Abstract
Food by-products contain a remarkable source of bioactive molecules with many benefits for humans; therefore, their exploitation can be an excellent opportunity for the food sector. Moreover, the revalorization of these by-products to produce value-added compounds is considered pivotal for sustainable growth based on a circular economy. Traditional extraction technologies have several drawbacks mainly related to the consumption of hazardous organic solvents, and the high temperatures maintained for long extraction periods which cause the degradation of thermolabile compounds as well as a low extraction efficiency of desired compounds. In this context, supercritical fluid extraction (SFE) has been explored as a suitable green technology for the recovery of a broad range of bioactive compounds from different types of agri-food wastes. This review describes the working principle and development of SFE technology to valorize by-products from different origin (marine, fruit, vegetable, nuts, and other plants). In addition, the potential effects of the extracted active substances on human health were also approached.
1. Introduction
During the production and processing of aquatic products, fruit, vegetables, and cereals, a huge amount of by-products are generated in the food industry. Although these by-products are rich in high-added-value biologically active substances, including proteins, oils, vitamins, and polyphenols, they are usually only employed for feed processing, biological fertilizers, etc., and even a considerable part is directly discarded [1]. This not only reduces the use value of these by-products, but also causes severe pollution problems for the environment [1,2].
For the purpose of recycling waste and protecting the environment, researchers have evaluated the suitability of by-products generated during the food industry’s production process to recover their biologically active ingredients by various extraction methods [2,3]. Extracting nutrients and bioactive compounds from agricultural and sideline raw materials and processing them into different products is an important part of food production, whereas extraction technology is the key factor to determine the quality of the extracted compounds. Due to the shortcomings of traditional extraction technologies, such as low extraction efficiency, high temperatures, which can promote the degradation of thermolabile compounds, and residual toxic reagents, researchers are continuously explored the application of new extraction technologies to recover by-products from the agri-food industry [4].
Among the green technologies to extract nutrients and bioactive compounds, different studies have shown that supercritical fluid extraction (SFE) technology is simple to operate, has low extraction temperature, and is non-polluting [5]. SFE has been widely applied in the extraction of different nutrients and bioactive compounds from several types of food processing by-products, such as polyunsaturated fatty acids from marine fish by-products [6], carotenoids from vegetable wastes and marine microalgae [7,8], as well as polyphenol antioxidant bioactive substances from fruit processing by-products [9]. In addition to being used alone, SFE can be also combined with other extraction technologies, such as mechanical expression, to extract phenolic substances and oils from olive kernels, and improve the extraction efficiency, further protecting the biological activity of the extracted substances [10].
Besides the physical and chemical properties of recovered extracts, it is necessary to take into account their positive impact on human health, since one of the main objectives of their extraction is their incorporation into functional foods. Precisely, the feasibility of SFE technology is not only reflected in improving the extraction efficiency, but also has the effect of preserving the bioactivity of the recovered substances as well as to improve the functional characteristics of the extract. The low-temperature extraction conditions of supercritical fluids are very suitable for the recovery of biologically active compounds from agri-food by-products, allowing the recovery of thermolabile or easily oxidizable molecules [11]. In this sense, a recent study showed the superiority of supercritical CO2 extraction to obtain highly bioaccessible α-mangostin from mangosteen pericarp extract in comparison with conventional extraction using ethanol (91% vs. 30%) [12]. Other research also demonstrated that the application of SFE technology can enhance the extraction efficiency of fish oil, also preserving its antioxidant activity [13].
At this time of fast technological development, the relationship between SFE technology and the food industry will become closer in the future. From the perspective of social economic benefits, consumer′s requirements regarding the biological activity of extracted products from natural resources and their impact on human health is increasing. This review aims to summarize the application of supercritical fluid extraction technology to recover natural active substances from food processing by-products and underutilized biological resources, as well as their beneficial effects on human health. The main aspects reported in this review are shown in Figure 1.
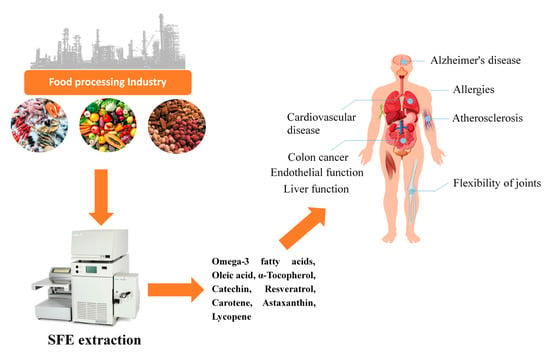
Figure 1.
Application of supercritical fluid extraction (SFE) in the recovery of natural active substances from food processing by-products and their beneficial effects on human health.
2. General Aspects of SFE Process
Supercritical fluid (SF) is defined as any substance that presents properties of both gas and liquid above its critical temperature and pressure (Figure 2). Under these conditions, SF has lower viscosity and higher diffusivity than traditional solvents, which allows increasing of the penetration of the solvent through the solid matrix and, therefore, enhancing of the extraction yields of a broad spectrum of biocompounds [3,14]. Another important characteristic of this type of fluids is the possibility of modifying its density by changing its pressure and/or temperature in a manner, thus facilitating the extraction of the desired compounds [12]. Various solvents can be used as SF; however, when selecting the most suitable solvent for a particular application, aspects such as the critical conditions of pressure and temperature, toxicity, cost, and solvation power should be considered [15]. Carbon dioxide (CO2) has become the most commonly used gas in SFE due to its multiple advantages such as its innocuous nature to human health, environmentally friendly, inexpensive, non-corrosive, readily available, and its reusability [16]. CO2 also has interesting physicochemical properties, since it is an inert, nonpolar, nonflammable, odorless, and tasteless gas. Moreover, it has low-value critical parameters (temperature of 31.1 °C and pressure of 73.8 bar). Its moderate critical temperature makes it especially suitable for the extraction of thermolabile bioactive molecules [12,17]. Another notable advantage of CO2 is that it is gaseous at room temperature and pressure, so once the extraction is finished and by decompression of the system, the total removal of CO2 is achieved, obtaining a solvent-free extract without the need for further expensive purification treatments [18].
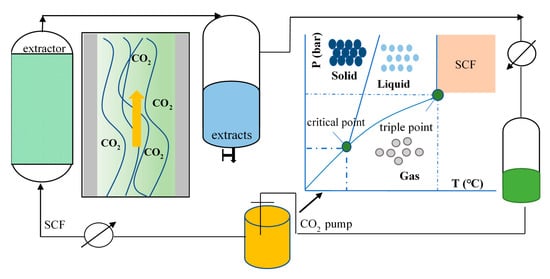
Figure 2.
Schematic diagram of the supercritical fluid phase transition curve.
Nevertheless, the low polarity of CO2 makes it less effective in extracting more polar phytochemicals from natural sources. In order to overcome this limitation, co-solvents (also called modifiers or entrainers) are used and added in small quantities that can improve the solubilizing capacity of CO2 and increase the extraction of more polar compounds [12,16]. Ethanol is commonly used as a modifier due to its low toxicity and enhanced extraction capacity of polar compounds such as polyphenols [3].
The extraction of phytochemicals from several natural sources by SFE is a complex process that is influenced by several operating variables: process parameters (temperature and/or pressure), extraction time, factors related to mass transfer (solvent to solid ratio and particle size), percentage of modifiers, and solvent flow rate [19]. Therefore, proper optimization of all of them is necessary in order to improve the extraction yields and selectivity of the extracted biomolecules. In fact, a great number of reviews and original works have focused on the optimization of the SFE process to recover valuable compounds from plant matrices [20,21,22,23]. The next sections discuss the more relevant information derived from these studies.
3. Use of SFE Technology for Aquatic By-Products
3.1. Current Status of Consumption of Aquatic Products and Utilization of the By-Products
Aquaculture is the fastest growing food production sector, representing about 50% of the fish that is used as food worldwide [24]. According to a recent report by the Food and Agriculture Organization of the United Nations (FAO), world fish production increased from 19 million metric tons (MMT) in 1950 to 171 MMT in 2016 [24]. In per capita terms, fish consumption has also risen from 9.0 kg in 1961 to 20.2 kg in 2015 [25]. As a consequence of industrial fish processing, a great amount of waste is generated each year, which can represent up to 75% of the total weight of the catch, although it differs according to the species, size, or the processing type [26]. The fishery by-products include skin, scales, bones, thorns, and internal organs, among others [27]. As shown in Figure 3, these marine by-products are generally discarded without trying to take advantage of them, causing serious environmental problems. However, several studies have indicated that they are a magnificent source of different active biomolecules with interesting health-promoting properties [1,27].
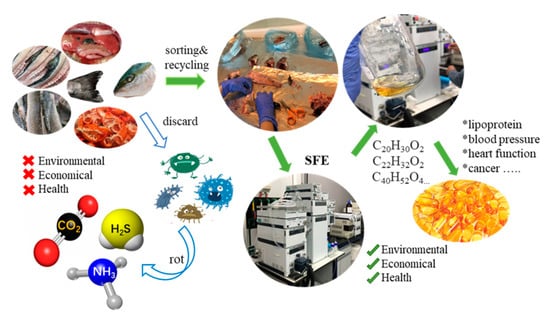
Figure 3.
The benefits of supercritical fluid technology to recover by-products from aquatic products.
3.2. Main Biologically Active Ingredients in Aquatic By-Products
According to the characteristics of SFE, by-product extracts from aquatic industries are mainly non-polar components. Unsaturated fatty acids rich in omega-3, which is one of the main extracted compounds from aquatic fish and shrimp by-products, are recognized for their outstanding human health benefits [28]. Omega-3 fatty acids, mainly containing two types of fatty acids—eicosapentaenoic (EPA) and docosahexaenoic acids (DHA)—present multiple biological activities on lipoprotein, blood pressure, heart function, cancer, endothelial function, vascular reactivity, and cardiac electrophysiology, as well as effective anti-inflammatory effects [29]. Recently, Bettadahalli et al. demonstrated the retinoprotective potential of oleic acid and EPA + DHA in hyperlipidemia-induced retinal dysfunction [30]. Some authors have also reported that omega-3 PUFAs supplementation has a positive impact on the prevention and/or treatment of neurological diseases, mainly depression and anxiety [31]. An epidemiological study conducted by Kim et al. demonstrated that the consumption of long-chain omega-3 PUFAs had beneficial effects in preventing distal large bowel cancer [32]. More recently, Zheng et al. reported that Antarctic krill oil rich in EPA and DHA exhibited a notable inhibitory effect on the growth of several tumor cells (U937, K562, SMMC-7721, PC-3, MDA-MB-231, HL60, and MCF-7) [29]. The role of omega-3 on the gut microbiota has also been studied by many researchers. For instance, omega-3 acids have been evaluated by their capacity to attenuate clinical colitis and colonic immunopathology in animal models through decreasing proinflammatory cytokine synthesis and improving epithelial barrier function [33]. In different clinical studies, it has been shown that supplementation with omega-3 PUFAs led to a remarkable increase in Bifidobacterium, Roseburia, and Lactobacillus and decreases in Firmicutes/Bacteroidetes ratio as well as levels of Coprococcus and Faecalibacterium [34,35].
Astaxanthin, a biologically active substance that can be obtained from shrimp and crab by-products, has a very strong antioxidant capacity, even 6000 times higher than vitamin C [36]. Astaxanthin has a high potential and promising applications in nutrition as it presents multiple beneficial effects on human health. This compound is effective to treat chronic inflammation, cardiovascular disease, diabetes, metabolic syndrome, neurodegenerative diseases, cancer, and skin and eyes diseases [37]. Hence, the valorization of this waste to obtain high-added-value compounds is an interesting strategy, since it satisfies environmental concerns and improves the viability of the fishing industry.
3.3. Application of SFE in Extraction of Aquatic By-Products
Up to now, most of the research studies that have assessed the potential of SFE to recover valuable molecules from fish wastes have mainly brought into focus the extraction of polyunsaturated fatty acids and antioxidants compounds [27,38,39]. Some relevant works based on SFE for the isolation of bioactive compounds from these by-products are summarized in Table 1 and discussed below.

Table 1.
Application of supercritical fluid extraction (SFE) in aquatic by-products or unexploited resources.
A survey of the literature showed that the efficiency of the SFE process depends on several operating variables (type of supercritical fluid and CO2 pressure, temperature, time, entrainer, and flow rate) that need to be optimized to reach high extraction rates as well as to ensure the quality of the recovered compounds. For instance, Ferdosh et al. evaluated the effects of pressure (20–40 MPa), temperature (45–65 °C), and flow rate (1–3 mL/min) on the efficient recovery of oil from tuna head using a central composite rotatable design [42]. The SFE process at the combined conditions of 65 °C, 40 MPa, and 3 mL/min resulted in an optimal oil yield of 35.5%. The authors also reported that the fatty acid profile was formed by 41.6, 24.7, and 26.8% of saturated, monounsaturated, and polyunsaturated fatty acids (PUFAs), respectively. Among PUFAs, 22.3% corresponded to omega-3 fatty acids. Lisichkov et al. also applied an experimental design based on response surface methodology to study the effect of some working parameters (pressure: 200, 300, 350, and 400 bars; temperature: 40, 50, and 60 °C; mass flow of CO2: 0.194, 0.274, and 0.354 kg/h; and time: 30, 60, 120, and 180 min) on the extraction of PUFAs from common carp (Cyprinus carpio L.) viscera. Analysis of the 3D response surfaces revealed that the increase in pressure, CO2 flow rate, and extraction time had a positive impact on the extraction yield; however, the temperature displaying different behaviors relied upon the operating pressure values at isobaric conditions. SFE using 400 bar, 60 °C, at a CO2 flow rate of 0.354 kg/h for 180 min resulted in an optimal yield of PUFAs (25.24%) [51].
Rubio-Rodríguez et al. assessed the efficiency of SFE to recover omega-3 rich oil from hake (Merluccius capensis - Merluccius paradoxus) by-products in comparison with conventional solvent [40]. Under optimal extraction conditions (25 MPa, 10 kg CO2/h, and 40 °C), more than 96% of the total oil present in the raw material (determined by Soxhlet extraction) was extracted after 3 h. The results indicated that the use of SFE at low temperature resulted in a higher content of total fatty acids, mainly unsaturated, than the oil extracted with hexane. This behavior can be attributed to the fact that the Soxhlet extraction is carried out at high temperatures (69 °C), which can lead to thermal degradation of fatty acids. In another work, Sahena et al. compared various SFE techniques (co-solvent extraction, soaking, and pressure swing), using different pressures (20–35 MPa) and temperatures (45–75 °C) for oil extraction from ground skin of Indian mackerel (Rastrelliger kanagurta) [41]. The results indicated that the oil extractability raised with pressure and temperature, achieving for all extraction modes values between 52.3 and 53.2% at 35 MPa and 75 °C, which are very close to those obtained with the Soxhlet method (53.6%). In this study, the authors also stated that the addition of small amounts of an entrainer like ethanol to CO2 increased the solubility of the oil, improving the extraction of relatively more polar unsaturated triglycerides.
Compared with traditional methods, SFE shows extraordinary advantages in fish oil extraction [52]. It was found that SFE under mild conditions (25 MPa and 40 ºC) is a suitable technology for obtaining fish oil with a high content of omega-3 PUFAs, whether compared with conventional extraction methods (cold extraction, wet reduction, enzymatic extraction). The results revealed that SFE prevented lipid oxidation and considerably reduced the levels of certain pollutants like arsenic. Similar findings were also reported by Taati et al. who indicated that SFE led to high oil extraction yields and reduced the damage of PUFAs and vitamins, resulting in a better quality product [53]. The authors attributed these results to the absence of atmospheric oxygen in the SFE process and the mild temperatures used during extraction. Kuvendziev et al. [6] proved that SFE (400 bar, 60 °C, and CO2 flow rate of 0.194 kg/h) was more selective to isolate mono- and polyunsaturated fatty acids from carp (Cyprinus carpio L.) by-products (viscera) than solid–liquid extraction by Soxhlet.
In addition to fish, industrial shrimp processing also produces large amounts of waste (in particular heads, shells, and tails) that correspond with approximately 50–60% of the catch [45]. Several studies have reported that these by-products are an excellent source of omega-3 PUFAs, carotenoids, and astaxanthin that can be extracted by SFE [1,27,54]. In this context, Treyvaud Amiguet et al. applied SFE at moderate conditions (35 MPa, 40 °C) to extract deep red oil rich in omega-3 PUFAs from these by-products [54]. In this research, conventional extraction resulted in higher oil extraction yields (206 mg oil/g for acetone and 178 mg oil/g for hexane) than SFE (137 mg oil/g). However, the extract obtained by SFE had a higher content of total fatty acids (TFA: 795 mg/g) including eicosapentaenoic acid (EPA: 78 mg/g) and docosahexaenoic acid (DHA: 79.7 mg/g), as compared to extractions with acetone (TFA: 627 mg/g; EPA: 69.9 mg/g; DHA: 66.8 mg/g) and hexane (TFA: 705 mg/g; EPA: 72 mg/g; DHA: 69.7 mg/g). In addition, SFE presented two important advantages over Soxhlet extraction: reduction in extraction time and avoidance of additional solvent removal. Similarly, Nguyen et al. also highlighted the potential of SFE to recover lipids with a high level of PUFAs and low concentration of heavy metals from the livers of Australian Rock Lobsters (Jasus edwardsii) compared with Soxhlet extraction [55]. More interestingly, Mezzomo et al. analyzed the cost of the SFE to extract carotenoids from pink shrimp (Penaeus brasiliensis and P. paulensis) by-products and their report showed this technology is suitable for the valorization of pink shrimp wastes, since it allows obtaining of an extract with high yield and quality, and with a low cost of the target product [46].
In summary, we can suggest that SFE is a suitable technology to develop sustainable and efficient extraction processes compared to conventional extraction not only for waste management from the aquatic industries but also to satisfy the high demand for valuable compounds such as PUFA.
4. Use of SFE Technology for Fruits and Vegetables By-Products
4.1. Current Status of Consumption of Fruits and Vegetables and Utilization of the By-Products
Fruit demand has raised significantly during the last decade, mostly due to several epidemiological studies that have linked the dietary intake of fruits with a reduction in the incidence of cancer and cardiovascular disease mortality [2,56]. According to FAO data, the global production of fruits consists of 27.4 MMT of melon, 86.1 MMT of apples, 115.7 MMT of bananas, 138.6 MMT of citrus (including oranges, lemons, limes, grapefruit, and tangerines and mandarins), 79.12 MMT of grapes, 55.38 MMT of mangoes, mangosteens, and guavas, and 28.25 MMT of pineapples [24]. In industrial fruit processing, an important amount of waste is generated as skins, pulp, seeds, stones, and pomace, which accounts for 10% to 35% of processed material [57]. Vegetable processing also produces a large amount of waste that is an exceptional natural source of phytochemicals, including phenolic compounds, flavonoids, carotenoids, and vitamins [56]. For example, tomato is one of the most consumed worldwide, and its processing to produce ketchup and sauce generates great amounts of by-products including peels, pulp, and seeds, which corresponds to 40% of the net weight of tomatoes [58]. Tomatoes present a high content of carotenoids (5.1–6.3 mg/100 g), lycopene being the main component (accounting for 70–80% of the total carotenoids) and it is responsible for the deep-red color of ripe fruit [59]. It is important to note that the discarded tomato peel contains up to five times more lycopene than pulp [58]. Onion is also an important crop worldwide, and its annual production in Europe is estimated at about 6 MMT, generating each year approximately 500,000 tons of waste. These by-products are a high-quality source of active biomolecules such as flavonoids, phenolic acids, anthocyanins, and thiosulfinates, among others [1,60]. As shown in Figure 4, there is also a huge waste of other fruit and vegetable by-products. These by-products lack value-added industrial applications and are often discarded, causing not only serious environmental problems due to their high biodegradability, but also important economic loss.
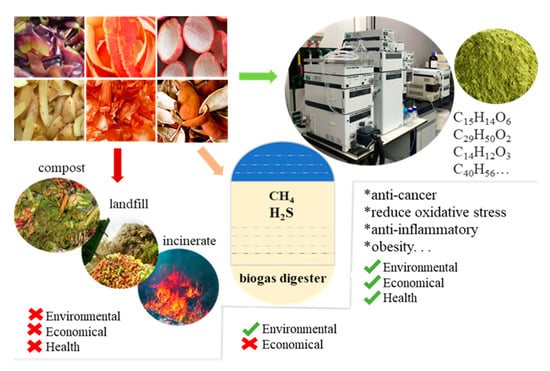
Figure 4.
The benefits of supercritical fluid technology to recover by-products from fruit and vegetable products.
4.2. Main Biologically Active Ingredients in Fruit and Vegetable By-Products
At present, SFE has been applied in the extraction of fruits and vegetables by-products. There are some reported bioactive components with added value for human health, such as catechin, α-tocopherol, resveratrol, and various natural pigments. α-tocopherol is the substance with the strongest antioxidant capacity among the eight forms of vitamin E, and is widely used in the treatment of illness related to human oxidative damage [61]. Besides its antioxidant properties, recent studies have confirmed the important role of α-tocopherol in other health problems, such as obesity [62], fatty liver [63], heart disease [64], lung function [65], inter alia. In addition, the results of some animal studies have shown that α-tocopherol reverses neurodegeneration by reducing oxidative stress, which promotes brain health [66]. Some studies have also revealed that α-tocopherol intake reduces the risk of colon, pancreatic, and bladder cancer [67]. Catechins are present in several fruits and herbs including tea, apples, cacaos, grapes, and berries. Catechins exhibit a plethora of pharmacological effects that have been demonstrated by both epidemiological and in vitro studies, and including antioxidants, anti-inflammatory, antibacterial, neuroprotective, cardiac protection, and anticancer activities [68]. For instance, Alshatwi stated apoptotic effects of catechin on MCF-7 breast cancer cells and Shahid et al. reported its protective effect in the lungs against toxins such as benzo (a) pyrene [69,70]. Due to their high antioxidant potential, catechins also have shown promising results in reducing neurotoxicity and oxidative stress in neurodegenerative disorders as in Alzheimer’s disease (AD) [71] and Parkinson’s disease (PD) [72]. Resveratrol (3,5,40-trihydroxy-trans-stilbene) is another important biomolecule that can be obtained from fruit by-products. Evidence in vitro, in vivo, and even clinical trials have shown that resveratrol possesses numerous therapeutic benefits, namely antioxidant, neuroprotective, cardioprotective, anti-inflammatory, anticancer, and antidiabetic properties [73]. Related research results show that resveratrol protects different organs from ischemia-reperfusion injury by up-regulating antioxidant enzymes, reducing cell death induced by hypoxia and oxidative stress [74,75]. However, a recent study revealed that the continuous administration of resveratrol (1000 mg/day or higher) in overweight older adults increases the levels of cardiovascular risk, which indicated high doses of resveratrol may also have a negative impact on human health [76].
Natural pigments, including liposoluble pigments such as β-carotene, astaxanthin, lycopene, and lutein, and water-soluble pigments such as anthocyanins are also present in several by-products. β-carotene is a natural pigment abundant in plants and fruits responsible for the yellow and orange colorations of these products. β-carotene is the major carotenoid in the human diet and is the main precursor of Vitamin A [77]. Several epidemiological studies have confirmed the myriad of health benefits related to the consumption of this compound including antioxidant activity, prevention of some types of cancer, cardiovascular disease, age-related macular degeneration, cataracts, and enhancement of immunological function [77]. Unlike β-carotene, lycopene is less stable because it is extremely sensitive to oxidation reactions. However, the antioxidant capacity of lycopene is much higher than other carotenoids and has superior physiological functions. Some epidemiological studies have indicated an inverse relationship between lycopene consumption and the risk of certain types of cancer [78]. Lycopene reduces the aggression of prostate tumors and prevents lung tumorigenesis [79,80]. Lycopene also has a key role in preventing cardiovascular diseases and arteriosclerosis, strengthening the human immune system, delaying aging, and can reverse neurobehavioral deficits. Besides its health-promoting properties, lycopene has been widely used in the food industry as a natural colorant due to its strong red color and non-toxicity [78].
Lutein is present in leafy green vegetables and brightly colored fruits, presenting an antioxidant activity 10 times more powerful than β-carotene and 15 times stronger than that of lycopene. Studies have shown that lutein has a positive effect on protecting eyesight, inhibiting oxygen free radical activity in the body, preventing tumor angiogenesis and cell proliferation, slowing the development of early arteriosclerosis, and decreasing the risk of diabetes [81]. The daily intake of lutein is well below the recommended dose (1.7 vs. 6–14 mg/dL) necessary to exert its health-promoting effects. Therefore, the development of foods enriched in lutein recovered from natural sources can be an interesting strategy for the modern food industry.
4.3. Application of SFE in Fruit By-Products
SFE is recognized as an efficient extraction method to selectively isolate valuable molecules from fruit by-products, mainly phenolic compounds and essential oils, such as in grape by-products. Casas et al. evaluated the impact of SFE on the isolation of resveratrol from grape pomace, an industrial residue from the wine process, consisting of grape seeds, skin, and stems [20]. In this case, the examined parameters were pressure (10–40 MPa) and temperature (35–55 °C), using 5% (v/v) of ethanol as the modifier. The authors proved that the addition of co-solvent improved the solubility of resveratrol, and hence increased the extraction performance. On the other hand, Marqués et al. applied SFE to extract antioxidants (catechins, epicatechin, gallic acid, and resveratrol) from grape seeds [82]. The results showed that operating under optimal conditions (15 MPa, 40 °C, and a molar fraction of CO2 of 0.98) from 1 kg of seeds, 51 mg of gallic acid, 49 mg of catechin, 53 mg of epicatechin, and 667 mg of resveratrol were recovered. In addition, the solids obtained after extraction showed remarkable values of total polyphenol content (TPC), ranging from 15.60 to 22.56 g equivalent gallic acid (GAE)/kg seed. This solid could be used as a source of antioxidant dietary fiber for the formulation of innovative foods, such as functional meat products. Furthermore, Farías-Campomanes et al. compared conventional extraction and supercritical CO2 to recover phenolic compounds, including syringic acid, vanillic acid, gallic acid, and p-hydroxybenzene formic acid, protocatechuic acid, p-coumaric acid, and quercetin, from grape bagasse [83]. Although the classic Soxhlet extraction led to a higher extraction yield (10.4%) than the SFE process (5.5%), analysis of the extracts’ composition evidenced that SFE containing 10% ethanol (w/w) at 40 °C and 20 MPa was more efficient to extract the target compounds (23 vs. 1.8 g/kg of extract). In addition, the authors carried out an economic analysis of the process, indicating that it is possible to design an industrial plant based on SFE to recover antioxidant compounds from grape bagasse. With the purpose to enhance the recovery of desired compounds from grape waste, some researchers have proposed the simultaneous combination of supercritical fluids with other extraction methods. Passos et al. [84] evaluated the effect of an enzymatic pre-treatment, containing cellulase, protease, xylanase, and pectinase, on the extraction yield of grape seed oil using supercritical CO2. The results indicated that total extraction rate augmented in the pre-treated seeds, attaining 16.5%, as compared 11.5% with untreated seeds, which represents a rise of 44%. These better results are due to the enzymatic action that causes the degradation of the cellular structure improving the subsequent extraction of the oil by supercritical CO2. Porto et al. applied a combined process based on ultrasound-assisted extraction followed by supercritical CO2 to isolate polyphenols from grape marc. The finding of this study revealed that this strategy allows for an increase in the recovery of polyphenols and total antioxidant activity by 28% and 62%, respectively, in comparison with extraction using only SFE [85].
Orange processing by-products have also been widely used to recover biologically active compounds by SFE. Benelli et al. compared Soxhlet, ultrasonic-assisted extraction, hydrodistillation, and SFE to obtain bioactive extracts of orange pomace [86]. The results showed that although the ultrasonic and Soxhlet extraction provided the highest yield, the extract obtained by SFE (20 MPa and 50 °C) exhibited better antioxidant and antibacterial properties. In a more recent study, Espinosa-Pardo et al. also investigated the extraction efficiency of SFE versus conventional Soxhlet extraction to isolate phenolic compounds from orange pomace [87]. It was found that despite the SFE enabling the acquirement of only half of the phenolic compounds obtained by Soxhlet, it is more cost-effective in terms of energy, time, and solvent consumption, since it requires 78% less time and 10 times less ethanol than Soxhlet.
Other fruits that are consumed in large quantities, such as apples, mangoes, and passion fruits, generate by-products that have also been valorized by supercritical extraction, and the main isolated biomolecules include polyphenols, tocopherols, and carotene, among others [38,88,89]. Hatami et al. [89] proposed, for the first time, a process based on the integration of SFE and supercritical adsorption (SESA) to obtain tocols from passion fruit by-products. Under optimized extraction conditions (35 MPa and 40 °C), SESA led to a yield of 19.1 g oil/100 g feed. In addition, the authors carried out the economic analysis and the results indicated that in order to obtain 1 kg of the extract, the cost of production would range from 35 to 54 USD, suggesting that the cost of raw materials (by-products and solvents) is the main factor that influences these costs. In another study, Ferrentino et al. applied SFE for the extraction of phenolics compounds from freeze-dried apple pomace [88]. According to the authors, SFE operating under optimized conditions (30 MPa and 45 °C) resulted in extracts with an antioxidant activity approximately 2.74 times greater than the Soxhlet extraction in a much shorter time (2 vs. 6 h). Sánchez-Camargo et al. optimized the SFE conditions for the isolation of carotenoids from mango peel using a Box–Behnken design [90]. SFE at 25.0 MPa, 60 °C, and 15% w/w ethanol resulted in an optimal carotenoids yield of 1.9 mg/g dry sample. In this study, the authors also highlighted the potential of this extract to protect the sunflower oil against lipid oxidation, suggesting that this application could be an interesting alternative for mango peel valorization. Valuable lipophilic constituents extraction from elderberry juice processing by-products was optimized by Kitryte et al. and they reported a recovery of this fraction of 14.05 g/100 g pomace when SFE was performed under optimal conditions (35 MPa, 53 °C, 45 min) [91]. In addition, this extract presented health-beneficial polyunsaturated linoleic (42.0%) and α-linolenic (34.1%) fatty acids. The authors also reported that the efficiency of SFE-CO2, in terms of time and of extraction yields, was higher compared to conventional Soxhlet and maceration (up to 4% higher yield in an 8-fold shorter time). However, compared to UAE, SFE turned out to be a less efficient technique (up to 29% lower yield in 3-fold longer time). Similarly, the use of SFE to recover bioactive compounds, mainly carotenoids and unsaturated fatty acids from raspberry, rowanberry, elderberry, and cranberry, has also been proposed. Table 2 details more studies about SFE for the recovery of several value-added molecules from fruit by-products.

Table 2.
Application of supercritical fluid extraction (SFE) in fruit by-products.
4.4. Application of SFE in Vegetables By-Products
Several studies have applied SFE to obtain lycopene from tomato by-product. Kehili et al. extracted lycopene from tomato peels at a temperature of 50–80 °C, pressure of 30–50 MPa, and flow rate of 3–5 g CO2/min for 105 min [23]. The extraction performance of lycopene and carotene was between 32.0–60.9% and 28.4–58.8%, respectively, and the results suggested that only the temperature had a significant influence on the extraction process. Moreover, SFE was compared to conventional maceration extraction using different solvents (hexane, ethyl acetate, and ethanol). The authors found that SFE (40 MPa, 80 °C, and 4 g CO2/min) led to a higher lycopene recovery (728.98 mg/kg of dry tomato peels) compared to conventional extraction (hexane: 608.94 mg/kg; ethyl acetate: 320.35 mg/kg; and ethanol: 284.53 mg/kg of dry tomato peels). In a previous study, Vági et al. evaluated the effect of the temperature and pressure on the recovery of lycopene from tomato by-products [59]. The extraction efficiency was higher at a temperature of 80 °C and a pressure of 46 MPa, obtaining an extract with a high concentration of lycopene (90.1%). In this study, the authors also studied the effect of different storage conditions on supercritical extraction of carotenoids from tomato by-products. The results revealed that the recovery of carotenoids from deep-frozen tomato waste was ten-fold higher in comparison with those of air-dried samples, indicating that the deep-frozen storage is the most suitable choice to guarantee a greater recovery of valuable biocompounds from tomato waste.
Extensive research has also been conducted on SFE from onion by-products. Devani et al. evaluated the effects of pressure (15–45 MPa), temperature (50–90 °C), time (30–150 min), and particle size (0.4–1.2 mm) on oleoresin extraction yield, sulfur content, and pyruvate content from rotten onion waste through a central composite rotatable design [109]. Under optimized extraction conditions (40 MPa, 80 °C, particle size 0.53 mm for 60 min) 1.012% oleoresin, 31 g of sulfur/kg of oleoresin, and 10.41 μ mol pyruvate/g fresh weight of onion were recovered. Furthermore, the authors also compared the efficiency of SFE with conventional extraction using hexane as the solvent. The results displayed that SFE permitted the yield of twice the sulfur content (responsible for the characteristics of the flavor of onion and some of the bioactivities associated with its consumption) in the oleoresin if compared to Soxhlet extraction. In another study, Campone et al. applied SFE to recover flavonoids from brown onion peels [60]. The extract obtained under optimized conditions (10 MPa, 40 °C and 85% ethanol) presented high content of quercetin and quercetin derivatives. The SFE extract exhibited stronger antioxidant capacity (measured by DPPH and ABTS) than that obtained using ultrasound-assisted extraction. Table 3 collects more studies about the extraction of valuable compounds from other vegetable processing by-products using SFE.

Table 3.
Application of supercritical fluid extraction (SFE) in vegetables and beans by-products.
Soybean is one of the most important leguminous crops worldwide, and nowadays, it is mainly used for the production of vegetable oil [60]. During soybean oil processing, a residue called soybean cake is generated, which is used as animal feed due to its high protein content. However, important amounts of polyphenols and other biologically active ingredients remain in the solid residue after oil extraction [116]. Several studies have shown that SFE is a good technique for the recovery of active substances from soybean by-products. Kao et al. compared the extraction efficiency of isoflavones from soybean cake using SFE and conventional solvent extraction [117]. The results showed that solvent extraction enabled a higher yield of malonylglucoside and glucoside from soybean cake, while SFE operating at 350 bar and 80 °C led to a higher amount of acetylglucoside and aglycone using this same by-product. In another study, Fang et al. used SFE to concentrate tocopherols from methyl esterified oil deodorizer distillate (ME-DOD) [121]. The authors found that a pressure of 20 MPa led to an extract with a high content of tocopherols (>50%) and with high recovery (>80%). Taking these results into account, the authors concluded that SFE fractionation is suitable for concentrating natural tocopherols from by-products from the soybean oil refining process. Recently, Alvarez et al. studied the effectiveness of SFE and the use of ethanol as a co-solvent for the recovery of phytochemicals with antioxidant capacity from soybean oil extraction by-products (soybean expellers) [116]. The best results in terms of yield, maximum extraction of polyphenols and flavonoids, and maximum antioxidant activity were achieved using CO2 as solvent (5 kg CO2/kg expeller) at 40 MPa and 35 °C using ethanol (25% w/w expeller), as modifier. The authors concluded that SFE is a safe and green technology to recover phytochemicals from soybean expellers.
As shown in this section, the use of the SFE process presents a lot of potential for the recovery of a wide range of valuable compounds from vegetable by-products. However, optimization of extraction parameters is key not only to maximize extraction performance but also to achieve greater selectivity of the target compounds isolated from this food waste.
5. Use of SFE Technology for Nuts and Other Plant By-Products Industries
Edible nuts have excellent effects on human and development, enhance physical fitness, and prevent diseases. In some nut processing industries, a huge amount of by-products is also produced. For example, tiger nuts, in the process of making “horchata”, generate waste rich in oleic acid, polyunsaturated fatty acids (linoleic acid and linolenic acid), vitamins C and E (especially α-tocopherol), and minerals (such as potassium, calcium, and magnesium) [122,123]. Therefore, the recovery of these heat-sensitive substances by SFE is an interesting alternative, since the oxidation potential of the extracted solutes is reduced.
In this context, Roselló-Soto et al. investigated the effect of SFE pressure (10–40 MPa) on the recovery of oil from “horchata” by-products [123]. Through component analysis, it was found that monounsaturated fatty acids (MUFA) were the main compounds in the by-products of “horchata”, accounting for about 70% of total fatty acids. The content of saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA) was higher and MUFA was lower in the oil extracted at 10 MPa compared to that obtained at 20, 30, or 40 MPa. The results showed that the oil yield increased from 0.61 g to 7.36 g per 100 g of “horchata” by-products applying 10 and 40 MPa, respectively. In addition, in contrast with conventional extraction, the content of α-tocopherol after SC-CO2 treatment was significantly higher than that of the traditional method, regardless of the pressure applied. The level of polyphenols and total antioxidant activity raised with the increase in SC-CO2 extraction pressure, and the data showed a linear correlation. Furthermore, they analyzed the phenolic profile of oils, and the results revealed that the predominant compound in the oil extracted by SC-CO2 was isohydroxymatrine, especially at 30 and 40 MPa, while 3-vinylphenol was the main phenolic compound in the oil recovered by traditional method. Overall, the increase in SC-CO2 extraction pressure improved both the recovery of phenolic compounds as well as the antioxidant potential and oxidation quality of the extracted oil [122].
Recently, Salinas et al. optimized SFE to extract oil from chañar almonds, a residue from “arrope” production [124]. In this study, the influence of pressure (20, 30, and 40 MPa) and temperature (40 and 60 °C) on the extraction yield and oil composition was tested. The results indicated that the best conditions to obtain a high oil yield (40%) from chañar almonds were 60 °C and 40 MPa. With these conditions, high amounts of MUFAs (363 mg/g oil) and PUFAs (468 mg/g oil) were recovered. More recently, Mazzutti et al. combined SFE with the pressurized liquid extraction (PLE) method to extract antioxidant compounds from cocoa bean hulls [125]. Compared with single separation method extracts, PLE extracted from SC-CO2 residue had higher total phenol content and antioxidant properties. TPC values ranged from 35 to 51 mg GAE/g, and EC50 (concentration that gives half-maximal response) determined by DPPH values range from 115 to 177 μg/mL. The combined application of SC-CO2 and PLE could selectively recover biologically active extracts from cocoa bean shells, which represented a green method that was promising and could provide consumables for the cosmetics and food industries. Table 4 collects more studies about the extraction of valuable compounds from nuts and other food processing by-products using SFE.

Table 4.
Application of supercritical fluid extraction (SFE) in nuts by-products and other plants.
6. Conclusions and Some Technical Considerations
This review paper summarizes the application of supercritical fluid technology in the extraction of valuable compounds with potential effects on human health from comprehensive agricultural food processing by-products. The rational use of agricultural and sideline products, using green advanced technologies, could allow for the implementation of a circular economy in the food industry in the future. New green extraction technologies are indispensable tools in the food industry nowadays. The extraction technology represented by SFE not only improved the efficiency of food processing, but also saved energy and reduced pollution.
Despite the advantages reported in the literature for the extraction of bioactive molecules from agricultural food processing by-products using SFE, there are still some challenges to overcome. The first is related to the scaling up of this technology and a better understanding of the kinetic mechanisms involved in the extraction process to improve the process yield of the bioactive compounds. The second is regarding a deeper assessment of the interaction of these molecules with food components if they are incorporated into a food matrix as well as their bioavailability in the human body and the main mechanisms employed to exert their beneficial effect on health. Third, the solvent commonly used in supercritical fluid technology is carbon dioxide fluid, and the corresponding target substances are mainly non-polar components. Therefore, how to efficiently match the entrainer to extract more polar substances is still a topic worth discussing. Fourth, due to the density of the fluid itself, the extraction of macromolecular substances from by-products, such as proteins, is still restricted. Therefore, whether it is possible to extract substances with relatively large molecular masses through supercritical fluid technology in the future is also a challenge.
Author Contributions
Conceptualization, J.M.L. and F.J.B.; writing—original draft preparation, J.Z., M.W. and F.J.B.; writing—review and editing, J.Z., B.G., M.W., P.G., J.M.L. and F.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Thanks to GAIN (Axencia Galega de Innovación) for supporting this review (grant number IN607A2019/01). J.M.L. and F.J.B. would also like to acknowledge the EU Commission and BBI-JU Horizon H2020, through AQUABIOPRO-FIT project (Aquaculture and agriculture biomass side stream proteins and bioactives for feed, fitness and health promoting nutritional supplements) grant number 790956. J.M.L. is a member of the HealthyMeat network, funded by CYTED (ref. 119RT0568). B.G. would like to express her gratitude to the Spanish Ministry of Economy and Competitiveness for the financial support (Grant reference RYC2018-026177-I). J.Z. was supported by a PhD fellowship from the China Scholarship Council (CSC) (No. 201908420246). Moreover, M.W. would also like to thank China Scholarship Council (CSC) for her PhD fellowship (No. 201908420245). In addition, Francisco J. Barba thanks Generalitat Valenciana for the support (IDIFEDER/2018/046-Procesos innovadores de extracción y conservación: pulsos eléctricos y fluídos supercríticos) through European Union ERDF funds (European Regional Development Fund).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Montefusco, A.; Marrese, P.P.; Soccio, M.; Pastore, D.; Piro, G.; Mita, G.; Lenucci, M.S. Seeds of pomegranate, tomato and grapes: An underestimated source of natural bioactive molecules and antioxidants from agri-food by-products. J. Food Compos. Anal. 2017, 63, 65–72. [Google Scholar] [CrossRef]
- Baldino, L.; Della Porta, G.; Reverchon, E. Supercritical CO2 processing strategies for pyrethrins selective extraction. J. CO2 Util. 2017, 20, 14–19. [Google Scholar] [CrossRef]
- Kuvendziev, S.; Lisichkov, K.; Zekovi, Z.; Marinkovski, M.; Musliu, Z.H. Supercritical fluid extraction of fish oil from common carp (Cyprinus carpio L.) tissues. J. Supercrit. Fluids 2018, 133, 528–534. [Google Scholar] [CrossRef]
- Matrices, V.W. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef]
- Abrahamsson, V.; Cunico, L.P.; Andersson, N.; Nilsson, B.; Turner, C. Multicomponent inverse modeling of supercritical fluid extraction of carotenoids, chlorophyll A, ergosterol and lipids from microalgae. J. Supercrit. Fluids 2018, 139, 53–61. [Google Scholar] [CrossRef]
- Kelly, N.P.; Kelly, A.L.; O’Mahony, J.A. Strategies for enrichment and purification of polyphenols from fruit-based materials. Trends Food Sci. Technol. 2019, 83, 248–258. [Google Scholar] [CrossRef]
- Misra, N.N.; Koubaa, M.; Roohinejad, S.; Juliano, P.; Alpas, H.; Inàcio, R.S.; Saraiva, J.A.; Barba, F.J. Landmarks in the historical development of twenty first century food processing technologies. Food Res. Int. 2017, 97, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Fabrowska, J.; Ibañez, E.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Supercritical CO2 extraction of bioactive compounds from Hibiscus sabdariffa. J. Supercrit. Fluids 2019, 147, 213–221. [Google Scholar] [CrossRef]
- Melgosa, R.; Sanz, M.T.; Benito-román, Ó.; Illera, A.E.; Beltrán, S. Supercritical CO2 assisted synthesis and concentration of monoacylglycerides rich in omega-3 polyunsaturated fatty acids. J. CO2 Util. 2019, 31, 65–74. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae – An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical fluid extraction of bioactive compounds from plant materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae-A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Tita, G.J.; Navarrete, A.; Martín, Á.; Cocero, M.J. Model assisted supercritical fluid extraction and fractionation of added-value products from tobacco scrap. J. Supercrit. Fluids 2021, 167. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; De Ossa, E.J.M.; Roldán, A.; De Ory, I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Supercritical fluid extraction of polyphenols from grape seed (Vitis vinifera): Study on process variables and kinetics. J. Supercrit. Fluids 2017, 130, 239–245. [Google Scholar] [CrossRef]
- Trabelsi, D.; Aydi, A.; Wüst, A.; Della, G.; Scognamiglio, M.; Cricchio, V.; Langa, E.; Abderrabba, M.; Mainar, A.M. Supercritical extraction from Citrus aurantium amara peels using CO2 with ethanol as co-solvent. J. Supercrit. Fluids 2016, 117, 33–39. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.J.; Kousoulaki, K.; Barba, F.J. Improved extraction efficiency of antioxidant bioactive compounds from Tetraselmis chuii and Phaedoactylum tricornutum using pulsed electric fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef] [PubMed]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, S.M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Cao, W.; Yang, B.; Mu, Y.; Dong, Y.; Xiu, Z. E-configuration structures of EPA and DHA derived from Euphausia superba and their significant inhibitive effects on growth of human cancer cell lines in vitro. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 47–53. [Google Scholar] [CrossRef]
- Bettadahalli, S.; Acharya, P.; Ramaiyan, B. Evidence on oleic acid and EPA + DHA role in retinal antioxidant defense, leukocyte adhesion, and vascular permeability: Insight from hyperlipidemic rat model. J. Funct. Foods 2020, 67, 103864. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain. Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- Kim, S.; Sandler, D.P.; Galanko, J.; Martin, C.; Sandler, R.S. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. Am. J. Epidemiol. 2010, 171, 969–979. [Google Scholar] [CrossRef]
- Balbas, J.; Hamid, N.; Liu, T.; Kantono, K.; Robertson, J.; White, W.L.; Ma, Q.; Lu, J. Comparison of physicochemical characteristics, sensory properties and volatile composition between commercial and New Zealand made wakame from Undaria pinnatifida. Food Chem. 2015, 186, 168–175. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Balfegò, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martínez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N.; et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Iwata, S.; Imai, T.; Shimazawa, M.; Ishibashi, T.; Hayashi, M.; Hara, H.; Nakamura, S. Protective effects of the astaxanthin derivative, adonixanthin, on brain hemorrhagic injury. Brain Res. 2018, 1698, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F.A. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids 2011. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Rubio-rodríguez, N.; De Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of the omega-3 rich oil contained in Study of the influence of process parameters on the extraction yield and oil quality. J. Supercrit. Fluids 2008, 47, 215–226. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Jahurul, M.H.A.; Khatib, A.; Norulaini, N.A.N. Extraction of fish oil from the skin of Indian mackerel using supercritical fluids. J. Food Eng. 2010, 99, 63–69. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, Z.I.; Norulaini, N.; Ab, N.; Akand, J.H.; Ghafoor, K.; Awang, M.B.; Omar, M.; Kadir, A. Supercritical carbon dioxide extraction of oil from Thunnus tonggol head by optimization of process parameters using response surface methodology. Korean J. Chem. Eng. 2013, 30, 1466–1472. [Google Scholar] [CrossRef]
- Esqu, M.M.; Bandarra, N.M.; Fontan, I.; Bernardo-gil, M.G.; Batista, I.; Nunes, M.L.; Esqu, M.M. Supercritical carbon dioxide extraction of sardine Sardina pilchardus oil. LWT Food Sci. Technol. 1997, 720, 715–720. [Google Scholar]
- Catchpole, O.J.; Grey, J.B.; Noermark, K.A. Fractionation of fish oils using supercritical CO2 and CO2 + ethanol mixtures. J. Supercrit. Fluids 2000, 19, 25–37. [Google Scholar] [CrossRef]
- Treyvaud, V.; Kramp, K.L.; Mao, J.; Mcrae, C.; Goulah, A.; Kimpe, L.E.; Blais, J.M.; Arnason, J.T. Supercritical carbon dioxide extraction of polyunsaturated fatty acids from Northern shrimp (Pandalus borealis Kreyer) processing by-products. Food Chem. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Mezzomo, N.; Martínez, J.; Maraschin, M.; Ferreira, S.R.S. Pink shrimp (P. brasiliensis and P. paulensis) residue: Supercritical fluid extraction of carotenoid fraction. J. Supercrit. Fluids 2013, 74, 22–33. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Sapatinha, M.; Pinheiro, J.; Lemos, M.F.L.; Bandarra, N.M.; Batista, I.; Paulo, M.C.; Coutinho, J.; Saraiva, J.A.; Silva, C.M. Supercritical CO2 extraction of Aurantiochytrium sp. biomass for the enhanced recovery of omega-3 fatty acids and phenolic compounds. J. CO2 Util. 2020, 38, 24–31. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskait, J.; Ba, L. Recovery of lipophilic products from wild cyanobacteria (Aphanizomenon flos-aquae) isolated from the Curonian Lagoon by means of supercritical carbon dioxide extraction. Algal Res. 2018, 35, 10–21. [Google Scholar] [CrossRef]
- Gouveia, L. Food Chemistry Functional food oil coloured by pigments extracted from microalgae with supercritical CO2. Food Chem. 2007, 101, 717–723. [Google Scholar] [CrossRef]
- Cansell, M.; Subra-paternault, P.; Thongdeng, H.; Gr, A. Extraction of phospholipids from scallop by-product using supercritical CO2 /alcohol mixtures. LWT Food Sci. Technol. 2015, 60, 990–998. [Google Scholar] [CrossRef]
- Lisichkov, K.; Kuvendziev, S.; Zeković, Z.; Marinkovski, M. Influence of operating parameters on the supercritical carbon dioxide extraction of bioactive components from common carp (Cyprinus carpio L.) viscera. Sep. Purif. Technol. 2014, 138, 191–197. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; De Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Taati, M.M.; Shabanpour, B.; Ojagh, M. Extraction of oil from tuna by-product by supercritical fluid extraction (SFE) and comparison with wet reduction method. J. Food Eng. 2017, 10, 1546–1553. [Google Scholar]
- Trung, T.S.; Phuong, P.T.D. Bioactive compounds from by-products of shrimp processing industry in Vietnam. J. Food Drug Anal. 2012, 20, 194–197. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Significant enrichment of polyunsaturated fatty acids (PUFAs) in the lipids extracted by supercritical CO2 from the livers of Australian rock lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Machmudah, S.; Winardi, S.; Sasaki, M.; Goto, M.; Kusumoto, N. Lycopene extraction from tomato peel by-product containing tomato seed using supercritical carbon dioxide. J. Food Eng. 2012, 108, 290–296. [Google Scholar] [CrossRef]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.P.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. J. Supercrit. Fluids 2007, 40, 218–226. [Google Scholar] [CrossRef]
- Campone, L.; Celano, R.; Lisa, A.; Pagano, I.; Carabetta, S.; Di, R.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response surface methodology to optimize supercritical carbon dioxide / co- solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef]
- Tucker, J.M.; Townsend, D.M. Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed. Pharm. 2005, 59, 380–387. [Google Scholar] [CrossRef]
- Hamułka, J.; Górnicka, M.; Sulich, A.; Frąckiewicz, J. Weight loss program is associated with decrease α-tocopherol status in obese adults. Clin. Nutr. 2019, 38, 1861–1870. [Google Scholar] [CrossRef]
- Bartolini, D.; Torquato, P.; Barola, C.; Russo, A.; Rychlicki, C.; Giusepponi, D.; Bellezza, G.; Sidoni, A.; Galarini, R.; Svegliati-Baroni, G.; et al. Nonalcoholic fatty liver disease impairs the cytochrome P-450-dependent metabolism of α-tocopherol (vitamin E). J. Nutr. Biochem. 2017, 47, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Kumar, R.; Ferrie, R.P.; Balmert, L.C.; Kienzl, M.; Rifas-Shiman, S.L.; Gold, D.R.; Sordillo, J.E.; Kleinman, K.; Camargo, C.A.; Litonjua, A.A.; et al. Associations of α- and γ-tocopherol during early life with lung function in childhood. J. Allergy Clin. Immunol. 2020, 13–16. [Google Scholar] [CrossRef]
- Morris, M.C.; Schneider, J.A.; Li, H.; Tangney, C.C.; Nag, S.; Bennett, D.A.; Honer, W.G.; Barnes, L.L. Brain tocopherols related to Alzheimer’s disease neuropathology in humans. Alzheimers Dement. 2015, 11, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ranard, K.M.; Erdman, J.W. Effects of dietary RRR α-tocopherol vs all-racemic α-tocopherol on health outcomes. Nutr. Rev. 2018, 76, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J. Exp. Clin. Cancer Res. 2010, 29, 167. [Google Scholar] [CrossRef]
- Shahid, A.; Ali, R.; Ali, N.; Hasan, S.K.; Bernwal, P.; Afzal, S.M.; Vafa, A.; Sultana, S. Modulatory effects of catechin hydrate against genotoxicity, oxidative stress, inflammation and apoptosis induced by benzo(a)pyrene in mice. Food Chem. Toxicol. 2016, 92, 64–74. [Google Scholar] [CrossRef]
- Ejaz Ahmed, M.; Khan, M.M.; Javed, H.; Vaibhav, K.; Khan, A.; Tabassum, R.; Ashafaq, M.; Islam, F.; Safhi, M.M. Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochem. Int. 2013, 62, 492–501. [Google Scholar] [CrossRef]
- Teixeira, M.D.A.; Souza, C.M.; Menezes, A.P.F.; Carmo, M.R.S.; Fonteles, A.A.; Gurgel, J.P.; Lima, F.A.V.; Viana, G.S.B.; Andrade, G.M. Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol. Biochem. Behav. 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420–10428. [Google Scholar] [PubMed]
- Guan, P.; Sun, Z.M.; Wang, N.; Zhou, J.; Luo, L.F.; Zhao, Y.S.; Ji, E.S. Resveratrol prevents chronic intermittent hypoxia-induced cardiac hypertrophy by targeting the PI3K/AKT/mTOR pathway. Life Sci. 2019, 233, 116748. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults–A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, encapsulation, and health benefits of β-carotene-A review. Cogent Food Agric. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT-Food Sci. Technol. 2020, 127, 109323. [Google Scholar] [CrossRef]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato lycopene and lung cancer prevention: From experimental to human studies. Cancers 2011, 3, 2333–2357. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Sharafabad, F.; Ghoreishi, Z.; Maleki, V.; Tarighat-Esfanjani, A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies. Pharmacol. Res. 2019, 149, 104477. [Google Scholar] [CrossRef] [PubMed]
- Marqués, J.L.; Della Porta, G.; Reverchon, E.; Renuncio, J.A.R.; Mainar, A.M. Supercritical antisolvent extraction of antioxidants from grape seeds after vinification. J. Supercrit. Fluids 2013, 82, 238–243. [Google Scholar] [CrossRef]
- Farías-campomanes, A.M.; Rostagno, M.A.; Meireles, M.A.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Passos, C.P.; Silva, R.M.; Da, F.A.; Coimbra, M.A.; Silva, C.M. Enhancement of the supercritical fluid extraction of grape seed oil by using enzymatically pre-treated seed. J. Supercrit. Fluids 2009, 48, 225–229. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT-Food Sci. Technol. 2015, 61, 98–104. [Google Scholar] [CrossRef]
- Benelli, P.; Riehl, C.A.S.; Smânia, A.; Smânia, E.F.A.; Ferreira, S.R.S. Bioactive extracts of orange (Citrus sinensis L. Osbeck) pomace obtained by SFE and low pressure techniques: Mathematical modeling and extract composition. J. Supercrit. Fluids 2010, 55, 132–141. [Google Scholar] [CrossRef]
- Espinosa-pardo, F.A.; Mayumi, V.; Alves, G.; Alves, J.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2016, 101, 1–10. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Hatami, T.; Cristina, L.; Leone, G.; Virginia, P.; Pontes, D.A.; Augusto, E.; Batista, C.; Helena, L.; Mei, I.; Martínez, J. Integrated supercritical extraction and supercritical adsorption processes from passion fruit by-product: Experimental and economic analyses. J. Supercrit. Fluids 2020, 162, 162. [Google Scholar] [CrossRef]
- Sánchez-camargo, P.; Gutiérrez, L.; Milena, S.; Martinez-correa, H.A.; Parada-alfonso, F.; Narváez-cuenca, C. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Kitryt, V.; Syrpas, M.; Pukalskas, A. Modeling and optimization of supercritical carbon dioxide extraction for isolation of valuable lipophilic constituents from elderberry (Sambucus nigra L.) pomace. J. CO2 Util. 2020, 35, 225–235. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Augusta, A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Fiori, L.; Lavelli, V.; Simon, K.; Siva, P.; Sri, C.; Ben, H.; Guella, G. Supercritical CO2 extraction of oil from seeds of six grape cultivars: Modeling of mass transfer kinetics and evaluation of lipid profiles and tocol contents. J. Supercrit. Fluids 2014, 94, 71–80. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Extraction of proanthocyanidins from grape marc by supercritical fluid extraction using CO2 as solvent and ethanol – water mixture as. J. Supercrit. Fluids 2014, 87, 59–64. [Google Scholar] [CrossRef]
- Cristina, L.; Gabrí, R.; De Tarso, P.; Martínez, J. Fluid phase equilibria solubility of passion fruit (Passiflora edulis Sims) seed oil in supercritical CO2. Fluid Phase Equilib. 2019, 493, 174–180. [Google Scholar] [CrossRef]
- Massias, A.; Boisard, S.; Baccaunaud, M.; Leal, F.; Subra-paternault, P. Recovery of phenolics from apple peels using CO2 + ethanol extraction: Kinetics and antioxidant activity of extracts. J. Supercrit. Fluids 2015, 98, 172–182. [Google Scholar] [CrossRef]
- Montañés, F.; Catchpole, O.J.; Tallon, S.; Mitchell, K.A.; Scott, D.; Webby, R.F. Extraction of apple seed oil by supercritical carbon dioxide at pressures up to 1300 bar. J. Supercrit. Fluids 2018, 141, 128–136. [Google Scholar] [CrossRef]
- Meneses, M.A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J. Food Eng. 2015, 163, 45–53. [Google Scholar] [CrossRef]
- Prado, I.M.; Prado, G.H.C.; Prado, J.M.; Meireles, M.A.A. Supercritical CO2 and low-pressure solvent extraction of mango (Mangifera indica) leaves: Global yield, extraction kinetics, chemical composition and cost of manufacturing. Food Bioprod. Process. 2013, 91, 656–664. [Google Scholar] [CrossRef]
- Castro-vargas, H.I.; Rodríguez-varela, L.I.; Parada-alfonso, F. Guava (Psidium guajava L.) seed oil obtained with a homemade supercritical fluid extraction system using supercritical CO2 and co-solvent. J. Supercrit. Fluids 2011, 56, 238–242. [Google Scholar] [CrossRef]
- Dias, J.L.; Mazzutti, S.; De Souza, J.A.L.; Ferreira, S.R.S.; Soares, L.A.L. Extraction of umbu (Spondias tuberosa) seed oil using CO2, ultrasound and conventional methods: Evaluations of composition profiles and antioxidant activities. J. Supercrit. Fluids 2019, 145, 10–18. [Google Scholar] [CrossRef]
- Kraujalien, V. Biorefining of blackcurrant pomace into high value functional ingredients using supercritical CO2, pressurized liquid and enzyme assisted extractions. J. Supercrit. Fluids 2017, 124, 10–19. [Google Scholar] [CrossRef]
- Pezo, L. Supercritical fluid extraction of raspberry seed oil: Experiments and modelling sko Mari c. J. Supercrit. Fluids 2020, 157, 157. [Google Scholar] [CrossRef]
- Kraujalis, P.; Tamkut, L.; Urbonavi, D.; Vi, P.; Venskutonis, P.R. Recovery of bioactive substances from rowanberry pomace by consecutive extraction with supercritical carbon dioxide and pressurized solvents. Ind. Eng. Chem. Res. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Luis, J.; Reátegui, P.; Paula, A.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar] [CrossRef]
- Tamkut, L. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 159. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Alminger, M.; Ahrné, L. Supercritical CO2 extraction of bilberry (Vaccinium myrtillus L.) seed oil: Fatty acid composition and antioxidant activity. J. Supercrit. Fluids 2018, 135, 91–97. [Google Scholar] [CrossRef]
- Devani, B.M.; Jani, B.L.; Balani, P.C.; Akbari, S.H. Optimization of supercritical CO2 extraction process for oleoresin from rotten onion waste. Food Bioprod. Process. 2020, 119, 287–295. [Google Scholar] [CrossRef]
- Scaglia, B.; Incecco, P.D.; Squillace, P.; Dell, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2 - supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT-Food Sci. Technol. 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Romo-Hualde, A.; Yetano-Cunchillos, A.I.; González-Ferrero, C.; Sáiz-Abajo, M.J.; González-Navarro, C.J. Supercritical fluid extraction and microencapsulation of bioactive compounds from red pepper (Capsicum annum L.) by-products. Food Chem. 2012, 133, 1045–1049. [Google Scholar] [CrossRef]
- Shi, X.; Wu, H.; Shi, J.; Jun, S.; Wang, D.; Wang, W.; Cheng, A.; Gong, Z.; Chen, X.; Wang, C. Effect of modifier on the composition and antioxidant activity of carotenoid extracts from pumpkin (Cucurbita maxima) by supercritical CO2. LWT-Food Sci. Technol. 2013, 51, 433–440. [Google Scholar] [CrossRef]
- Fabian, H.; Lasta, B.; Lentz, L.; Mezzomo, N.; Regina, S.; Ferreira, S. Supercritical CO2 to recover extracts enriched in antioxidant compounds from beetroot aerial parts. Biocatal. Agric. Biotechnol. 2019, 19, 101169. [Google Scholar] [CrossRef]
- Lima, M.D.A.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Cabred, S.; Ramirez, C.L.; Fanovich, M.A. Valorization of an agroindustrial soybean residue by supercritical fluid extraction of phytochemical compounds. J. Supercrit. Fluids 2019, 143, 90–96. [Google Scholar] [CrossRef]
- Kao, T.; Chien, J.; Chen, B. Extraction yield of isoflavones from soybean cake as affected by solvent and supercritical carbon dioxide. Food Chem. 2008, 107, 1728–1736. [Google Scholar] [CrossRef]
- Fang, T.; Goto, M.; Wang, X.; Ding, X.; Geng, J.; Sasaki, M.; Hirose, T. Separation of natural tocopherols from soybean oil byproduct with supercritical carbon dioxide. J. Supercrit. Fluids 2007, 40, 50–58. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Qiu, A.; Wang, X. Preparation of isoflavones enriched soy protein isolate from defatted soy hypocotyls by supercritical CO2. LWT Food Sci. Technol. 2007, 40, 800–806. [Google Scholar] [CrossRef]
- Li, X.; Song, L.; Xu, X.; Wen, C.; Ma, Y.; Yu, C.; Du, M. One-step coextraction method for flavouring soybean oil with the dried stipe of Lentinus edodes (Berk.) sing by supercritical CO2 fluid extraction. LWT-Food Sci. Technol. 2020, 120, 108853. [Google Scholar] [CrossRef]
- Shen, X.; Fang, T.; Gao, F.; Guo, M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocoll. 2017, 63, 668–676. [Google Scholar] [CrossRef]
- Roselló-soto, E.; Barba, F.J.; Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Carlos, J. Phenolic profile of oils obtained from “horchata” by-products assisted by supercritical-CO2 and its relationship with antioxidant and lipid oxidation parameters: Triple TOF-LC-MS-MS characterization. Food Chem. 2019, 274, 865–871. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Lorenzo, J.M.; Dominguez, R.; Pateiro, M.; Mañes, J.; Moltó, J.C. Evaluating the impact of supercritical-CO2 pressure on the recovery and quality of oil from “horchata” by-products: Fatty acid profile, α-tocopherol, phenolic compounds, and lipid oxidation parameters. Food Res. Int. 2019, 120, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenéz, D.; Muñoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal- Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado A., D.; del Pilar Sánchez-Camargo, A. Study of the fatty acid profile and the aroma composition of oil obtained from roasted Colombian coffee beans by supercritical fluid extraction. J. Supercrit. Fluids 2016, 113, 44–52. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Veggi, P.C.; Angela, M.; Meireles, A. Supercritical fluid extraction with a modifier of antioxidant compounds from jabuticaba (Myrciaria cauliflora) by-products: Economic viability. Ital. Oral Surg. 2011, 1, 1672–1678. [Google Scholar] [CrossRef]
- Santos-zea, L.; Gutiérrez-uribe, J.A.; Benedito, J. Effect of ultrasound intensification on the supercritical fluid extraction of phytochemicals from Agave salmiana bagasse. J. Supercrit. Fluids 2019, 144, 98–107. [Google Scholar] [CrossRef]
- Guoliang, L.; Junyou, S.; Yourui, S.; Zhiwei, S.; Lian, X.; Jie, Z.; Jinmao, Y. Supercritical CO2 cell breaking extraction of Lycium barbarum seed oil and determination of its chemical composition by HPLC/APCI/MS and antioxidant activity. LWT Food Sci. Technol. 2011, 44, 1172–1178. [Google Scholar] [CrossRef]
- Garcia-mendoza, P.; Espinosa-pardo, F.A.; Mara, A.; Fernández, G.; Roberto, M.; Junior, M.; Ariel, M.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juc (Euterpe edulis Mart) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).