | 10.39 | 11.53 | 12.00 | 10.20 |
| 6 | 6.69 | 7.17 | 7.24 | 8.15 | 9.39 | 10.37 | 10.92 | 8.31 |
| 7 | 6.48 | 7.57 | 7.86 | 8.05 | 9.69 | 10.87 | 10.84 | 9.78 |
| 8 | 7.00 | 8.05 | 8.24 | 8.56 | 10.43 | 11.43 | 11.74 | 10.21 |
| 9 | 7.00 | 8.05 | 8.24 | 8.56 | 10.43 | 11.43 | 11.74 | 10.21 |
| 10 | 6.81 | 7.51 | 9.51 | 8.10 | 10.70 | 11.93 | 12.46 | 10.59 |
| 11 | 6.94 | 7.59 | 7.53 | 8.20 | 9.71 | 10.77 | 11.38 | 8.69 |
| 12 | 6.47 | 8.12 | 8.30 | 9.01 | 10.53 | 11.21 | 11.71 | 10.50 |
| 13 | 6.94 | 8.61 | 8.68 | 9.24 | 11.27 | 11.78 | 12.61 | 10.92 |
| 14 | 6.94 | 8.61 | 8.68 | 9.24 | 11.27 | 11.78 | 12.62 | 10.92 |
| 15 | 7.02 | 8.07 | 9.95 | 9.03 | 11.55 | 12.26 | 13.33 | 11.30 |
| 16 | 6.97 | 8.14 | 7.97 | 9.07 | 10.55 | 11.11 | 11.42 | 9.40 |
| Program | Correlation Equation | r | SD |
|---|---|---|---|
| ALOGPS | logPTLC = 1.454 LogPcalc − 2.458 | 0.864 | 0.373 |
| ACLOGP | logPTLC = 0.871 LogPcalc + 0.584 | 0.824 | 0.421 |
| ALOGP | logPTLC = 0.513 LogPcalc + 3.068 | 0.638 | 0.571 |
| milogP | logPTLC = 1.001 LogPcalc − 1.236 | 0.702 | 0.528 |
| XLOGP2 | logPTLC = 0.666 LogPcalc + 0.475 | 0.842 | 0.401 |
| XLOGP3 | logPTLC = 0.653 LogPcalc + 0.037 | 0.829 | 0.415 |
| ACD/logP | logPTLC = 0.575 LogPcalc + 0.633 | 0.815 | 0.430 |
| MolLogP | logPTLC = 0.482 LogPcalc + 2.513 | 0.642 | 0.569 |
| Compound | M (g/mol) | nHD | nHA | nRT | TPSA (Å2) | logBB |
|---|---|---|---|---|---|---|
| 1 | 442.73 | 2 | 2 | 2 | 40.46 | −0.421 |
| 2 | 634.29 | 1 | 5 | 4 | 75.96 | −0.335 |
| 3 | 676.32 | 0 | 6 | 6 | 82.03 | −0.674 |
| 4 | 676.32 | 0 | 6 | 6 | 82.56 | −0.667 |
| 5 | 686.32 | 0 | 6 | 7 | 82.56 | −0.634 |
| 6 | 734.36 | 0 | 8 | 9 | 108.86 | −1.124 |
| 7 | 648.31 | 1 | 5 | 4 | 75.96 | −0.344 |
| 8 | 690.35 | 0 | 6 | 6 | 82.56 | −0.683 |
| 9 | 690.35 | 0 | 6 | 6 | 82.56 | −0.676 |
| 10 | 700.34 | 0 | 6 | 7 | 82.56 | −0.643 |
| 11 | 748.38 | 0 | 8 | 9 | 108.86 | −1.133 |
| 12 | 633.30 | 1 | 4 | 4 | 63.60 | −0.524 |
| 13 | 675.33 | 0 | 5 | 6 | 69.67 | −0.439 |
| 14 | 675.33 | 0 | 5 | 6 | 69.67 | −0.434 |
| 15 | 685.33 | 0 | 5 | 7 | 69.67 | −0.401 |
| 16 | 733.37 | 0 | 7 | 9 | 95.97 | −0.893 |
| Compound | A549/IC50 (µM) [19] | NQO1 Activity (µmolNADPH/µmolNQO1/min) |
|---|---|---|
| 1 | Neg. | NO |
| 2 | 8.58 ± 1.70 | 1685 ± 12 |
| 3 | 14.34 ± 0.31 | 1451 ± 9 |
| 4 | 13.46 ± 1.45 | 1419 ± 6 |
| 5 | 0.45 ± 0.20 | 2192 ± 21 |
| 6 | 3.30 ± 0.48 | 1505 ± 15 |
| 7 | 1.62 ± 0.91 | 1705 ± 19 |
| 8 | 0.64 ± 0.04 | 2008 ± 19 |
| 9 | 0.59 ± 0.13 | 1744 ± 14 |
| 10 | 18.41 ± 1.91 | 1784 ± 7 |
| 11 | 0.84 ± 0.01 | 1839 ± 23 |
| 12 | 10.63 ± 1.45 | 1459 ± 18 |
| 13 | 1.15 ± 0.10 | 1784 ± 6 |
| 14 | 0.77 ± 0.12 | 2009 ± 17 |
| 15 | 1.28 ± 0.06 | 1870 ± 13 |
| 16 | 53.9 ± 8.02 | 1040 ± 9 |
| Streptonigrin | NO | 621 ± 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Boryczka, S. Chromatographic and Computational Screening of Lipophilicity and Pharmacokinetics of Newly Synthesized Betulin-1,4-quinone Hybrids. Processes 2021, 9, 376. https://doi.org/10.3390/pr9020376
Kadela-Tomanek M, Jastrzębska M, Chrobak E, Bębenek E, Boryczka S. Chromatographic and Computational Screening of Lipophilicity and Pharmacokinetics of Newly Synthesized Betulin-1,4-quinone Hybrids. Processes. 2021; 9(2):376. https://doi.org/10.3390/pr9020376
Chicago/Turabian StyleKadela-Tomanek, Monika, Maria Jastrzębska, Elwira Chrobak, Ewa Bębenek, and Stanisław Boryczka. 2021. "Chromatographic and Computational Screening of Lipophilicity and Pharmacokinetics of Newly Synthesized Betulin-1,4-quinone Hybrids" Processes 9, no. 2: 376. https://doi.org/10.3390/pr9020376
APA StyleKadela-Tomanek, M., Jastrzębska, M., Chrobak, E., Bębenek, E., & Boryczka, S. (2021). Chromatographic and Computational Screening of Lipophilicity and Pharmacokinetics of Newly Synthesized Betulin-1,4-quinone Hybrids. Processes, 9(2), 376. https://doi.org/10.3390/pr9020376






