Biodegradable Polyester Synthesis in Renewed Aqueous Polycondensation Media: The Core of the New Greener Polymer-5B Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymes and Chemicals
2.2. Methods
2.2.1. Activity of the Free and Immobilized Enzyme Preparations
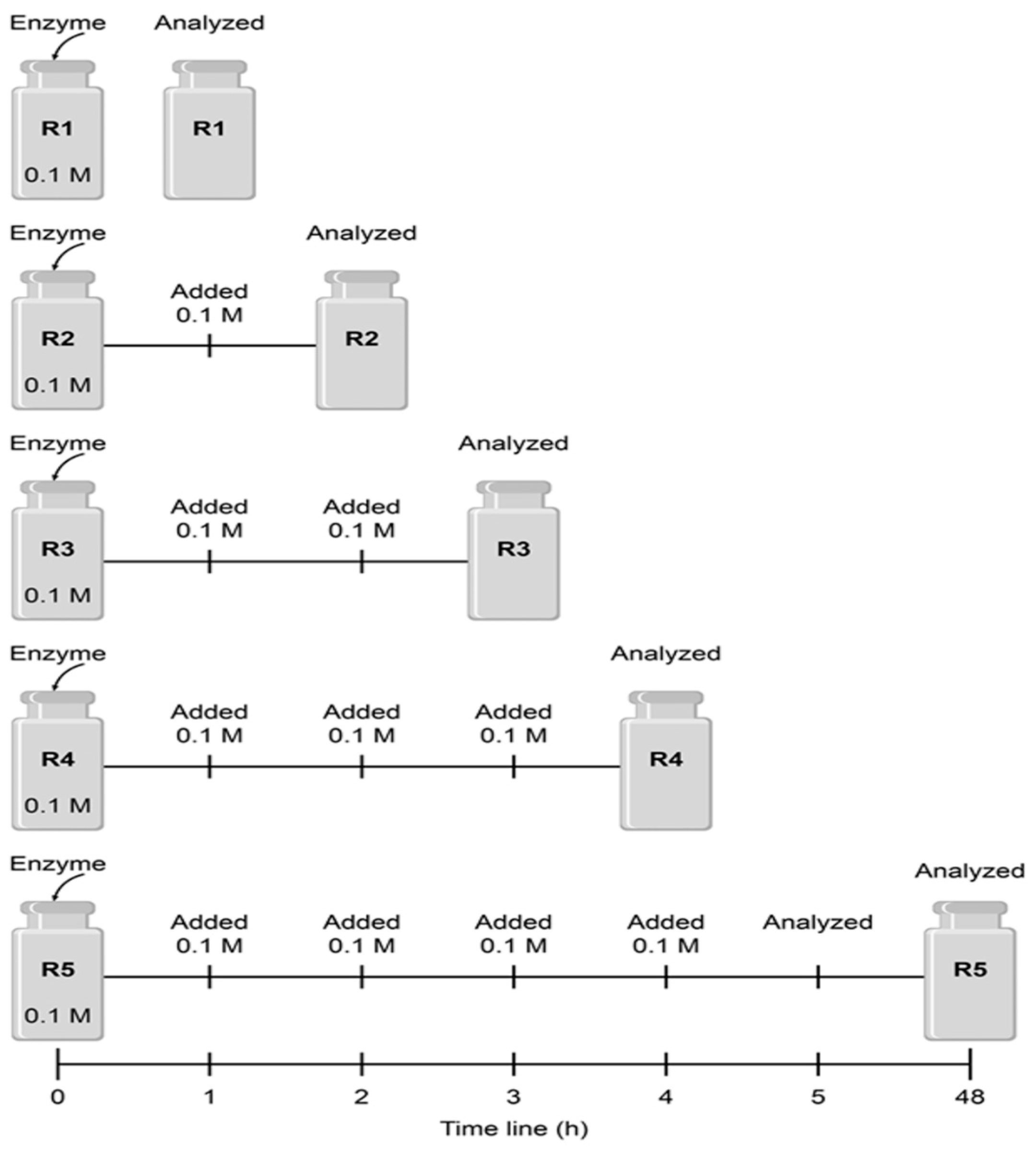
2.2.2. Miniemulsion Preparation
2.2.3. Polyester Synthesis in Miniemulsion
2.2.4. Poly(octamethylene suberate) (POS) Synthesis with the PBLI Biocatalyst
2.2.4.1. In Batch Operation Mode
2.2.4.2. In Fed-Batch Operation Mode
2.2.4.3. Effect of the Stirring Type
2.2.5. Biocatalyst Reutilization (PBLI) in a Reactor with an Impeller (RI)
2.2.6. Determination of Acidity and Conversion
2.2.7. Polymer Characterization
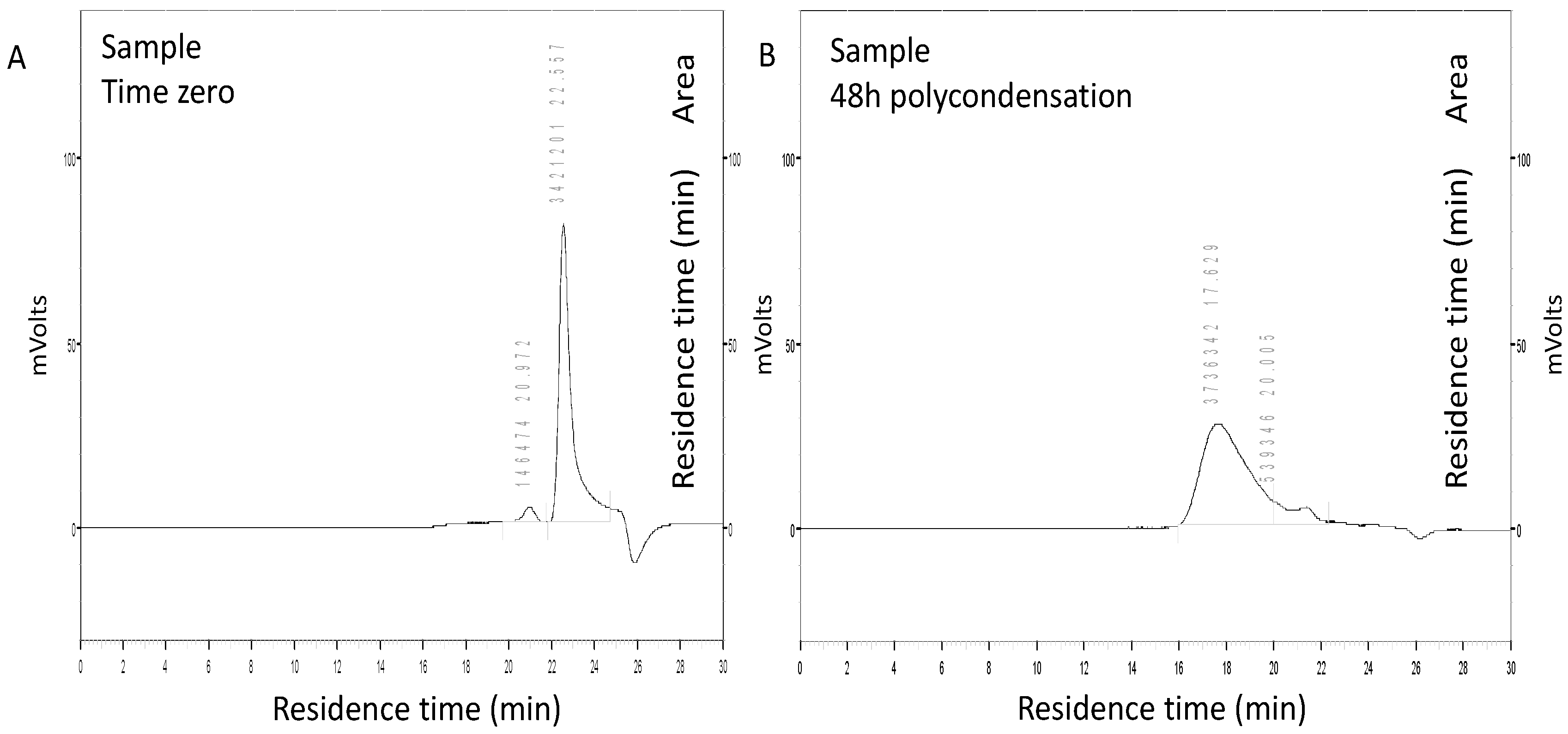
2.2.7.1. Polyester Molecular Weight Assay by Size-Exclusion Chromatography (SEC)
2.2.7.2. Nuclear Magnetic Resonance (1H NMR) Spectroscopy
2.2.7.3. Thermogravimetric Analysis (TGA)
2.2.7.4. Differential Scanning Calorimetry (DSC) Analysis
3. Results and Discussion
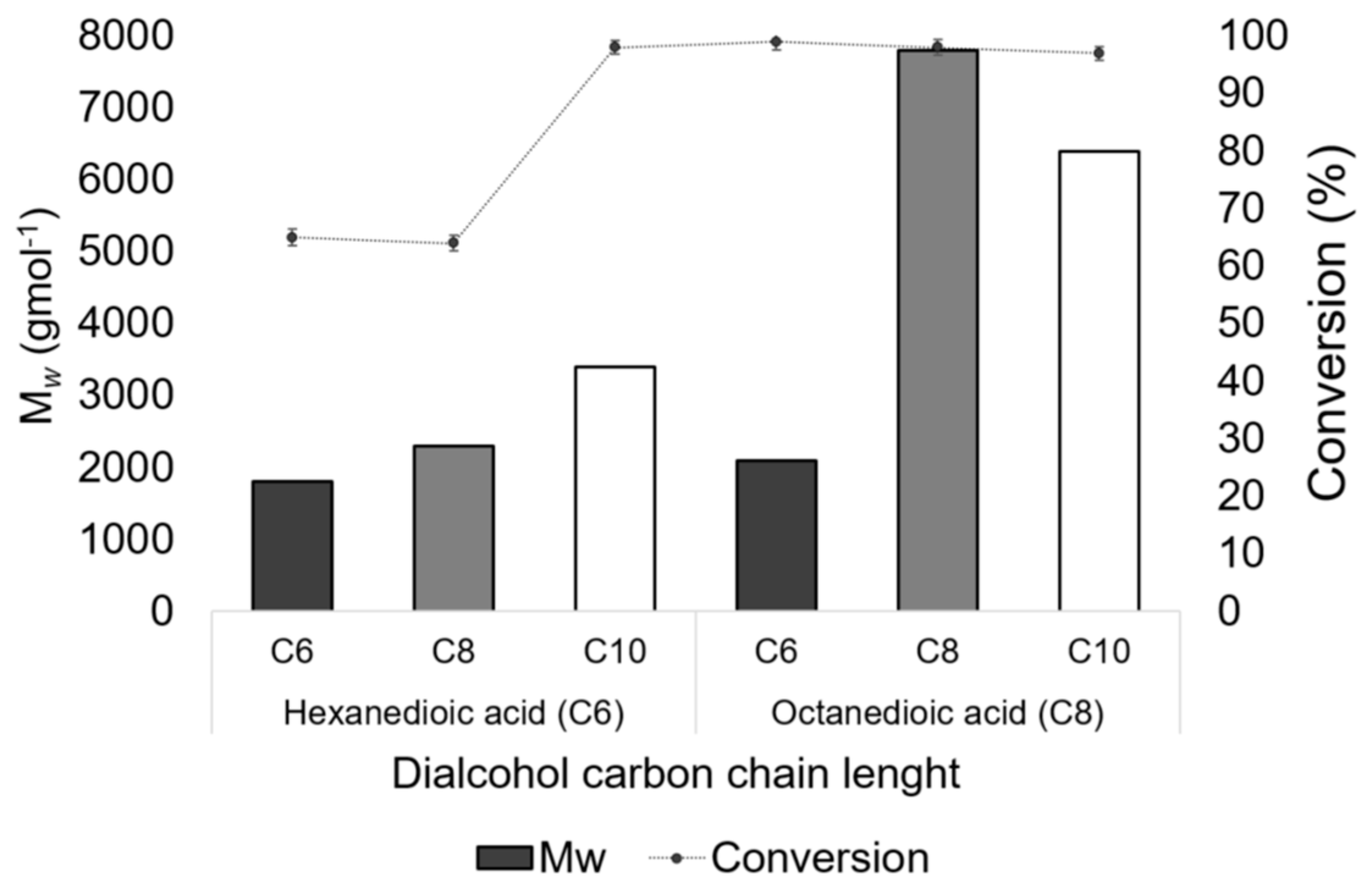
3.1. Polyester Synthesis in the Miniemulsion
3.2. Effect of Reactor Conditions on POS Synthesis in the Miniemulsion
3.3. Effect of the Reaction Operation Mode: Batch vs. Fed-Batch for Different Polymerization Media
3.4. Effect of Stirring Type on POS Synthesis
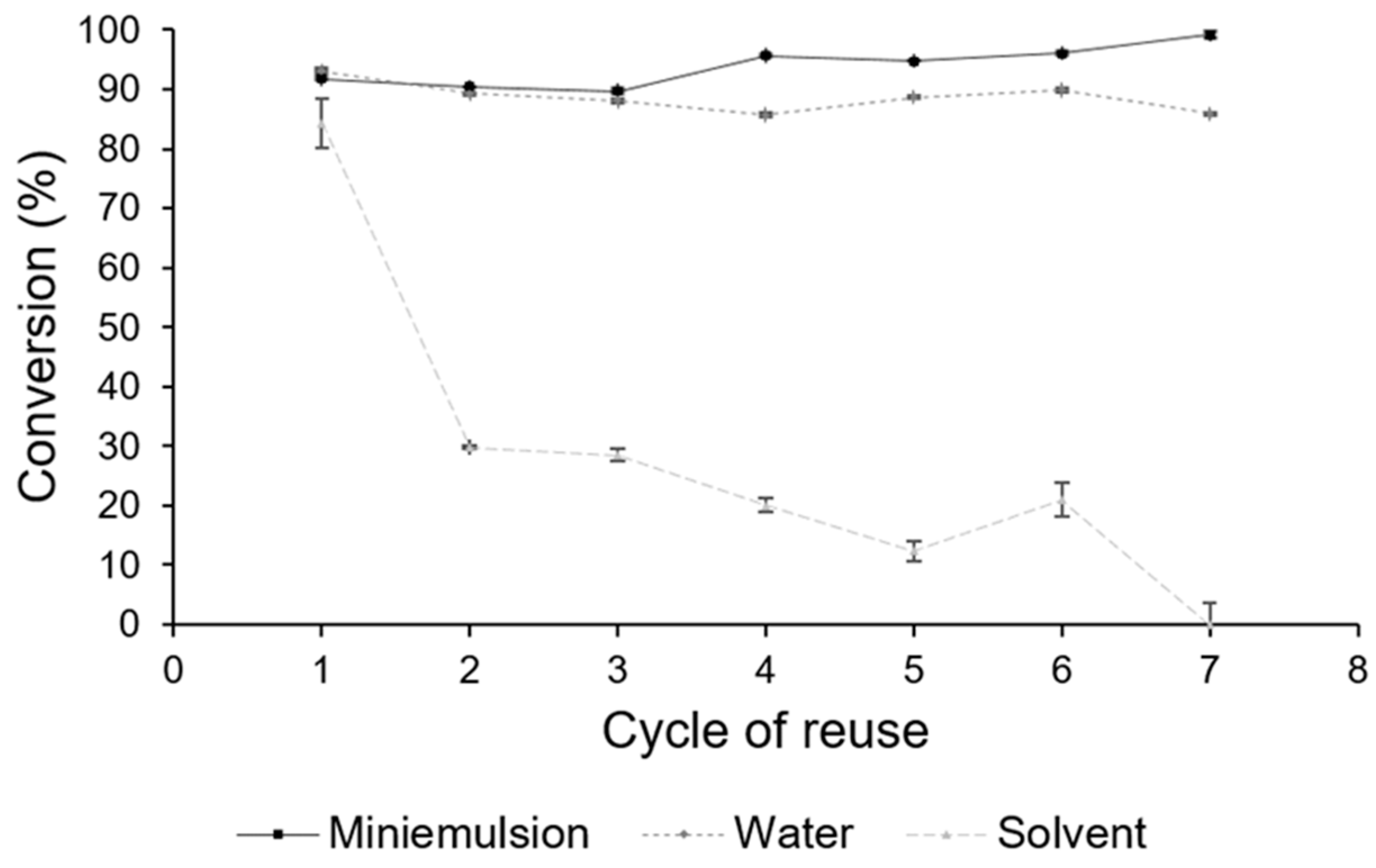
3.5. Reutilization of the PBLI Biocatalyst during POS Synthesis in the Reactor with an Impeller (RI)
3.6. Polymer Characterization
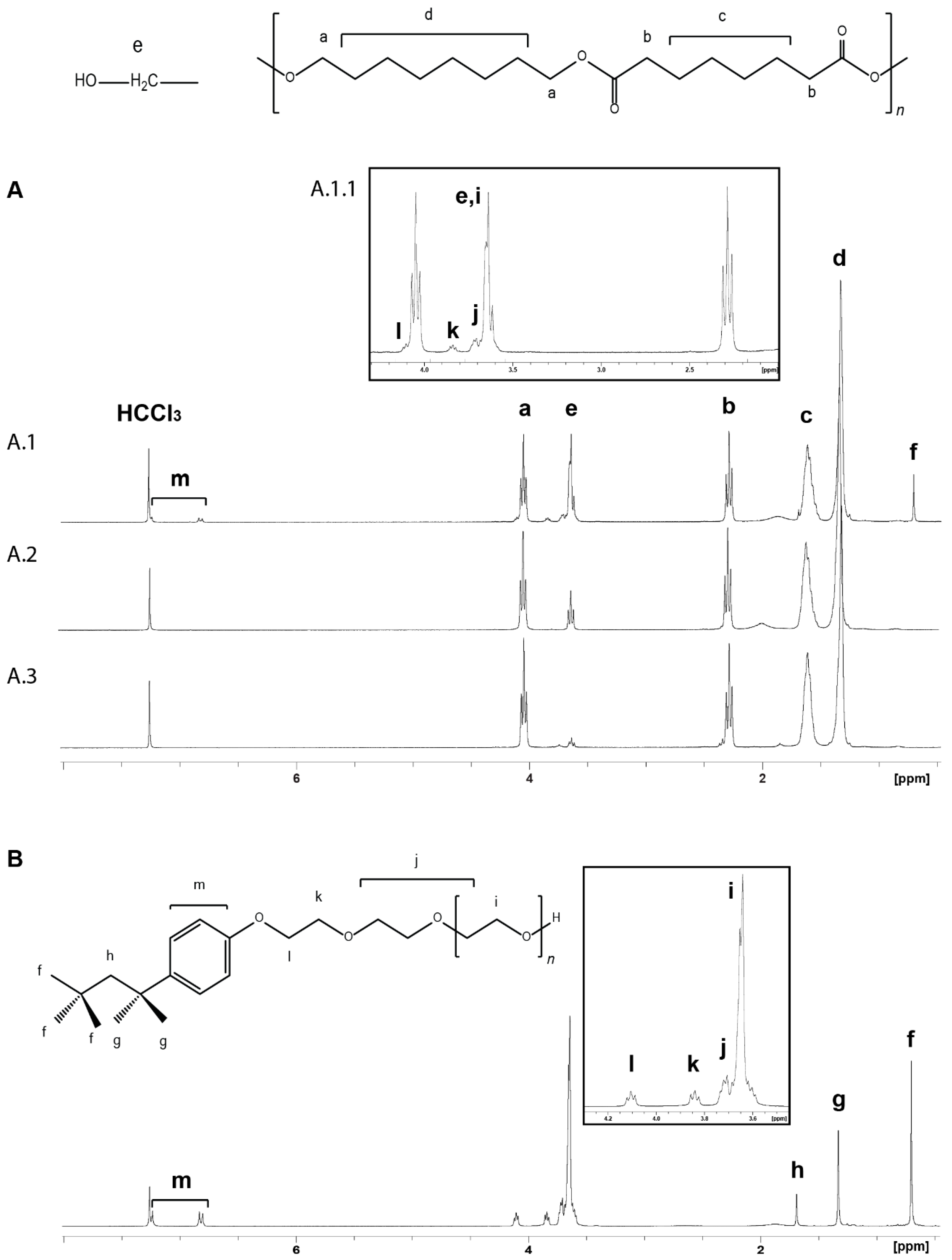
3.6.1. 1H NMR Analysis of the Polymer Molecular Structure (POS)
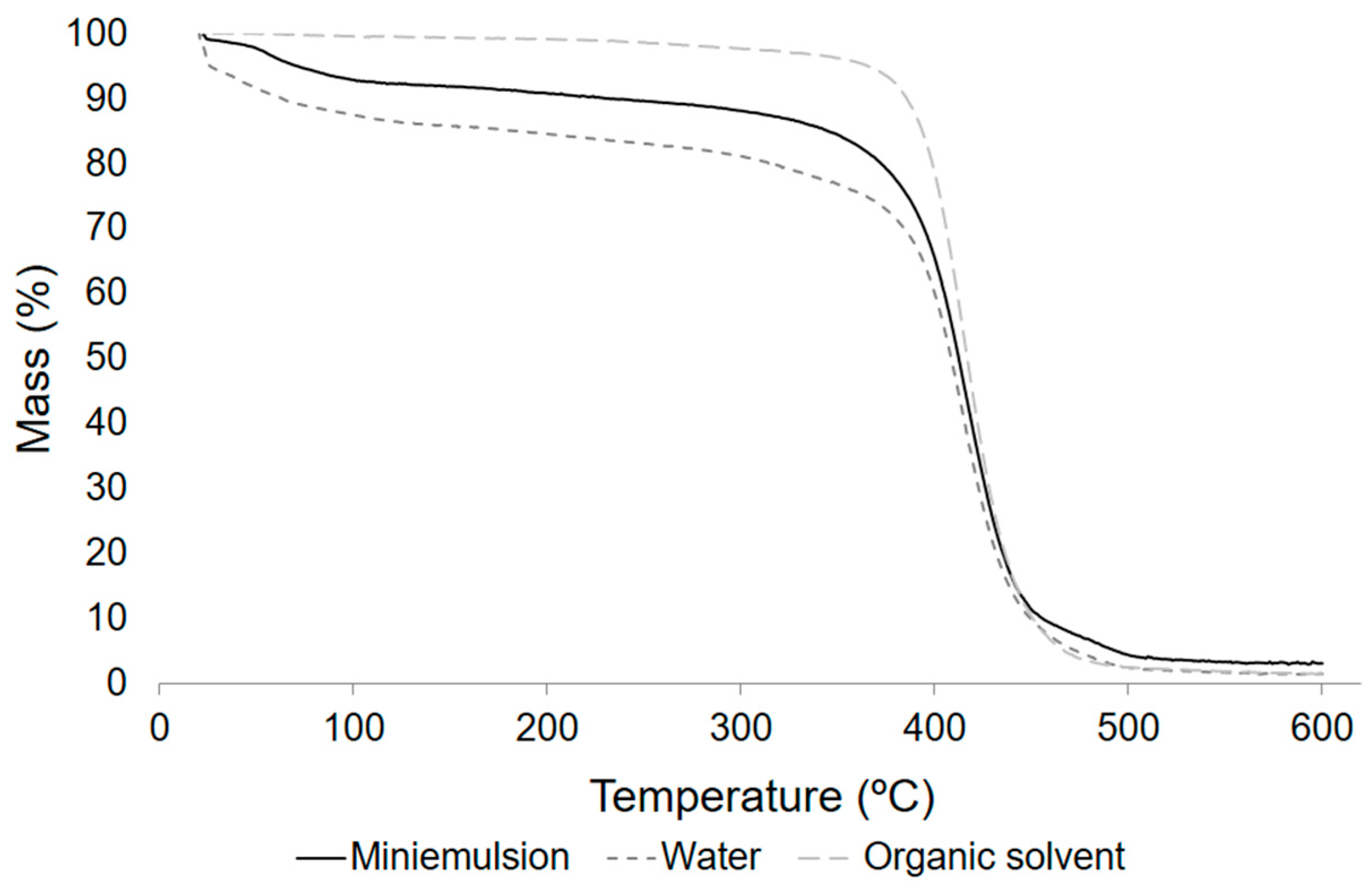
3.6.2. Thermogravimetric Analysis (TGA) of the POS Synthetized
3.6.3. Differential Scanning Calorimetry (DSC) of the POS Synthetized
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gestí, S.; Zanetti, M.; Lazzari, M.; Franco, L.; Puiggalí, J. Degradable polyoctamethylene suberate/clay nanocomposites. Crystallization studies by DSC and simultaneous SAXS/WAXD synchrotron radiation. Eur. Polym. J. 2009, 45, 398–409. [Google Scholar] [CrossRef]
- Philp, J.C.; Bartsev, A.; Ritchie, R.J.; Baucher, M.-A.; Guy, K. Bioplastics science from a policy vantage point. New Biotechnol. 2013, 30, 635–646. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, D.; Liu, C.; Zhao, Z.; Yang, Y.; Li, Q. Lipase/esterase-catalysed synthesis of aliphatic polyesters via polycondensation: A review. Process Biochem. 2012, 47, 1027–1036. [Google Scholar] [CrossRef]
- Pellis, A.; Acero, E.H.; Gardossi, L.; Ferrarioc, V.; Guebitza, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.-M.; Papaspyrides, C.D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Gestí, S.; Casas, M.T.; Puiggali, J. Single crystal morphology and structural data of a series of polyesters derived from 1,8-octanediol. Eur. Polym. J. 2008, 44, 2295–2307. [Google Scholar] [CrossRef]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, K.; Azadpour, P.; Moztarzadeh, F.; Urbanska, A.M.; Mozafari, M. Tissue-engineered chitosan/bioactive glass bone scaffolds integrated with PLGA nanoparticles: A therapeutic design for on-demand drug delivery. Mater. Lett. 2015, 138, 16–20. [Google Scholar] [CrossRef]
- Rim, N.; Lee, Y.; Kim, S.; Shin, H. Current status and prospect of biomaterials as tissue substitutes in regenerative medicine. Korean Ind. Chem. News 2010, 13, 2–17. [Google Scholar]
- Diaz, A.; Katsarava, R.; Puiggali, J. Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef]
- Barrère, M.; Landfester, K. Polyester synthesis in aqueous miniemulsion. Polymer 2003, 44, 2833–2841. [Google Scholar] [CrossRef]
- Takasu, A.; Takemoto, A.; Hirabayashi, T. Polycondensation of dicarboxylic acids and diols in water catalyzed by surfactant-combined catalysts and successive chain extension. Biomacromolecules 2006, 7, 6–9. [Google Scholar] [CrossRef]
- Moyori, T.; Tang, T.; Takasu, A. Dehydration polycondensation of dicarboxylic acids and diols using sublimating strong Brønsted acids. Biomacromolecules 2012, 13, 1240–1243. [Google Scholar] [PubMed]
- Komorowska-Durka, M.; Dimitrakis, G.; Bogdał, D.; Stankiewicz, A.I.; Stefanidis, G.D. A concise review on microwave-assisted polycondensation reactions and curing of polycondensation polymers with focus on the effect of process conditions. Chem. Eng. J. 2015, 264, 633–644. [Google Scholar] [CrossRef]
- Sharma, S.; Kanwar, S. Organic solvent tolerant lipases an applications. Sci. World J. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Duwensee, J.; Wenda, S.; Ruth, W.; Kragl, U. Lipase-catalyzed polycondensation in water: A new approach for polyester synthesis. Org. Process Res. Dev. 2010, 14, 48–57. [Google Scholar] [CrossRef]
- Kong, X.; Qi, H.; Curtis, J.M. Synthesis and characterization of high-molecular weight aliphatic polyesters from monomers derived from renewable resources. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar]
- Jiang, Y.; Loos, K. Enzymatic synthesis of biobased polyesters and polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Miletic, N.; Nastasovic, A.; Loos, K. Immobilization of biocatalysts for enzymatic polymerizations: Possibilities, advantages, applications. Bioresour. Technol. 2012, 115, 126–135. [Google Scholar] [CrossRef]
- Gross, R.A. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Zhao, H. Enzymatic polymerization to polyesters in non-aqueous solvents. Method. Enzymol. 2019, 627, 1–21. [Google Scholar] [CrossRef]
- Liu, W.; Chen, B.; Wang, F.; Tan, T.; Deng, L. Lipase-catalyzed synthesis of aliphatic polyesters and properties characterization. Proc. Biochem. 2011, 46, 1993–2000. [Google Scholar]
- Jiang, Z.; Zhang, J. Lipase-catalyzed synthesis of aliphatic polyesters via copolymerization of lactide with diesters and diols. Polymer 2013, 54, 6105–6113. [Google Scholar] [CrossRef]
- Azim, H.; Dekhterman, A.; Jiang, Z.; Gross, R.A. Candida antarctica lipase B-catalyzed synthesis of poly(butylene succinate): Shorter chain building blocks also work. Biomacromolecules 2006, 7, 3093–3097. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Wang, C.; Liu, J.; Shi, W.; Zhu, X.; Lu, L.; Li, Q. Cell debris self immobilized thermophilic lipase: A biocatalyst for synthesizing aliphatic polyester. Appl. Biochem. Biotechnol. 2013, 170, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Farmer, T.; Castle, R.; Clark, J.; Macquarrie, D. Synthesis of unsaturated polyester resins from various bio-derived platform molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef]
- Binns, F.; Roberts, S.M.; Taylor, A.; Williams, C.F. Enzymatic polymerization of an unactivated diol diacid system. J. Chem. Soc. Perkin Trans. 1993, 1, 899–904. [Google Scholar] [CrossRef]
- Linko, Y.Y.; Wang, Z.L.; Seppala, J. Lipase-catalyzed synthesis of poly(1,4-butyl sebacate) from sebacic acid or its derivatives with BDO. J. Biotechnol. 1995, 40, 133–138. [Google Scholar]
- Binns, F.; Harffey, P.; Roberts, S.M.; Taylor, A. Studies of lipase-catalyzed polyesterification of an unactivated diacid/diol system. J. Polym. Sci. A 1998, 36, 2069–2079. [Google Scholar] [CrossRef]
- Uyama, H.; Inada, K.; Kobayashi, S. Lipase-catalyzed synthesis of aliphatic polyesters by polycondensation of dicarboxylic acids and glycols in solvent-free system. Polym. J. 2000, 32, 440–443. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Feng, N.; Jiang, W.; Jin, Y.; Li, X. Recent advances in the synthesis of biodegradable polyesters by sustainable polymerization: Lipase catalyzed polymerization. RSC Adv. 2020, 10, 36230. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Namekawa, S. In vitro biosynthesis of polyesters with isolated enzymes in aqueous systems and organic solvents. Polym. Degrad. Stab. 1998, 59, 195–201. [Google Scholar] [CrossRef]
- MacDonald, J.P.; Shaver, M.P. An aromatic/aliphatic polyester prepared via ring-opening polymerisation and its remarkably selective and cyclable depolymerisation to monomer. Polym. Chem. 2016, 7, 553–559. [Google Scholar] [CrossRef]
- Varma, I.K.; Albertsson, A.-C.; Rajkhowa, R.; Srivastava, R.K. Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 2005, 30, 949–981. [Google Scholar]
- Stergiou, P.-Y.; Foukis, A.; Filippo, M.; Koukouritki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Målberg, S.; Finne-Wistrand, A.; Albertsson, A.-C. The environmental influence in enzymatic polymerization of aliphatic polyesters in bulk and aqueous mini-emulsion. Polymer 2010, 51, 5318–5322. [Google Scholar] [CrossRef]
- Lovell, P.A.; Schork, F.J. Fundamentals of emulsion polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef] [PubMed]
- De Barros, D.P.C.; Fonseca, L.P.; Cabral, J.M.S.; Aschenbrenner, E.M.; Weiss, C.K.; Landfester, K. Miniemulsion as efficient system for enzymatic synthesis of acid alkyl esters. Biotechnol. Bioeng. 2010, 93, 1338–1346. [Google Scholar] [CrossRef]
- De Barros, D.P.C.; Fernandes, P.; Cabral, J.M.S.; Fonseca, L.P. Synthetic application and activity of cutinase in an aqueous, miniemulsion model system: Hexyl octanoate synthesis. Catal. Today 2011, 173, 95–102. [Google Scholar]
- De Barros, D.P.C.; Pinto, F.; Pfluck, A.C.D.; Dias, A.S.A.; Fernandes, P.; Fonseca, L.P. Improvement of enzyme stability for alkyl esters synthesis in miniemulsion systems by using media engineering. J. Chem. Technol. Biotechnol. 2017, 106, 507–515. [Google Scholar] [CrossRef]
- Lourenço, N.M.T.; Matias, S.C.; Altas, M.C.; Fonseca, L.P. Low-temperature enzymatic hydrolysis resolution in mini-emulsion media. Chem. Pap. 2015, 69, 810–816. [Google Scholar] [CrossRef]
- De Lima, A.P.D.; Aschenbrenner, E.M.; Oliveira, S.N.; Doucet, J.B.; Weiss, C.K.; Ziener, U.; Fonseca, L.P.; Ricardo, N.M.P.S.; de Freitas, L.L.; Petzhold, C.L.; et al. Towards regioselective enzymatic hydrolysis and glycerolysis of tricaprylin in miniemulsion and the direct preparation of polyurethane from the hydrolysis products. J. Mol. Catal. B Enzym. 2013, 98, 127–137. [Google Scholar] [CrossRef]
- Birolli, W.G.; Porto, A.L.M.; Fonseca, L.P. Miniemulsion in biocatalysis, a new approach employing a solid reagent and an easy protocol for product isolation applied to the aldol reaction by Rhizopus niveus lipase. Bioresour. Technol. 2020, 297, 122441. [Google Scholar] [CrossRef]
- Divandari, M.; Pollard, J.; Dehghani, E.; Bruns, N.; Benetti, E.M. Controlling enzymatic polymerization from surfaces with switchable bioaffinity. Biomacromolecules 2017, 18, 4261–4270. [Google Scholar] [CrossRef] [PubMed]
- Schork, F.J.; Guo, J. Continuous miniemulsion polymerization. Macromol. React. Eng. 2008, 2, 287–303. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Zeaiter, J.; Romagnoli, J.A.; Barton, G.W.; Gomes, V.G.; Hawkett, B.S.; Gilbert, R.G. Operation of semi-batch emulsion polymerisation reactors: Modelling, validation and effect of operating conditions. Chem. Eng. Sci. 2002, 57, 2955–2969. [Google Scholar] [CrossRef]
- Pfluck, A.C.D.; de Barros, D.P.C.; Fonseca, L.P. Stability assay of Candida rugosa lipase in miniemulsion system to synthesis of biodegradable polymers. In Proceedings of the 2017 IEEE 5th Portuguese Meeting on Bioengineering (ENBENG), Coimbra, 16–18 February 2017. [Google Scholar] [CrossRef]
- Pfluck, A.C.D.; de Barros, D.P.C.; Fonseca, L.P.; Melo, E.P. Stability of lipases in miniemulsion systems: Between correlation secondary structure and activity. Enzym. Microb. Technol. 2018, 114, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zisis, T.; Freddolino, P.L.; Turunen, P.; van Teeseling, M.C.F.; Rowan, A.E.; Blank, K.G. Interfacial activation of Candida antarctica lipase B: Combined evidence from experiment and simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar] [CrossRef] [PubMed]
- Kirk, O.; Christensen, M.W. Lipases from Candida antarctica: Unique biocatalysts from a unique origin organic process. Org. Proc. Res. Dev. 2002, 6, 446–451. [Google Scholar] [CrossRef]
- Pellis, A.; Comerford, J.W.; Maneffa, A.J.; Sipponen, M.H.; Clark, J.H.; Farmer, T.J. Elucidating enzymatic polymerisations: Chain-length selectivity of Candida antarctica lipase B towards various aliphatic diols and dicarboxylic acid diesters. Eur. Polym. J. 2018, 106, 79–84. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Halling, P.J. Solid-to-solid biocatalysis: Thermodynamic feasibility and energy efficiency. Green Chem. 2004, 6, 488–496. [Google Scholar] [CrossRef]
- Van Herk, A.M. Chemistry and Technology of Emulsion Polymerisation, 2nd ed.; John Wiley & Sons, Ltd.: Singapore, 2013. [Google Scholar]
- Meneses, A.C.; Lerin, L.A.; Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Benzyl propionate synthesis by fed-batch esterification using commercial immobilized and lyophilized Cal B lipase. Bioprocess Biosyst. Eng. 2019, 42, 1625–1634. [Google Scholar] [CrossRef]
- Suda, S.; Uyama, H.; Kobayashi, S. Dehydration polycondensation in water for synthesis of polyesters by lipase catalyst. Proc. Jpn. Acad. 1999, 75, 201. [Google Scholar] [CrossRef]
- Meyer, T. Scale-up of polymerization process: A practical example organic process. Org. Proc. Res. Dev. 2003, 7, 297–302. [Google Scholar] [CrossRef]
- Asua, J.M. Polymer Reaction Engineering, 4th ed.; Wiley-Blackwell Publishing Ltd.: New York, NY, USA, 2008. [Google Scholar]
- Lerin, L.; Ceni, G.; Richetti, A.; Kubiak, G.; Oliveira, J.V.; Toniazzo, G.; Treichel, H.; Oestreicher, E.G.; Oliveira, D. Successive cycles of utilization of Novozym 435 in three different reaction systems. Braz. J. Chem. Eng. 2011, 28, 181–188. [Google Scholar] [CrossRef]
- Tang, D.; Noordover, B.A.J.; Sablong, R.J.; Koning, C.E. Metal-free synthesis of novel biobased dihydroxyl-terminated aliphatic polyesters as building blocks for thermoplastic polyurethanes. J. Polym. Sci. Polym. Chem. 2011, 49, 2959–2968. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 4th ed.; Wiley-Blackwell Publishing Ltd.: New York, NY, USA, 2004. [Google Scholar]



| Biocatalysts | Activity |
|---|---|
| μmol Butyric Acid (g min)−1 | |
| Free Candida rugosa lipase (CRL) | 1368 |
| Immobilized Candida rugosa lipase (CRLI) | 84 |
| Free Lipase Burkholderia cepacia lipase (BCL) | 10,140 |
| Immobilized Burkholderia cepacia lipase (BCLI) | 14,100 |
| Free Pseudozyma antarctica lipase B (PBL) | 5478 |
| Immobilized Pseudozyma antarctica lipase B (PBLI) | 1224 |
| pH | Temperature (°C) | Mw (g mol−1) | |
|---|---|---|---|
| PBL | PBLI | ||
| 3.3 * | 25 | 2600 | 2700 |
| 5 | 25 | 6950 | 7300 |
| 3.3 * | 45 | 2600 | 3450 |
| 5 | 45 | 3550 | 7600 |
| Experiment Condition | Temperature (°C) | pH | Stirring (rpm) | Polycondensation System | Conversion (%) |
|---|---|---|---|---|---|
| A | 45 | 3.3 * | Magnetic, 500 | Miniemulsion | 92 ± 0.2 |
| B | 45 | 5 | Magnetic, 500 | Miniemulsion | 99 ± 0.4 |
| C | 45 | 5 | Magnetic, 250 | Miniemulsion | 98 ± 0.2 |
| D | 45 | 5 | Orbital, 250 | Miniemulsion | 99 ± 0.6 |
| E | 25 | 5 | Magnetic, 250 | Miniemulsion | 99 ± 0.2 |
| F | 65 | 5 | Magnetic, 250 | Miniemulsion | 91 ± 0.4 |
| G | 45 | 5 | Magnetic, 250 | Emsulsion without sonication | 97 ± 1.2 |
| H | 45 | 3.3 * | Magnetic, 250 | Water | 94 ± 0.3 |
| Time (h) | Batch | Fed-Batch | |||
|---|---|---|---|---|---|
| Mw (g mol−1) | Conversion (%) | Mw (g mol−1) | Conversion (%) | ||
| Miniemulsion a | 1 | 2600 | 92.9 ± 1.0 | 2700 | 100 ± 0.5 |
| 24 | 3700 | 98.2 ± 1.1 | 2700 | 76.9 ± 1.3 | |
| 48 | 7800 | 98.3 ± 0.7 | 4600 | 96.2 ± 1.0 | |
| Water a | 1 | 2700 | 75.6 ± 1.8 | 1950 | 100 ± 0.4 |
| 24 | 3450 | 98.5 ± 1.5 | 3450 | 100 ± 0.3 | |
| 48 | 6900 | 94.3 ± 1.3 | 3800 | 100 ± 0.5 | |
| Organic solvent | 1 | 2700 | 53.0 ± 2.3 | 4500 | 74.4 ± 1.4 |
| 24 | 5900 | 87.5 ± 1.9 | 4600 | 93.2 ± 0.9 | |
| 48 | 5900 | 86.7 ± 1.8 | 5900 | 94.6 ± 1.2 | |
| Time (h) | Vial with a Magnetic Stirrer (RMS) | Reactor with Impeller (RI) | |||
|---|---|---|---|---|---|
| Mw (g mol−1) | Conversion (%) | Mw (g mol−1) | Conversion (%) | ||
| Miniemulsion a | 1 | 2690 | 92.3 ± 0.9 | 2690 | 45.3 ± 2.1 |
| 2 | 2690 | 90.3 ± 1.0 | 2690 | 90.2 ± 1.4 | |
| 4 | 2690 | 93.4 ± 1.2 | 2690 | 95.0 ± 0.9 | |
| 8 | 2690 | 100 ± 0.6 | 2690 | 94.4 ± 0.9 | |
| Water a | 1 | 2690 | 72.6 ± 1.4 | 2690 | 56.8 ± 2.0 |
| 2 | 2690 | 98.1 ± 0.8 | 2690 | 91.5 ± 1.2 | |
| 4 | 2500 | 100 ± 0.4 | 2690 | 88.1 ± 1.0 | |
| 8 | 3460 | 100 ± 0.6 | 2690 | 99.3 ± 0.4 | |
| Organic solvent | 1 | 2690 | 53.9 ± 2.0 | 2690 | 63.1 ± 1.8 |
| 2 | 3460 | 72.5 ± 1.7 | 2690 | 77.7 ± 1.7 | |
| 4 | 4900 | 82.2 ± 1.1 | 2690 | 79.7 ± 1.0 | |
| 8 | 4900 | 86.7 ± 1.3 | 2690 | 78.8 ± 1.3 | |
| System | Tmax (°C) | Tonset (°C) | Tm (°C) 2 | Weight Loss (%) |
|---|---|---|---|---|
| Miniemulsion | 488.6 | 387.7 | 59.2 | 97.5 |
| Water | 419.9 | 385.6 | 63.9 | 98.2 |
| Organic solvent 1 | 416.8 | 385.8 | 70.8 | 99.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfluck, A.C.D.; de Barros, D.P.C.; Fonseca, L.P. Biodegradable Polyester Synthesis in Renewed Aqueous Polycondensation Media: The Core of the New Greener Polymer-5B Technology. Processes 2021, 9, 365. https://doi.org/10.3390/pr9020365
Pfluck ACD, de Barros DPC, Fonseca LP. Biodegradable Polyester Synthesis in Renewed Aqueous Polycondensation Media: The Core of the New Greener Polymer-5B Technology. Processes. 2021; 9(2):365. https://doi.org/10.3390/pr9020365
Chicago/Turabian StylePfluck, Ana C. D., Dragana P.C. de Barros, and Luis P. Fonseca. 2021. "Biodegradable Polyester Synthesis in Renewed Aqueous Polycondensation Media: The Core of the New Greener Polymer-5B Technology" Processes 9, no. 2: 365. https://doi.org/10.3390/pr9020365
APA StylePfluck, A. C. D., de Barros, D. P. C., & Fonseca, L. P. (2021). Biodegradable Polyester Synthesis in Renewed Aqueous Polycondensation Media: The Core of the New Greener Polymer-5B Technology. Processes, 9(2), 365. https://doi.org/10.3390/pr9020365








