A Bioreactor Designed for Restricting Oversize of Aerobic Granular Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculated Sludge and Wastewater
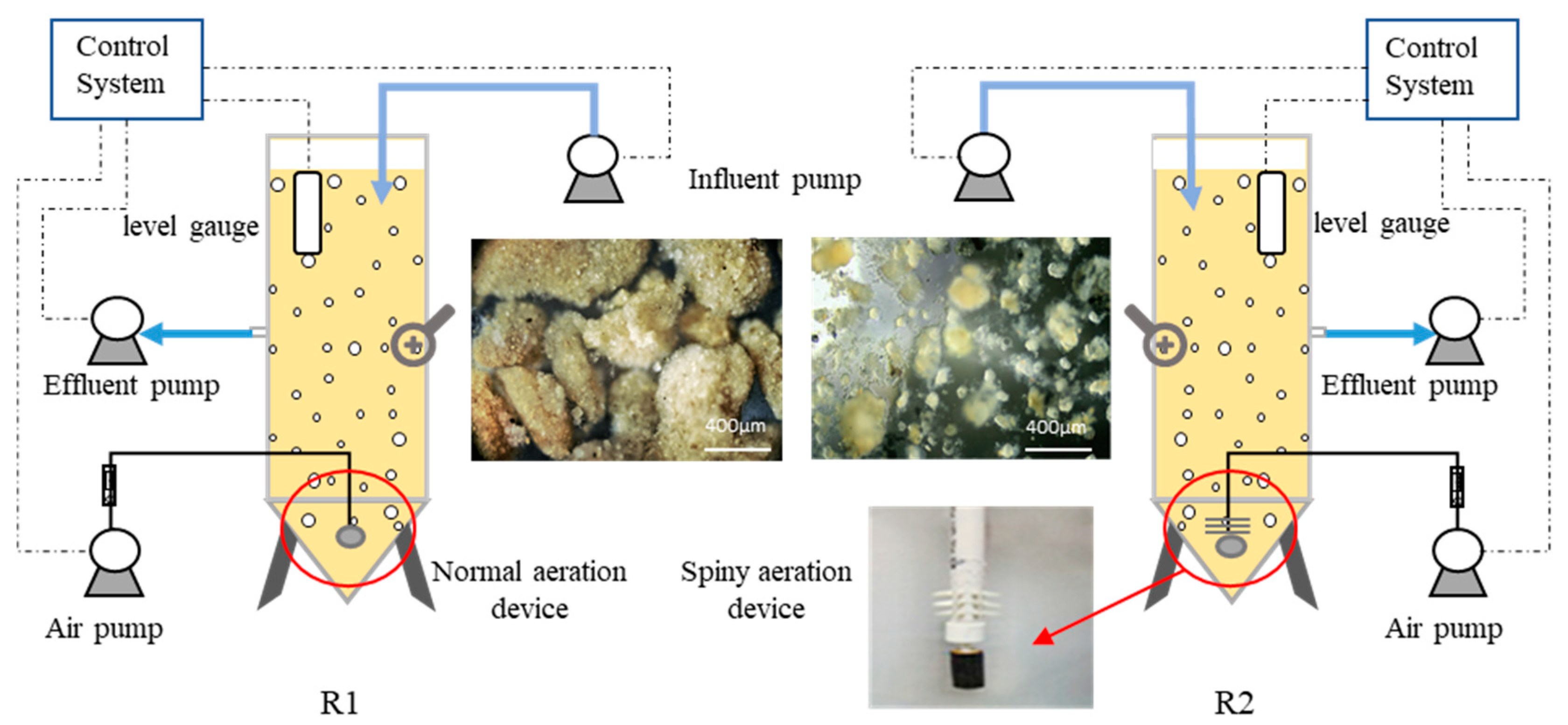
2.2. Experimental Set-Up and Operation
2.2.1. Lab-Scale Sequencing Batch Reactors
2.2.2. Pilot-Scale Sequencing Batch Reactors
2.3. Analytical Methods
3. Results
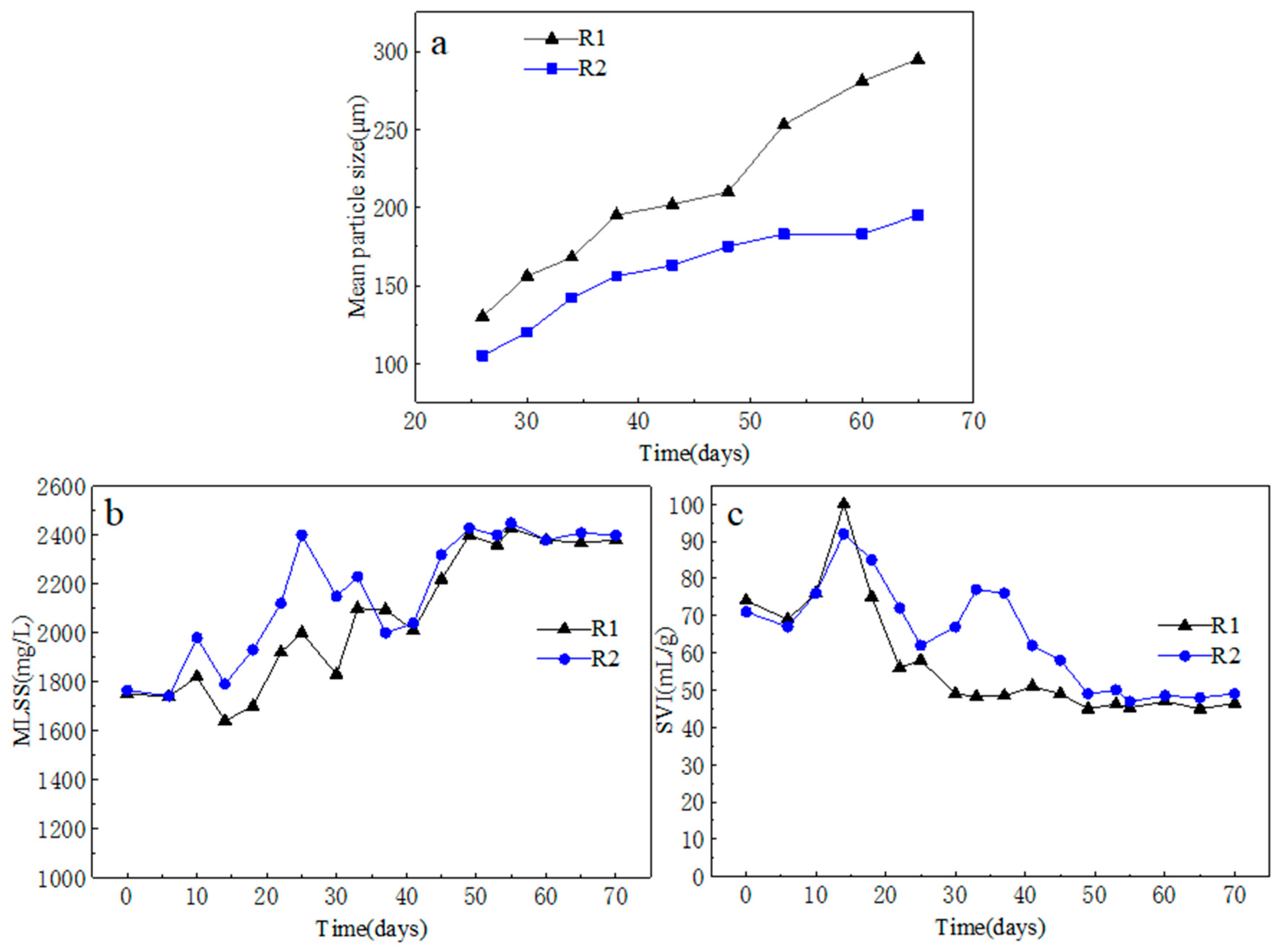
3.1. Comparison of Sludge Characteristics
3.1.1. Sludge Particle Size
3.1.2. Sludge Sedimentation
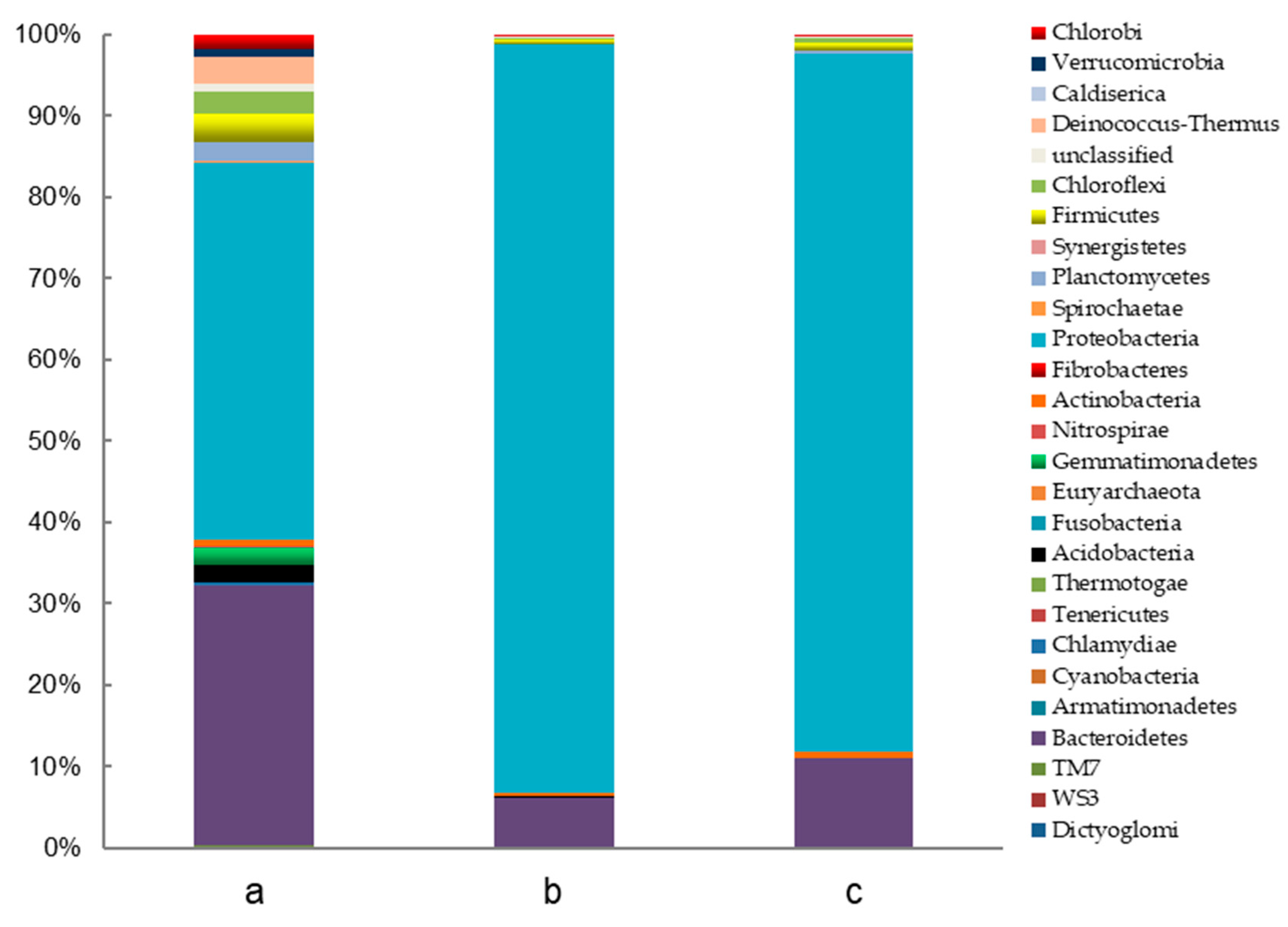
3.1.3. Microbial Community Analysis
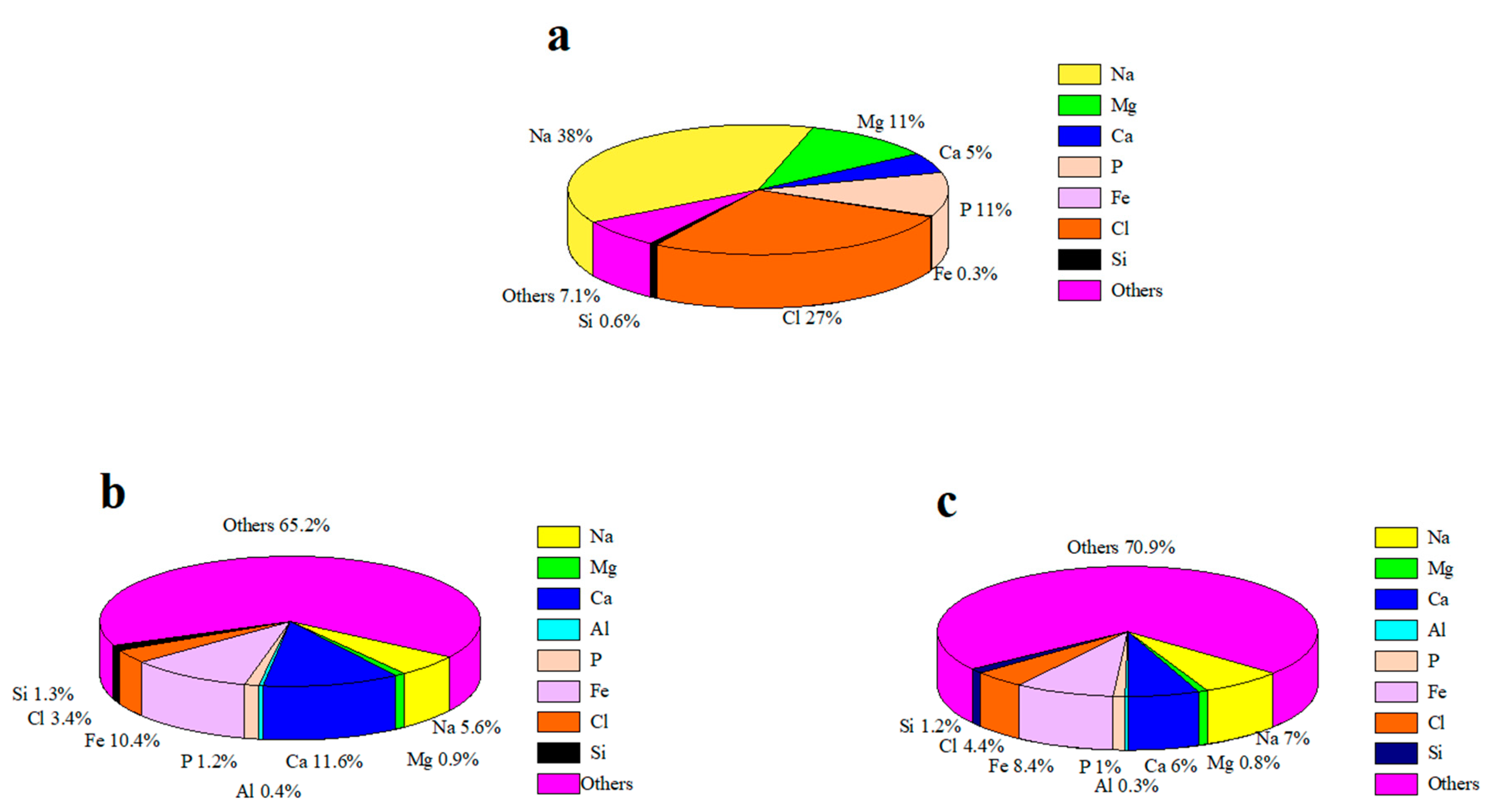
3.1.4. The X-ray Fluorescence Analysis of Sludge
3.2. Pollutant Removal Performance
3.3. An Attempt at the Pilot-Scale System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Kreuk, M.K.; Kishida, N.; Van Loosdrecht, M.C.M. Aerobic granular sludge—State of the art. Water Sci. Technol. 2007, 55, 75–81. [Google Scholar] [CrossRef]
- Bhunia, P.; Ghangrekar, M. Required minimum granule size in UASB reactor and characteristics variation with size. Bioresour. Technol. 2007, 98, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Nakamura, M. Self-Immobilization of Aerobic Activated Sludge–A Pilot Study of the Aerobic Upflow Sludge Blanket Process in Municipal Sewage Treatment. Water Sci. Technol. 1991, 23, 981–990. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J. Aerobic granular processes: Current research trends. Bioresour. Technol. 2016, 210, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lotito, A.M.; De Sanctis, M.; Di Iaconi, C.; Bergna, G. Textile wastewater treatment: Aerobic granular sludge vs activated sludge systems. Water Res. 2014, 54, 337–346. [Google Scholar] [CrossRef]
- Rosman, N.H.; Anuar, A.N.; Othman, I.; Harun, H.; Sulong, M.Z.; Elias, S.H.; Hassan, M.A.H.M.; Chelliapan, S.; Ujang, Z. Cultivation of aerobic granular sludge for rubber wastewater treatment. Bioresour. Technol. 2013, 129, 620–623. [Google Scholar] [CrossRef]
- Corsino, S.F.; Di Biase, A.; Devlin, T.R.; Munz, G.; Torregrossa, M.; Oleszkiewicz, J.A. Effect of extended famine conditions on aerobic granular sludge stability in the treatment of brewery wastewater. Bioresour. Technol. 2017, 226, 150–157. [Google Scholar] [CrossRef]
- Chen, C.; Ming, J.; Yoza, B.A.; Liang, J.; Li, Q.X.; Guo, H.; Liu, Z.; Deng, J.; Wang, Q. Characterization of aerobic granular sludge used for the treatment of petroleum wastewater. Bioresour. Technol. 2019, 271, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Y.; Zhang, S.; Gao, Y.; Jiang, Y.; Horn, H.; Li, J. Successful granulation and microbial differentiation of activated sludge in anaerobic/anoxic/aerobic (A2O) reactor with two-zone sedimentation tank treating municipal sewage. Water Res. 2020, 178, 115825. [Google Scholar] [CrossRef]
- Guo, T.; Ji, Y.; Zhao, J.; Horn, H.; Li, J. Coupling of Fe-C and aerobic granular sludge to treat refractory wastewater from a membrane manufacturer in a pilot-scale system. Water Res. 2020, 186, 116331. [Google Scholar] [CrossRef]
- Li, J.; Ding, L.-B.; Cai, A.; Huang, G.-X.; Horn, H. Aerobic Sludge Granulation in a Full-Scale Sequencing Batch Reactor. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pronk, M.; De Kreuk, M.; De Bruin, B.; Kamminga, P.; Kleerebezem, R.; Van Loosdrecht, M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Franca, R.D.; Pinheiro, H.M.; Van Loosdrecht, M.C.; Lourenço, N.D. Stability of aerobic granules during long-term bioreactor operation. Biotechnol. Adv. 2018, 36, 228–246. [Google Scholar] [CrossRef] [PubMed]
- Jungles, M.K.; Figueroa, M.; Morales, N.; Del Río, Á.V.; Da Costa, R.H.R.; Campos, J.L.; Mosquera-Corral, A.; Méndez, R. Start up of a pilot scale aerobic granular reactor for organic matter and nitrogen removal. J. Chem. Technol. Biotechnol. 2011, 86, 763–768. [Google Scholar] [CrossRef]
- Farooqi, I.; Basheer, F. Treatment of Adsorbable Organic Halide (AOX) from pulp and paper industry wastewater using aerobic granules in pilot scale SBR. J. Water Process. Eng. 2017, 19, 60–66. [Google Scholar] [CrossRef]
- Lee, D.-J.; Chen, Y.-Y.; Show, K.-Y.; Whiteley, C.G.; Tay, J.-H. Advances in aerobic granule formation and granule stability in the course of storage and reactor operation. Biotechnol. Adv. 2010, 28, 919–934. [Google Scholar] [CrossRef]
- Toh, S.K.; Tay, J.H.; Moy, B.Y.P.; Ivanov, V.; Tay, S.T.L. Size-effect on the physical characteristics of the aerobic granule in a SBR. Appl. Microbiol. Biotechnol. 2003, 60, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Xu, B.; Wu, J. Experimental and mathematical simulation study on the effect of granule particle size distribution on partial nitrification in aerobic granular reactor. Biochem. Eng. J. 2018, 134, 22–29. [Google Scholar] [CrossRef]
- Verawaty, M.; Tait, S.; Pijuan, M.; Yuan, Z.; Bond, P.L. Breakage and growth towards a stable aerobic granule size during the treatment of wastewater. Water Res. 2013, 47, 5338–5349. [Google Scholar] [CrossRef]
- Long, B.; Xuan, X.; Yang, C.; Zhang, L.; Cheng, Y.; Wang, J. Stability of aerobic granular sludge in a pilot scale sequencing batch reactor enhanced by granular particle size control. Chemosphere 2019, 225, 460–469. [Google Scholar] [CrossRef]
- Yang, H.G.; Li, J.; Ding, L.B.; Chen, T.; Huang, G.X.; Shen, J.Y.; Liu, J. A case for aerobic sludge granulation: From pilot to full scale. J. Water Reuse Desalination 2015, 6, 188–194. [Google Scholar] [CrossRef]
- Eaton, A.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M. APHA: Standard Methods for the Examination of Water and Wastewater; Centennial Edition; APHA, AWWA, WEF: Washington, DC, USA, 2005. [Google Scholar]
- Ministry of Ecological Environment of the People’s Republic of China; State Administration for Market Regulation. Discharge Standard of Water Pollutants for Leather and Fur Making Industry. Available online: http://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/swrwpfbz/201312/t20131227_265766.shtml (accessed on 11 December 2020).
- Zhao, Y.; Huang, J.; Zhao, H.; Yang, H. Microbial community and N removal of aerobic granular sludge at high COD and N loading rates. Bioresour. Technol. 2013, 143, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, B.; Zhang, C.; Zhang, Z.; Lei, Z.; Lu, B.; Zhou, B. Effect of algae growth on aerobic granulation and nutrients removal from synthetic wastewater by using sequencing batch reactors. Bioresour. Technol. 2015, 179, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, J.; Guo, X.; Li, L. Micro-environment characteristics and microbial communities in activated sludge flocs of different particle size. Bioresour. Technol. 2012, 124, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Lan, G.-H.; Zeng, P. Size-dependent calcium carbonate precipitation induced microbiologically in aerobic granules. Chem. Eng. J. 2016, 285, 341–348. [Google Scholar] [CrossRef]
- Ren, T.-T.; Liu, L.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q.; Zhang, M.-C.; Zhu, J.-R. Calcium spatial distribution in aerobic granules and its effects on granule structure, strength and bioactivity. Water Res. 2008, 42, 3343–3352. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Yang, H.; Sheng, J.; Pan, Z.; Li, J. A Bioreactor Designed for Restricting Oversize of Aerobic Granular Sludge. Processes 2021, 9, 374. https://doi.org/10.3390/pr9020374
Feng H, Yang H, Sheng J, Pan Z, Li J. A Bioreactor Designed for Restricting Oversize of Aerobic Granular Sludge. Processes. 2021; 9(2):374. https://doi.org/10.3390/pr9020374
Chicago/Turabian StyleFeng, Hongbo, Honggang Yang, Jianlong Sheng, Zengrui Pan, and Jun Li. 2021. "A Bioreactor Designed for Restricting Oversize of Aerobic Granular Sludge" Processes 9, no. 2: 374. https://doi.org/10.3390/pr9020374
APA StyleFeng, H., Yang, H., Sheng, J., Pan, Z., & Li, J. (2021). A Bioreactor Designed for Restricting Oversize of Aerobic Granular Sludge. Processes, 9(2), 374. https://doi.org/10.3390/pr9020374





