Abstract
The enormous library of pharmaceutical compounds presents endless research avenues. However, several factors limit the therapeutic potential of these drugs, such as drug resistance, stability, off-target toxicity, and inadequate delivery to the site of action. Extracellular vesicles (EVs) are lipid bilayer-delimited particles and are naturally released from cells. Growing evidence shows that EVs have great potential to serve as effective drug carriers. Since EVs can not only transfer biological information, but also effectively deliver hydrophobic drugs into cells, the application of EVs as a novel drug delivery system has attracted considerable scientific interest. Recently, EVs loaded with siRNA, miRNA, mRNA, CRISPR/Cas9, proteins, or therapeutic drugs show improved delivery efficiency and drug effect. In this review, we summarize the methods used for the cargo loading into EVs, including siRNA, miRNA, mRNA, CRISPR/Cas9, proteins, and therapeutic drugs. Furthermore, we also include the recent advance in engineered EVs for drug delivery. Finally, both advantages and challenges of EVs as a new drug delivery system are discussed. Here, we encourage researchers to further develop convenient and reliable loading methods for the potential clinical applications of EVs as drug carriers in the future.
1. Introduction
Multicellular organisms communicate intercellularly via soluble cell-secreted factors, and the direct interactions between cell surfaces are an essential part of exchanging genetic information. Distant cell–cell communication in eukaryotic cells utilizes membrane-derived vesicles secreted into the extracellular space [1]. Extracellular vesicles (EVs), nano-sized secreted cellular particles, were first purified and validated from various cells during their discovery in the 1980s. EVs were considered simple membrane fragments until Raposo et al. characterized the antigen-presenting EVs secreted by B lymphocytes in 1996 [2]. Generally, EVs are classified into exosomes, microvesicles, and apoptotic bodies according to their diameters and intracellular origins [3,4]. Exosomes are the smallest EVs with a size of 40–100 nm. They originate from multivesicular endosomes (MVEs), which contain intraluminal vesicles (ILVs) incorporated with RNA, DNA, proteins, and lipid. MVEs exit the cell through plasma membrane fusion, which allows intracellular molecules to traffic through extracellular spaces to distant cells. While microvesicles with a size of 50–1000 nm are derived directly from the plasma membrane, apoptotic bodies are typically 1000–5000 nm in diameter and secreted from the cells under apoptosis [3,4]. Although each of them has specific characteristics and forming processes, distinguishing them experimentally presents a challenge due to their overlapping sizes and lack of distinct subtype markers.
The effects of EVs and their cargo have been studied in many diseases such as cancer [5], cardiovascular diseases [6], autoimmune diseases [7], inflammatory diseases [8], and type 2 diabetes mellitus [9]. Their usability and effectiveness are also under evaluation in clinical trials [10]. Therapeutic agents, including siRNA [11], miRNA [12], mRNA/proteins [13], CRISPR/Cas9 [14], and chemical drugs [15] were loaded into EVs for treating diseases. It has been reported that an exosome-based delivery system could target cancer stem cells with improved efficiency and specificity [16]. Although technical difficulties still exist in EVs preparation and cargo-loading efficiency, the therapeutic platform of EVs has promise as a novel drug delivery method. Considering their potential applications for human diseases, we summarize not only the EVs loading methods, but also introduce the advantages and challenges of these loading methods.
2. EV Loading Methods
Cargos can be packaged into EVs with or without the help of donor cells. Thus, methods for encapsulating cargo into EVs can be roughly divided into two types: cell-based loading methods and non-cell-based loading methods. In the cell-based loading approach, cargos are usually delivered into the donor cells first. After being packaged into EVs, the cargos can be secreted and collected in an EV-carrying manner for therapeutic use [17]. The non-cell-based loading approach involves directly loading chemical or biomolecules into isolated EVs through electroporation, sonication, incubation, and/or transfection [18]. In the following content, these methods are reviewed according to the cargos.
2.1. Cell-Based Loading Methods
Cell-based loading, also known as endogenous loading, employs the indirect deposition of a therapeutic cargo into EVs through donor cell manipulation (Figure 1). Incubation and transfection are widely used to load the cargo into donor cells. After purification of released EVs, EV-carrying cargos can be used for therapeutic purposes [19,20,21]. This method provides a convenient and effective way for loading biological materials and drug therapies into EVs.
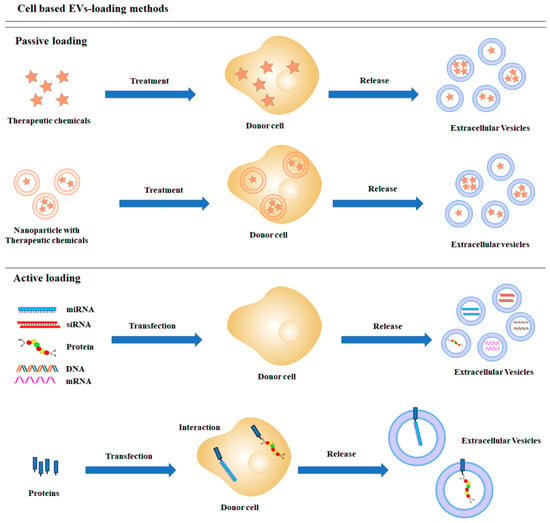
Figure 1.
Schematic diagrams of cell-based cargo loading methods.
2.1.1. Doxorubicin
The chemotherapeutic agent doxorubicin is both highly efficacious and cytotoxic, specifically to cardiac cells. Efficacy has been limited by insufficient cellular uptake, instability in the acidic tumor environment, and intermediate solubility due to amphoteric property [22,23]. EVs can mitigate these limitations, and doxorubicin can be loaded into EVs simply by application to cells in vitro. Jang et al. and Kanchanapally et al. treated various cells with doxorubicin, including U937 monocytes, macrophages, and cancer cells. After incubation for 24 to 48 h, doxorubicin-loaded EVs could be isolated from culture media [24,25]. Silva et al. developed hybrid EVs, which consist of components of macrophage-derived EVs and iron oxide nanoparticles. This team treated THP-1 macrophages with magnetic iron oxide nanoparticles and doxorubicin, which results in the generation of EVs containing both iron oxide and chemotherapeutic agents. These hybrid EVs could be manipulated by magnetic force for delivering the doxorubicin to the cancer cells [23]. Moreover, Yong et al. used an electrochemical etching method to load doxorubicin into luminescent porous silicon nanoparticles (PSiNPs) for cell treatment. Through this process, exosome-sheathed PSiNPs (E-PSiNPs) containing doxorubicin were constructed as the drug delivery system [26].
2.1.2. Curcumin
Currently, it has been demonstrated that natural products have beneficial effects for multiple human diseases. However, their commercial development is limited due to low biocompatibility, poor solubility, and poor absorption [27]. Curcumin, a natural polyphenolic compound, has been shown to suppress cancer cell growth both in vitro and in vivo (e.g., tumor stem cells). However, clinical curcumin use is problematic due to its low solubility and cellular uptake [28]. Perteghella et al. sought to remove these barriers by loading curcumin into EVs. They used silk nanoparticles to enhance loading efficiency. The nanoparticles were conjugated with curcumin and then applied to mesenchymal stem cells. After incubating for several days, EVs containing silk/curcumin nanoparticles were observed [29].
2.1.3. Other Drugs
Besides the doxorubicin, Silva et al. loaded tissue-plasminogen activator (t-PA), disulfonated tetraphenyl chlorin (TPCS2a), and, 5,10,15,20-tetra(m-hydroxyphenyl)chlorin (mTHPC) into magnetic iron oxide hybrid EVs by adding the magnetic nanoparticles and drugs to the differentiated THP-1 cells. They also showed that magnetically engineered-EVs did not disturb the cellular uptake of drugs and the uptake of engineered EVs by cancer cells could be kinetically modulated and spatially controlled under a magnetic field [23]. Millard et al. loaded mTHPC into EVs isolated from human umbilical vascular endothelial cells (HUVEC). To isolate EVs containing mTHPC from cell-culture media, they treated HUVECs with mTHPC for 2 h and maintained the cells in fresh media for 72 h [30].
2.1.4. miRNA
miRNA is a group of small non-coding RNA containing ~22 nucleotides that bind to the 3′-UTR of target mRNA to suppress gene expression [31]. The role of altered miRNAs has been characterized in many pathologies, such as viral infections, inflammatory diseases, and cancer [32,33]. Currently, transfection is a reproducible method for miRNAs loading into EVs through a cell-based method. For example, the transient transfection of miRNAs into donor cells can package miRNAs as a cargo into secreted EVs. Through this simple transfection, EV-loaded miRNAs such as miR-1-1, miR-210, miR-584, and miR-21-5p have shown therapeutic effects by binding to disease-induced mRNA [34,35,36,37]. On the other hand, stable transfection has also been used to generate miRNA-loaded EVs. For example, Monfared et al. generated a miR-21 expressing stable cell line using HEK 293T cells. miR-21-loaded EVs were isolated from culture media and applied therapeutically in a glioblastoma rat model [38]. Zeh et al. made an immortalized human amniocyte cell line, stably releasing EV-encapsulated-miR-493 or miR-744 [39]. Furthermore, lentiviral packaging systems can overexpress miRNAs in the donor cells, which are difficult to transfect, such as stem cells, lymphocytes, and some cancer cells. After lentiviral transduction, miR-335, miR-302a, miR-3188, miR-101-3p, and miR-125-5p were successfully encapsulated into donor cell EVs [40,41,42,43,44,45].
2.1.5. siRNA
siRNA is a powerful tool to block gene expression. Different from permanent genome editing technology, siRNA inhibits gene expression at the post-transcriptional level. This specificity enables tight control of siRNA-based therapies [46]. However, the delivery of negatively charged siRNA throughout the cell membrane is challenging, and prolonged siRNA stability is also a concern [47]. EVs present a solution to these therapeutic issues inherent to siRNA. Similarly, transfection is an effective way for packaging siRNA into EVs via donor cells. For example, Zhang et al. found that transfected siRNA can be encapsulated into secreted EVs. Subsequently, they were able to identify hepatocyte growth factor (HGF) siRNA loaded EVs after donor cell transfection [48].
2.1.6. mRNA/Protein
Even though mRNA-based gene therapy possesses many advantages regarding easy manipulation and expression, the unstable structure and ubiquitous presence of RNase are obstacles to overcome [49]. EVs could serve as an ideal carrier for functional mRNA delivery [50]. Given the larger size of mRNA, the development of mRNA as EV cargo can be more difficult compared with miRNA and siRNA, since most full-length mRNAs in EVs are smaller than 1 kb [51]. Thus, the length of desired mRNAs must be taken into consideration for packaging into EVs. Mizrak et al. first reported the therapeutic potential of EV-encapsulated mRNA/protein. They transfected prodrug-converting enzyme cytosine deaminase (CD)-uracil phosphoribosyltransferase (UPRT) into HEK 293T cells and isolated EVs loaded with CD-UPRT-EGFP mRNA/protein [52]. Kanada et al. observed luciferase loading of EVs isolated from luciferase transfected HEK 293FT cells [17]. Vituret et al. loaded cystic fibrosis transmembrane conductance regulator (CFTR) glycoprotein and its encoding mRNA into cystic fibrosis cells to improve the function of CFTR chloride channel. They isolated EVs from CFTR-positive Calu-3 cells and A549 cells transfected with an adenoviral CFTR expressing vector [53]. Khan et al. and Forterre et al. used HEK 293T cells to load mRNA/protein into EVs that achieved successful cellular delivery and targeting [54,55]. Unique molecular mechanisms have also been reported to induce specific mRNA/protein loading into EVs in HEK 293T models. Nedd4 family-interacting protein 1 (Ndfip1) induced EV loading of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) by Nedd4 family proteins [56,57]. Using this EVs releasing mechanism, Sterzenbach et al. loaded protein into EVs. They linked Cre recombinase with WW domains of the Nedd4 binding site of Ndfip1 allowing connected WW domains to be loaded into EVs and secreted into the extracellular area [58].
2.1.7. DNA
DNA modifications are mainstays of gene therapy, and recent research has significantly improved vector delivery and safety in gene therapy [59]. EVs are attractive delivery mechanisms for DNA modifications due to the higher efficiency and safety of EVs. Kanada et al. transfected Cre-encoding plasmid DNA into HEK 293T, and the successfully isolated EVs contained the plasmid DNA [17]. The same group also loaded minicircle DNA that encodes prodrug-converting enzymes into 4T1 cells and then isolated EVs containing the DNA [60]. Similarly, Haney et al. isolated EVs containing DNA from IC21 macrophages after transfecting TPP1-encoding plasmid DNA [61]. Although only a few attempts have been reported, Tran et al. demonstrated effective DNA loading conditions in transfected E. coli using several plasmids. They showed that a higher copying ability of the plasmid induced more DNA loading, and the plasmid origin affected loading efficiency, while plasmid size was not a significant factor in the range of 3.5 kb to 15 kb. [62]. The CRISPR/Cas9 system allows for precise DNA manipulation. This system has been commonly encapsulated through viral vectors [63]. The loading of CRISPR/Cas9 into EVs presents a novel area for improving safety and stability issues inherent to viral vectors. Li et al. successfully loaded C/EBPα CRISPR/Cas9 into EVs. [64] Since RNA-binding proteins can increase the incorporation of RNA into EVs [65], they fused RNA-binding protein HuR with exosomal membrane protein CD9 to increase the loading efficiency. To engineer the CRISPR/Cas9 cargo, they transfected CD9-HuR and CRISPR/Cas9 with packing plasmid psPAX2 and pMD2G into HEK 293T cells and isolated EVs [64].
Taken together, researchers have developed numerous cell-based loading approaches so far. For small molecules drugs, passive loading methods can be applied with anticipated efficiency. In contrast, biomolecules have to be delivered into the donor cells with active loading methods, such as transfection and vial transduction.
2.2. Non-Cell-Based EVs Loading Methods
Non-cell-based EV loading methods, also known as exogenous or direct loading, deposits a therapeutic cargo into EVs after isolation. Different siRNA, miRNA, proteins, CRISPR/Cas9, hydrophobic compound derived natural product, and anticancer drugs can be loaded into EVs through sonication, electroporation, transfection reagents, and a specific buffer agent (Figure 2). Some compounds can be loaded into EVs by mixing at room temperature. Although these loading methods can be classified as passive and active loading, they also can be combined for optimized loading efficiency [66]. Passive loading involves loading the therapeutic cargo into EVs through diffusion, whereas active loading consists of the disruption of EV membranes through electroporation or sonication, allowing entry into the EVs. After completing the reaction, the membrane is recovered by incubation for a while at room temperature or 37 °C. Through this process, EVs can be stabilized [67].
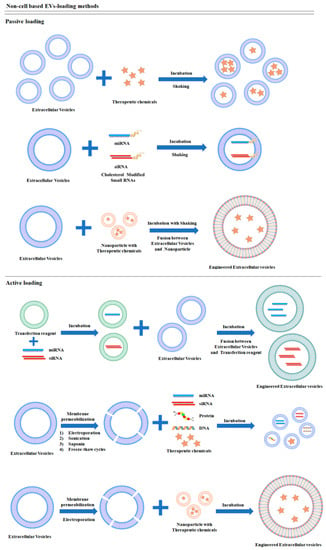
Figure 2.
Schematic diagrams of non-cell-based cargo loading methods.
2.2.1. Doxorubicin
Ingato et al. and Wu et al. loaded doxorubicin into EVs derived from cancer cells by incubating 25 μg/mL EVs in 1 mL PBS (by protein content) and 1 mg/mL doxorubicin mixture at 37 °C for several hours [68,69]. Triethylamine solution has also been used for incubating EVs and doxorubicin [70,71]. Saponin induces permeabilization of the EV membrane, creating pores by removing membrane cholesterol, and can be used for loading EV cargo without destroying the membrane [72]. Goh et al. used saponin to load doxorubicin into EVs isolated from U937 cells. They compared the efficiency of several methods, including simple mixture incubation at room temperature for 24 h, simple mixture incubation at 37 °C for 24 h, saponin-added incubation at room temperature for 5 min, and three freeze-thaw cycles. Even though these methods incorporated doxorubicin into EVs, they differed in loading efficiency. When loading 200 µg/mL doxorubicin, the saponin method showed the most efficient loading (~50%) [73]. Doxorubicin was loaded into EVs derived from HEK 293T cells and HeLa cells via electroporation [74,75,76]. Qi et al. and Zhang et al. conjugated magnetic iron oxide with EVs to improve targeting efficiency and then mixed doxorubicin via moderating stirring incubation or electroporation [77,78]. Alternatively, Li et al. loaded doxorubicin into EVs first and then conjugated magnetic iron oxide [79]. Both methods improved loading efficiency. Srivastava et al. applied gold nanoparticles (GNPs), which combine well with small molecules but have limitations as a drug carrier, so their incorporation as an EV cargo can address the limitation. First, they conjugated GNPs with doxorubicin using hydrazone pH-sensitive linkers. After then, doxorubicin was simply incubated at 37 °C at 250 revolutions per minute (rpm) for 2 h [80]. Kim et al. and Rayamajhi et al. synthesized hybrid EVs using simple thin-film hydration and then loaded doxorubicin via sonicator [81,82].
2.2.2. Curcumin
Carobolante et al. encapsulated curcumin into EVs isolated from cow skim milk and Caco-2 cells by mixing curcumin ethanol solution with EVs in PBS at room temperature overnight with stirring [83]. Tian et al. also loaded curcumin into EVs via mixing. To improve targeting, they conjugated cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide, which has a high affinity for integrin αvβ3 [84]. Zou et al. used saponin for membrane permeabilization and engineered the surface of EVs through donor cell transfection. They transfected 10E8-pDisplay expressing vector, which targets Chinese hamster ovary cells. Using EVs isolated from these engineered cells, they encapsulated curcumin into EVs via saponin permeabilization [85]. Others have used repeated freeze-thaw cycling to load curcumin [84,86]. Magnetic iron oxide nanoparticles have also been used with curcumin. Mixing EVs, curcumin, and magnetic iron oxide nanoparticles followed by electroporation successfully encapsulates them into EVs [87].
2.2.3. Paclitaxel
Paclitaxel is an anticancer drug that inhibits cell division, but poor cellular uptake and side effects limit its efficacy. Several EV loading methods of paclitaxel can improve therapeutic efficiency and minimize side effects. Saari et al. and Agrawal et al. loaded paclitaxel into EVs via simple incubation [88,89]. Sonication successfully loads paclitaxel, as demonstrated by Kim et al. and Salarpour et al. in which paclitaxel and EVs were mixed and sonicated [90,91]. In EVs derived from IC21 macrophages, sonication resulted in roughly 30% more loading efficiency than saponin permeabilization [61]. A similar tendency was confirmed by others in EVs from RAW264.7 cells when evaluating with mixing and EV incubation, electroporation, and sonication. For the incubation, the mixture was incubated at 37 °C with 1 h of shaking. The mixture was electroporated at 1000 kV for 5 ms and incubated at 37 °C for 30 min to promote recovery of the exosome membrane. The mixture was sonicated for 6 cycles of 30 s on and off with 2 min of cooling between each cycle and then incubated at 37 °C for 60 min for exosome membrane recovery. The loading efficiency of paclitaxel into EVs was as follows: simple incubation < electroporation << sonication [81].
2.2.4. Aspirin
Delivering functional aspirin to target cells is difficult due to poor water solubility [92]. These limitations have prevented aspirin use in severe diseases such as cancer, even though its effects as an anticancer agent have been long known. To resolve the low water-solubility issue, Tran et al. loaded aspirin into EVs for cancer treatment. Before loading aspirin into EVs, they constructed a nanoparticle of poloxamer 407 with MgO or TPGS. This nanoparticle was mixed with aspirin and EVs isolated from HT29 and MDA-MB-231 cells and incubated at room temperature for two hours with shaking [93]. Aspirin has also been loaded into EVs via freeze-thaw cycling with incubation and sonication [92]. Kalinec et al. loaded aspirin into EVs isolated from auditory HEI-OC1 cells. They incubated EVs and aspirin at 25 °C for 1 h with 5 min sonication [94].
2.2.5. Other Drugs
Yang et al. reported a method for the loading of the antibacterial linezolid into EVs derived from RAW264.7 cells. Linezolid was mixed with EVs, and the mixture was incubated at 37 °C for 1 h [95]. Dexamethasone was successfully loaded into EVs isolated from HEI-OC1 cells by room temperature incubation and 5 min of sonication [96]. Using this protocol, they loaded various drugs, including aspirin, arachidonic, eicosapentaenoic, docosahexaenoic, and linoleic acids, lipoxin A4, and resolvin D1 [94]. Erastin is a chemotherapeutics drug inducing ferroptosis. Even though erastin is a small molecule drug, poor water solubility and nephrotoxicity limit its applications. Similarly, Yu et al. encapsulated erastin into EVs to treat triple-negative breast cancer using sonication [97].
2.2.6. miRNA
EVs can be loaded with miRNA for safe and efficient delivery to target cells. For incorporating miRNAs into EVs, liposome-based transfection reagents can be applied to isolated EVs [98]. miRNAs, miR-335-5p, miR-21, and miR-143 have been successfully transfected into EVs with transfection reagents [99,100,101]. Electroporation is one of the most commonly used methods for loading miRNA directly into EVs. For example, miR-155, miR-26a, miR-155, miR-31, miR-451a, miR-939, miR-128-3p, miR-223 mimic, and miR-142 mimic have been successfully loaded [102,103,104,105,106,107,108]. Several methods have been devised to improve loading efficiency. Zhang et al. developed a method to load miRNA into EVs using modified calcium chloride transfection. EVs, miRNA mimic, and calcium chloride were mixed with PBS and placed on ice for 30 min, followed by miRNA incorporation by heat shock at 42 °C for 60 s [109]. According to the miRNA copy number per EV, the loading efficiency of modified calcium chloride transfection is comparable with the electroporation [110]. It has been reported that a cholesterol group is attached to the 3′ end of the miRNA to facilitate in vivo delivery [111]. Interestingly, Gong et al. identified that cholesterol-modified miRNA can quickly internalize into EVs by itself due to the hydrophobic-moiety modification. They incubated cholesterol-modified miRNA with EVs in PBS (37 °C, 90 min, 500 rpm shaking). The loading efficiency of miR-159 was 1.2%, whereas cholesterol-modified miR-159 improved to 5.33% [70]. Zhang et al. used peptide-conjugated EVs as well as cholesterol-modified miRNA. To improve the targeting property of EVs, they conjugated cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyK)], which can detect ischemic brain, onto the surface of EVs. Then, they incubated the engineered EVs with cholesterol modified miR-210 in PBS (37 °C, 60 min) and successfully obtained the miR-210-loaded EVs for therapy [112]. Jeyaram et al. loaded miRNA by pH gradient EV modification. EVs were isolated from HEK293T cells and dehydrated, followed by incubation in citrate buffer of various pH for one hour. After, EVs were dialyzed in HEPES buffered saline (pH 7.0) for 24 h. Modified EVs were incubated with miR-93 in PBS at various time points and temperatures. In a citrate buffer with a pH of 2.5, a two-hour incubation with 22 °C PBS resulted in the most efficient loading of miR-93 [12].
2.2.7. siRNA
Similar as miRNA, non-cell-based EV loading methods are widely used for siRNA loading by researchers. Some studies reported that transfection reagents promote siRNA loading into EVs. After isolating EVs, siRNA was transfected with EVs using commercial transfection reagents. EVs, siRNA, and reagents were incubated together for several hours at 37 °C allowing siRNA incorporation in EVs. [113,114]. Another popular method of loading siRNA directly into EVs is electroporation. The external electric field increases cellular membrane permeability by creating a hydrophilic pore and allows siRNA to enter EVs. This method has allowed EVs to knock down gene expression in target cells using siRNA cargo, including several genes, GAPDH, cyclophilin B, BACE1a, BACE1b, luciferase, PLK-1, VEGF, KrasG12D, and TPD52 [115,116,117,118,119,120]. Some studies have attempted to increase the silencing efficiency by allowing siRNA to reach target cells with high probability. They conjugated EVs with peptides that bind specific receptors of target cells. The transfection of donor cells can create EVs with specific peptides within their membrane. Rabies virus glycoprotein (RVG)-derived peptide, which binds p75 neurotrophin receptor, was conjugated to EVs, and then siRNA was loaded through electroporation [1,121,122]. Chen et al. conjugated klotho protein to EVs, which binds circulating endothelial progenitor cells (EPCs). Then, adenosine kinase (ADK) siRNA was loaded into EVs through electroporation to develop a cardiovascular disease therapeutic [123]. However, studies found electroporation can induce siRNA aggregation, which may cause overestimation of loading efficiency [124,125]. Lamichhane et al. loaded siRNA into EVs using a water bath sonicator by mixing EVs and siRNA in PBS, incubating for 30 min at room temperature, followed by sonication at 35 kHz for 30 s. Sonication of more than 30 s degraded the EVs [126]. Liu et al. loaded opioid receptor Mu (MOR) siRNA into RVG-EVs by sonication, and sonication achieved higher efficiency than electroporation [127]. Milk-derived EVs can inhibit cell growth of various cell lines [128], and several studies have used milk EVs loaded with siRNA for anticancer effects. After isolating milk-derived EVs, siRNA can be loaded with transfection reagents [129,130,131,132]. Cholesterol-conjugated hydrophobic siRNA can be loaded into EVs by simple incubation [11,133]. Biscans et al. conjugated several natural lipids, including fatty acids, sterols, and vitamin E, with siRNA. They suggested that vitamin E-conjugated siRNA showed the most efficient loading and delivery to neurons [134]. Haraszti et al. elucidated that the type of linker used to conjugate cholesterol to hydrophobic siRNA affected loading efficiency. For the huntingtin siRNA targeting neurons, the TEG (triethyl glycerol) linker showed better efficiency than the C7 (2-aminobutyl-1-3-propanediol) linker [135]. Jhan et al. fused several lipids such as DOTAP, POPC, DPPC, and POPG with EVs to make a hybrid-lipid membrane structure and then loaded siRNA into EVs through electroporation. These engineered EVs showed a higher gene silencing effect than lipofectamine transfection [136]. Jeyaram et al. loaded GAPDH siRNA through pH gradient modification of EVs. HEK 293T-derived EVs were incubated in pH 2.5 citrate buffer for one hour. Modified EVs were incubated with siRNA in PBS for 2 h at 22 °C. The modified EVs showed 54% knockdown efficiency, whereas unmodified EVs did not decrease GAPDH expression level [12].
2.2.8. Protein
Haney et al. loaded recombinant human TPP1 protein into EVs derived from IC21 macrophages through sonication or saponin permeabilization methods. For the sonication method, 500 μL EVs suspension (1011 particles per mL) were supplemented with 5 μL TPP1 (20 μg/100 μL) and sonicated at room temperature for 30 min. For the saponin permeabilization, 500 μL EVs suspension (1011 particles per mL) were supplemented with 5 μL TPP1 (20 μg/100μL) and 10 μL saponin solution to final concentration 0.4 mg/mL, and incubated at RT for 30 min. Although sonication loaded 40% more protein than saponin permeabilization, both methods loaded 7-times and 5-times more than donor cell transfection, respectively [61].
2.2.9. DNA
Exogenous DNA can be loaded into EVs via electroporation. Lamichhane et al. isolated EVs from HEK 293T cells and loaded 5 µg of dsDNA into 10 µg of EVs in a final volume of 50 µL electroporation buffer [19]. Kao et al. isolated EVs from CHRF cells and loaded Cy5-labeled pGFPns via electroporation [137]. Jeyaram et al. used HEK 293T cell EVs after incubating in pH 2.5 citrate buffer for one hour. Modified EVs were mixed with single-stranded DNA in PBS for 2 h at 22 °C [12]. Kim et al. isolated EVs from HEK293 and SKOV3 cells and loaded CRISPR/Cas9 targeting PARP-1 through electroporation. A total of 10 μg of DNA was mixed with 30 μg of exosomes and Cas9- and sgRNA-expressing plasmids were loaded by electroporation (1000 V, 10 ms, 2 pulses) [138].
Comparing with the cell-based methods, non-cell-based loading approaches have more flexibility without affecting the donor cells. Nevertheless, these methods may alter the integrity of the EV membrane and affect the bioactivity of cargos depending on the approaches (Table 1).

Table 1.
Comparison of cargo loading methods.
3. Conclusions and Future Directions
Dysregulated gene expression promotes the pathogenesis of many human diseases. Gene therapy is considered an ideal treatment for various disorders, such as inherited diseases and many cancers [139]. However, the immune rejection response against foreign substances and the limited stability of the vectors limits optimal application [140]. Artificially engineered nanoparticles, such as liposomes, polymeric nanoparticles, and magnetic nanoparticles, have been widely used for drug delivery. Nanoparticles have many applicable properties, such as high loading efficacy, safety, and cell targeting benefits as target-specific drug carriers. Even though nanodrugs are being investigated in clinical trials, the immune responses, toxicity issues, and high cost have limited development [141]. However, the EV delivery system has distinct advantages over vector delivery and artificial nanoparticles. EVs are natural products originating from the cell surface, and thus better at avoiding activating immune responses [142]. Another advantage of EVs is specificity for target cells. The tissue-homing characteristics of EVs allow the transport of their therapeutic cargo to distant target cells [143]. In diseases where drug receptors lack sufficient expression due to mutations or situations involving biological barriers such as the blood-brain barrier, the drug-loaded EVs can facilitate drug delivery by fusing with the membrane of target cells [144]. For example, many have studied the treatment of triple-negative breast cancer, which lacks estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 [67,145]. EVs present a targeting system that could overcome this kind of barrier for treatment. For this reason, they are expected to be safer and more efficient for delivering oligonucleotides and plasmids [146].
Despite these advantages, there are several developmental challenges. Although several methods for isolating EVs, such as ultracentrifugation, immunoaffinity, size exclusion, precipitation, and microfluidics techniques, have been reported, there are still no efficient isolation methods to achieve pure EVs. Jeppesen et al. reassessed the characteristics of EVs reported by other research groups. They showed at least 20 different results from existing reports [4]. Another disadvantage is the diverse mechanisms of cellular uptake, depending on the cell type and administration method [147]. Some researchers conjugated EVs with functional ligands to target the cells to improve specific cellular uptake [1,112,121,122]. Sometimes loading methods affect EV stability. The integrity of EVs can become damaged by sonication or permeabilization agents [66]. Additionally, electroporation has been known to aggregate siRNA during the loading process. To improve this technical problem, Kooijmans et al. added EDTA to the electroporation buffer [125]. Even though the effect of a low amount of EVs cargo is similar to that of conventional methods, the loading efficiency may be improved for better results [148]. To increase the loading efficiency, several researchers conjugated nanovesicles, which have high loading capacity [23,77,78,87,149]. Many challenges remain in designing the most efficient and effective EV loading methods.
The field of EVs is exponentially growing, as many studies focus on technical optimization of loading methods in the pre-clinical stage. In this review, we mainly summarized the methods for cargo loading into naturally generated EVs. However, some engineered EVs, such as gesicles [150], artificial EVs [151], and mimetic EVs [152] are not discussed in the current review.
Human cell/tissue-derived EVs have been applied in clinical trials as anti-cancer drug vehicles [153,154]. For instance, in a clinical trial (NCT03608631), KrasG12D siRNA was proposed to be loaded into mesenchymal stromal cells (MSC)-derived EVs for the treatment of patients with metastatic pancreas cancer. Besides the cancer studies, in the clinical trial NCT03608631, miR-124-enriched EVs from MSC cells were proposed to treat acute ischemic stroke.
Although safety and tolerance of EVs as carriers have been observed in most clinical trials [153,154,155,156], many challenges remain before applying EVs as drug carriers in clinical trials, such as loading efficiency and the purity of EVs for clinical use. Little is known about the mechanisms of cell-based or non-cell-based drug loading methods. Therefore, further investigations are expected not only to develop novel methods but also to explore the molecules that improve cargo loading efficiency through mechanistic studies.
Author Contributions
Conceptualization and supervision D.Z.; resources and writing Y.H. with the contributions of D.Z.; critical revision of the manuscript T.W.J., S.D., Y.Z., X.W., S.P.N., and S.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institutes of Health (NIH) grants NIH/NHLBI R00 HL141685 and NIH/NIAID R03 AI152003.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Andaloussi, S.; Lee, Y.; Lakhal-Littleton, S.; Li, J.; Seow, Y.; Gardiner, C.; Alvarez-Erviti, L.; Sargent, I.L.; Wood, M.J.A. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012, 7, 2112–2126. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Kalluri, R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, A.; Koenen, R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef]
- Turpin, D.; Truchetet, M.-E.; Faustin, B.; Augusto, J.-F.; Contin-Bordes, C.; Brisson, A.; Blanco, P.; Duffau, P. Role of extracellular vesicles in autoimmune diseases. Autoimmun. Rev. 2016, 15, 174–183. [Google Scholar] [CrossRef]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Pardo, F.; Villalobos-Labra, R.; Sobrevia, B.; Toledo, F.; Sobrevia, L. Extracellular vesicles in obesity and diabetes mellitus. Mol. Asp. Med. 2018, 60, 81–91. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- O’Loughlin, A.J.; Mäger, I.; De Jong, O.G.; Varela, M.A.; Schiffelers, R.M.; El Andaloussi, S.; Wood, M.J.; Vader, P. Functional Delivery of Lipid-Conjugated siRNA by Extracellular Vesicles. Mol. Ther. 2017, 25, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Lamichhane, T.N.; Wang, S.; Zou, L.; Dahal, E.; Kronstadt, S.M.; Levy, D.; Parajuli, B.; Knudsen, D.R.; Chao, W.; et al. Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Mol. Ther. 2020, 28, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; De Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018, 5, 1700611. [Google Scholar] [CrossRef]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Kanada, M.; Bachmann, M.H.; Hardy, J.W.; Frimannson, D.O.; Bronsart, L.; Wang, A.; Sylvester, M.D.; Schmidt, T.L.; Kaspar, R.L.; Butte, M.J.; et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA 2015, 112, E1433–E1442. [Google Scholar] [CrossRef]
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019, 459, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef]
- Hung, M.E.; Leonard, J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles 2016, 5, 31027. [Google Scholar] [CrossRef]
- Melling, G.E.; Carollo, E.; Conlon, R.; Simpson, J.C.; Carter, D.R.F. The Challenges and Possibilities of Extracellular Vesicles as Therapeutic Vehicles. Eur. J. Pharm. Biopharm. 2019, 144, 50–56. [Google Scholar] [CrossRef]
- Swietach, P.; Hulikova, A.; Patiar, S.; Vaughan-Jones, R.D.; Harris, A.L. Importance of Intracellular pH in Determining the Uptake and Efficacy of the Weakly Basic Chemotherapeutic Drug, Doxorubicin. PLoS ONE 2012, 7, e35949. [Google Scholar] [CrossRef]
- Silva, A.K.; Luciani, N.; Gazeau, F.; Aubertin, K.; Bonneau, S.; Chauvierre, C.; Letourneur, D.; Wilhelm, C. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapally, R.; Deshmukh, S.K.; Chavva, S.R.; Tyagi, N.; Srivastava, S.K.; Patel, G.K.; Singh, A.P.; Singh, S. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: A comparative analysis. Int. J. Nanomed. 2019, 14, 531–541. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017, 9, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Perteghella, S.; Crivelli, B.; Catenacci, L.; Sorrenti, M.; Bruni, G.; Necchi, V.; Vigani, B.; Sorlini, M.; Torre, M.L.; Chlapanidas, T. Stem cell-extracellular vesicles as drug delivery systems: New frontiers for silk/curcumin nanoparticles. Int. J. Pharm. 2017, 520, 86–97. [Google Scholar] [CrossRef]
- Millard, M.; Yakavets, I.; Piffoux, M.; Brun, A.; Gazeau, F.; Guigner, J.-M.; Jasniewski, J.; Lassalle, H.-P.; Wilhelm, C.; Bezdetnaya, L. mTHPC-loaded extracellular vesicles outperform liposomal and free mTHPC formulations by an increased stability, drug delivery efficiency and cytotoxic effect in tridimensional model of tumors. Drug Deliv. 2018, 25, 1790–1801. [Google Scholar] [CrossRef]
- Orang, A.V.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front. Immunol. 2020, 10, 3081. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012, 4, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Bronisz, A.; Wang, Y.; Nowicki, M.O.; Peruzzi, P.; Ansari, K.I.; Ogawa, D.; Balaj, L.; De Rienzo, G.; Mineo, M.; Nakano, I.; et al. Extracellular Vesicles Modulate the Glioblastoma Microenvironment via a Tumor Suppression Signaling Network Directed by miR-1. Cancer Res. 2014, 74, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Li, J.; Ma, C.; Chen, S.; Lei, W.; Yang, Y.; Liu, S.; Bihl, J.; Chen, C. Loading MiR-210 in Endothelial Progenitor Cells Derived Exosomes Boosts Their Beneficial Effects on Hypoxia/Reoxygeneation-Injured Human Endothelial Cells via Protecting Mitochondrial Function. Cell. Physiol. Biochem. 2018, 46, 664–675. [Google Scholar] [CrossRef]
- Kim, R.; Lee, S.; Lee, J.; Kim, M.; Kim, W.J.; Lee, H.W.; Lee, M.Y.; Kim, J.; Chang, W. Exosomes derived from microRNA-584 transfected mesenchymal stem cells: Novel alternative therapeutic vehicles for cancer therapy. BMB Rep. 2018, 51, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, S.; Zhu, J.; Hu, T.; Han, Z.; Zhang, S.; Zhao, J.; Chen, F.; Lei, P. Exosomes from MiR-21-5p-Increased Neurons Play a Role in Neuroprotection by Suppressing Rab11a-Mediated Neuronal Autophagy In Vitro After Traumatic Brain Injury. Med. Sci. Monit. 2019, 25, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Monfared, H.; Jahangard, Y.; Nikkhah, M.; Mirnajafi-Zadeh, S.J.; Mowla, S.J. Potential Therapeutic Effects of Exosomes Packed With a miR-21-Sponge Construct in a Rat Model of Glioblastoma. Front. Oncol. 2019, 9, 782. [Google Scholar] [CrossRef]
- Zeh, N.; Schneider, H.; Mathias, S.; Raab, N.; Kleemann, M.; Schmidt-Hertel, S.; Weis, B.; Wissing, S.; Strempel, N.; Handrick, R.; et al. Human CAP cells represent a novel source for functional, miRNA-loaded exosome production. PLoS ONE 2019, 14, e0221679. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.L.; Yao, J.L.; Wang, K.; Ai, H. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Zhang, J.; Jiao, Z.; Dong, N.; Wang, G.; Wang, Z.; Wang, L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 2019, 9, 2346–2360. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Yan, M.; Shi, J.; Xu, Q.; Li, Z.; Yang, W.; Zhang, J.; Chen, W. Loss of exosomal miR-3188 in cancer-associated fibroblasts contributes to HNC progression. J. Exp. Clin. Cancer Res. 2019, 38, 1–23. [Google Scholar] [CrossRef]
- Xie, C.; Du, L.-Y.; Guo, F.; Li, X.; Cheng, B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol. Cell. Biochem. 2019, 458, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Magenta, A.; Picozza, M.; Toietta, G. Extracellular Vesicles–Encapsulated MicroRNA-125b Produced in Genetically Modified Mesenchymal Stromal Cells Inhibits Hepatocellular Carcinoma Cell Proliferation. Cells 2019, 8, 1560. [Google Scholar] [CrossRef] [PubMed]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Bai, M.; Wang, J.; Zhu, K.; Liu, R.; Ge, S.; Li, J.; Ning, T.; Deng, T.; et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018, 109, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, S.; Fu, R.; Zhang, L.; Huang, K.; Peng, H.; Dai, L.; Chen, Q. Therapeutic Prospects of mRNA-Based Gene Therapy for Glioblastoma. Front. Oncol. 2019, 9, 1208. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Qixian, C.; Wang, Y.; Bruno, P.M.; Xiao, H.; Yu, Y.; Li, T.; Lauffer, S.; Wei, W.; Chen, Q.; Kang, X.; et al. Nanoparticle conjugates of a highly potent toxin enhance safety and circumvent platinum resistance in ovarian cancer. Nat. Commun. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Mizrak, A.; Bolukbasi, M.F.; Ozdener, G.B.; Brenner, G.J.; Madlener, S.; Erkan, E.P.; Ströbel, T.; Breakefield, X.O.; Saydam, O. Genetically Engineered Microvesicles Carrying Suicide mRNA/Protein Inhibit Schwannoma Tumor Growth. Mol. Ther. 2013, 21, 101–108. [Google Scholar] [CrossRef]
- Vituret, C.; Gallay, K.; Confort, M.-P.; Ftaich, N.; Matei, C.I.; Archer, F.; Ronfort, C.; Mornex, J.-F.; Chanson, M.; Di Pietro, A.; et al. Transfer of the Cystic Fibrosis Transmembrane Conductance Regulator to Human Cystic Fibrosis Cells Mediated by Extracellular Vesicles. Hum. Gene Ther. 2016, 27, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Lang, M.L.; Huang, M.-B.; Raymond, A.; Bond, V.C.; Shiramizu, B.; Powell, M.D. Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Aβ1–42 secretion in SH-SY5Y neural cells. J. NeuroVirol. 2016, 22, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Forterre, A.V.; Wang, J.-H.; Delcayre, A.; Kim, K.; Green, C.; Pegram, M.D.; Jeffrey, S.S.; Matin, A. Extracellular Vesicle–Mediated In Vitro Transcribed mRNA Delivery for Treatment of HER2+ Breast Cancer Xenografts in Mice by Prodrug CB1954 without General Toxicity. Mol. Cancer Ther. 2020, 19, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Putz, U.; Howitt, J.; Doan, A.; Goh, C.-P.; Low, L.-H.; Silke, J.; Tan, S.-S. The Tumor Suppressor PTEN Is Exported in Exosomes and Has Phosphatase Activity in Recipient Cells. Sci. Signal. 2012, 5, ra70. [Google Scholar] [CrossRef]
- Howitt, J.; Lackovic, J.; Low, L.-H.; Naguib, A.; MacIntyre, A.; Goh, C.-P.; Callaway, J.K.; Hammond, V.; Thomas, T.; Dixon, M.; et al. Ndfip1 regulates nuclear Pten import in vivo to promote neuronal survival following cerebral ischemia. J. Cell Biol. 2012, 196, 29–36. [Google Scholar] [CrossRef]
- Sterzenbach, U.; Putz, U.; Low, L.-H.; Silke, J.; Tan, S.-S.; Howitt, J. Engineered Exosomes as Vehicles for Biologically Active Proteins. Mol. Ther. 2017, 25, 1269–1278. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Kanada, M.; Kim, B.D.; Hardy, J.W.; Ronald, J.A.; Bachmann, M.H.; Bernard, M.P.; Perez, G.I.; Zarea, A.A.; Ge, T.J.; Withrow, A.; et al. Microvesicle-Mediated Delivery of Minicircle DNA Results in Effective Gene-Directed Enzyme Prodrug Cancer Therapy. Mol. Cancer Ther. 2019, 18, 2331–2342. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv. Health Mater. 2019, 8, e1801271. [Google Scholar] [CrossRef] [PubMed]
- Tran, F.; Boedicker, J.Q. Plasmid Characteristics Modulate the Propensity of Gene Exchange in Bacterial Vesicles. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019, 19, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, B.; Kowal, E.J.K.; Van Balkom, B.W.M.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; De Candia, P.; Chow, F.W.N.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA: An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef] [PubMed]
- Ingato, D.; Edson, J.A.; Zakharian, M.; Kwon, Y.J. Cancer Cell-Derived, Drug-Loaded Nanovesicles Induced by Sulfhydryl-Blocking for Effective and Safe Cancer Therapy. ACS Nano 2018, 12, 9568–9577. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Huang, C.-C.; Changou, C.A.; Lu, L.-S.; Goubran, H.; Burnouf, T. Clinical-grade cryopreserved doxorubicin-loaded platelets: Role of cancer cells and platelet extracellular vesicles activation loop. J. Biomed. Sci. 2020, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Wu, X.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y.; et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting of Doxorubicin and Exosome Derived from Mesenchymal Stem Cells for Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Jamur, M.C.; Oliver, C. Permeabilization of Cell Membranes. Methods Mol. Biol. 2010, 588, 63–66. [Google Scholar] [CrossRef]
- Goh, W.J.; Lee, C.K.; Zou, S.; Woon, E.C.; Czarny, B.; Pastorin, G. Doxorubicin-loaded cell-derived nanovesicles: An alternative targeted approach for anti-tumor therapy. Int. J. Nanomed. 2017, 12, 2759–2767. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Pinho, M.J.; Zuzarte, M.; Oliveira, C.; Pereira, P.; Sluijter, J.P.G.; Gomes, C.; Girao, H. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J. Extracell. Vesicles 2016, 5, 32538. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.; Collinson, A.; Matthews, C.; Pointon, A.; Jenkinson, L.; Minter, R.R.; Vaughan, T.J.; Tigue, N.J. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE 2019, 14, e0214545. [Google Scholar] [CrossRef]
- Takenaka, T.; Nakai, S.; Katayama, M.; Hirano, M.; Ueno, N.; Noguchi, K.; Takatani-Nakase, T.; Fujii, I.; Kobayashi, S.S.; Nakase, I. Effects of gefitinib treatment on cellular uptake of extracellular vesicles in EGFR-mutant non-small cell lung cancer cells. Int. J. Pharm. 2019, 572, 118762. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, Z.-L.; Wu, M.; Ren, J.-G.; Xia, H.-F.; Sa, G.-L.; Zhu, J.-Y.; Pang, D.-W.; Zhao, Y.-F.; Chen, G. Magnetic and Folate Functionalization Enables Rapid Isolation and Enhanced Tumor-Targeting of Cell-Derived Microvesicles. ACS Nano 2017, 11, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F.; Hu, C.; Zhang, L.; Guo, H.; Gao, S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1973–1985. [Google Scholar] [CrossRef]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N.; et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 2016, 6, 38541. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Carobolante, G.; Mantaj, J.; Ferrari, E.; Vllasaliu, D. Cow Milk and Intestinal Epithelial Cell-Derived Extracellular Vesicles as Systems for Enhancing Oral Drug Delivery. Pharmacy 2020, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yuan, M.; Zhang, T.; Wei, H.; Xu, S.; Jiang, N.; Zheng, N.; Wu, Z. Extracellular vesicles expressing a single-chain variable fragment of an HIV-1 specific antibody selectively target Env+ tissues. Theranostics 2019, 9, 5657–5671. [Google Scholar] [CrossRef]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; AlHakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Salarpour, S.; Forootanfar, H.; Pournamdari, M.; Ahmadi-Zeidabadi, M.; Esmaeeli, M.; Pardakhty, A. Paclitaxel incorporated exosomes derived from glioblastoma cells: Comparative study of two loading techniques. DARU J. Pharm. Sci. 2019, 27, 533–539. [Google Scholar] [CrossRef]
- Tran, P.H.; Wang, T.; Yin, W.; Tran, T.T.; Nguyen, T.N.; Lee, B.-J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Wang, T.; Yin, W.; Tran, T.T.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.; Lee, B.-J.; Duan, W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Gao, L.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Kalinec, F. Extracellular Vesicles from Auditory Cells as Nanocarriers for Anti-inflammatory Drugs and Pro-resolving Mediators. Front. Cell. Neurosci. 2019, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, G.; Guo, J.; Wang, C.; He, Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 8095–8104. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Kalinec, F.; Cohn, B.W. Preliminary Characterization of Extracellular Vesicles from Auditory HEI-OC1 Cells. Ann. Otol. Rhinol. Laryngol. 2019, 128, 52S–60S. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Piontek, K.; Sakaguchi, M.; Selaru, F.M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 2018, 67, 940–954. [Google Scholar] [CrossRef]
- Yang, L.; Niu, F.; Yao, H.; Liao, K.; Chen, X.; Kook, Y.; Ma, R.; Hu, G.; Buch, S. Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J. Neuroimmune Pharmacol. 2018, 13, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-Y.; Kim, H.; Mun, D.; Yun, N.; Joung, B. Therapeutic potential of miR-21 regulation by human peripheral blood derived-small extracellular vesicles in myocardial infarction. Clin. Sci. 2020, 134, 985–999. [Google Scholar] [CrossRef]
- Bala, S.; Csak, T.; Momen-Heravi, F.; Lippai, D.; Kodys, K.; Catalano, D.; Satishchandran, A.; Ambros, V.; Szabo, G. Biodistribution and function of extracellular miRNA-155 in mice. Sci. Rep. 2015, 5, 10721. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Kan, S.; Zhu, Y.; Feng, S.; Feng, W.; Gao, S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int. J. Nanomed. 2018, 13, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Asadirad, A.; Hashemi, S.M.; Baghaei, K.; Ghanbarian, H.; Mortaz, E.; Zali, M.R.; Amani, D. Phenotypical and functional evaluation of dendritic cells after exosomal delivery of miRNA-155. Life Sci. 2019, 219, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Shenoda, B.B.; Lin, Z.; Alexander, G.M.; Huppert, A.; Sacan, A.; Ajit, S.K. Inflammation potentiates miR-939 expression and packaging into small extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1650595. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, X.; Du, L.; Wang, Y.; Liu, X.; Tian, H.; Wang, L.; Li, P.; Zhao, Y.; Duan, W.; et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol. Cancer 2019, 18, 1–17. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Wang, X.; Groot, M.; Sharma, L.; Cruz, C.S.D.; Jin, Y. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax 2019, 74, 865–874. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Jin, Y. Delivery of Functional Small RNAs via Extracellular Vesicles In Vitro and In Vivo. Methods Mol. Biol. 2020, 2115, 107–117. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.S.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nat. Cell Biol. 2005, 438, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol. 2019, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fogarty, B.; LaForge, B.; Aziz, S.; Pham, T.; Lai, L.; Bai, S. Delivery of Small Interfering RNA to Inhibit Vascular Endothelial Growth Factor in Zebrafish Using Natural Brain Endothelia Cell-Secreted Exosome Nanovesicles for the Treatment of Brain Cancer. AAPS J. 2016, 19, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Niu, F.; Dagur, R.S.; He, M.; Tian, C.; Hu, G. Intranasal Delivery of lincRNA-Cox2 siRNA Loaded Extracellular Vesicles Decreases Lipopolysaccharide-Induced Microglial Proliferation in Mice. J. Neuroimmune Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Banizs, A.B.; Huang, T.; Dryden, K.; Berr, S.S.; Stone, J.R.; Nakamoto, R.K.; Shi, W. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int. J. Nanomed. 2014, 9, 4223–4230. [Google Scholar] [CrossRef]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G. PLK-1 Silencing in Bladder Cancer by siRNA Delivered with Exosomes. Urology 2016, 91, 241.e1–241.e7. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Tian, B.; Liu, J.; Yang, L.; Zeng, L.; Chen, T.; Hong, A.; Wang, X. Nucleolin-targeted Extracellular Vesicles as a Versatile Platform for Biologics Delivery to Breast Cancer. Theranostics 2017, 7, 1360–1372. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.; Hwang, D.W.; Lee, M. Delivery of High Mobility Group Box-1 siRNA Using Brain-Targeting Exosomes for Ischemic Stroke Therapy. J. Biomed. Nanotechnol. 2019, 15, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, M.; Bai, J.; Li, X.; Kong, X.; Gao, Y.; Bi, L.; Xiao, L.; Shi, B. Exosome-Modified Tissue Engineered Blood Vessel for Endothelial Progenitor Cell Capture and Targeted siRNA Delivery. Macromol. Biosci. 2017, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Stremersch, S.; Braeckmans, K.; De Smedt, S.C.; Hendrix, A.; Wood, M.J.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Liu, Z.; Zhou, Y.; Chu, D.; Li, X.; Jiang, X.; Hou, D.; Chen, X.; Chen, Y.; et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 2015, 5, 17543. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Matsuda, A.; Patel, T. Milk-derived Extracellular Vesicles for Therapeutic Delivery of Small Interfering RNAs. Methods Mol. Biol. 2018, 1740, 187–197. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk exosomes: Natural nanoparticles for siRNA delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef]
- Matsuda, A.; Moirangthem, A.; Angom, R.S.; Ishiguro, K.; Driscoll, J.; Yan, I.K.; Mukhopadhyay, D.; Patel, T. Safety of bovine milk derived extracellular vesicles used for delivery of RNA therapeutics in zebrafish and mice. J. Appl. Toxicol. 2020, 40, 706–718. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yan, I.K.; Lewis-Tuffin, L.; Patel, T. Targeting Liver Cancer Stem Cells Using Engineered Biological Nanoparticles for the Treatment of Hepatocellular Cancer. Hepatol. Commun. 2019, 4, 298–313. [Google Scholar] [CrossRef]
- Didiot, M.-C.; Hall, L.M.; Coles, A.H.; Haraszti, R.A.; Godinho, B.M.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R.; et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef]
- Biscans, A.; Haraszti, R.A.; Echeverria, D.; Miller, R.; Didiot, M.-C.; Nikan, M.; Roux, L.; Aronin, N.; Khvorova, A. Hydrophobicity of Lipid-Conjugated siRNAs Predicts Productive Loading to Small Extracellular Vesicles. Mol. Ther. 2018, 26, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Miller, R.; Didiot, M.-C.; Biscans, A.; Alterman, J.F.; Hassler, M.R.; Roux, L.; Echeverria, D.; Sapp, E.; DiFiglia, M.; et al. Optimized Cholesterol-siRNA Chemistry Improves Productive Loading onto Extracellular Vesicles. Mol. Ther. 2018, 26, 1973–1982. [Google Scholar] [CrossRef]
- Jhan, Y.-Y.; Prasca-Chamorro, D.; Zuniga, G.P.; Moore, D.M.; Kumar, S.A.; Gaharwar, A.K.; Bishop, C.J. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int. J. Pharm. 2020, 573, 118802. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Papoutsakis, E.T. Engineering human megakaryocytic microparticles for targeted delivery of nucleic acids to hematopoietic stem and progenitor cells. Sci. Adv. 2018, 4, eaau6762. [Google Scholar] [CrossRef]
- Kim, S.M.; Yang, Y.; Oh, S.J.; Hong, Y.; Seo, M.; Ja, O.S. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release 2017, 266, 8–16. [Google Scholar] [CrossRef]
- Cooper, T.A.; Wan, L.; Dreyfuss, G. RNA and Disease. Cell 2009, 136, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Zaldumbide, A.; Hoeben, R.C. How not to be seen: Immune-evasion strategies in gene therapy. Gene Ther. 2007, 15, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Phram. Ther. 2017, 42, 742–755. [Google Scholar]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Aryani, A.; Denecke, B. Exosomes as a Nanodelivery System: A Key to the Future of Neuromedicine? Mol. Neurobiol. 2016, 53, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2018, 23, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.-I.; Drummen, G.P.C.; Kuroda, M. Focus on Extracellular Vesicles: Development of Extracellular Vesicle-Based Therapeutic Systems. Int. J. Mol. Sci. 2016, 17, 172. [Google Scholar] [CrossRef]
- György, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic Applications of Extracellular Vesicles: Clinical Promise and Open Questions. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles as Nanomedicine: Hopes And Hurdles in Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef]
- Shimbo, K.; Miyaki, S.; Ishitobi, H.; Kato, Y.; Kubo, T.; Shimose, S.; Ochi, M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 2014, 445, 381–387. [Google Scholar] [CrossRef]
- Al Haydar, M.; Abid, H.R.; Sunderland, B.; Wang, S. Metal organic frameworks as a drug delivery system for flurbiprofen. Drug Des. Dev. Ther. 2017, 11, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Breakefield, X.O.; Frederickson, R.M.; Simpson, R.J. Gesicles: Microvesicle “Cookies” for Transient Information Transfer Between Cells. Mol. Ther. 2011, 19, 1574–1576. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Jones, M.-C.; Cox, S.C. Engineered Extracellular Vesicles: Tailored-Made Nanomaterials for Medical Applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-H.; Song, K.-M.; Limanjaya, A.; Choi, M.-J.; Ghatak, K.; Nguyen, N.M.; Ock, J.; Yin, G.N.; Kang, J.-H.; Lee, M.R.; et al. Embryonic stem cell-derived extracellular vesicle-mimetic nanovesicles rescue erectile function by enhancing penile neurovascular regeneration in the streptozotocin-induced diabetic mouse. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-derived Exosomes Combined With GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).