Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Basis and Process Scenarios
- Upstream Section
- Primary Recovery Section
- Purification Section
- Isopropanol Recycle Section
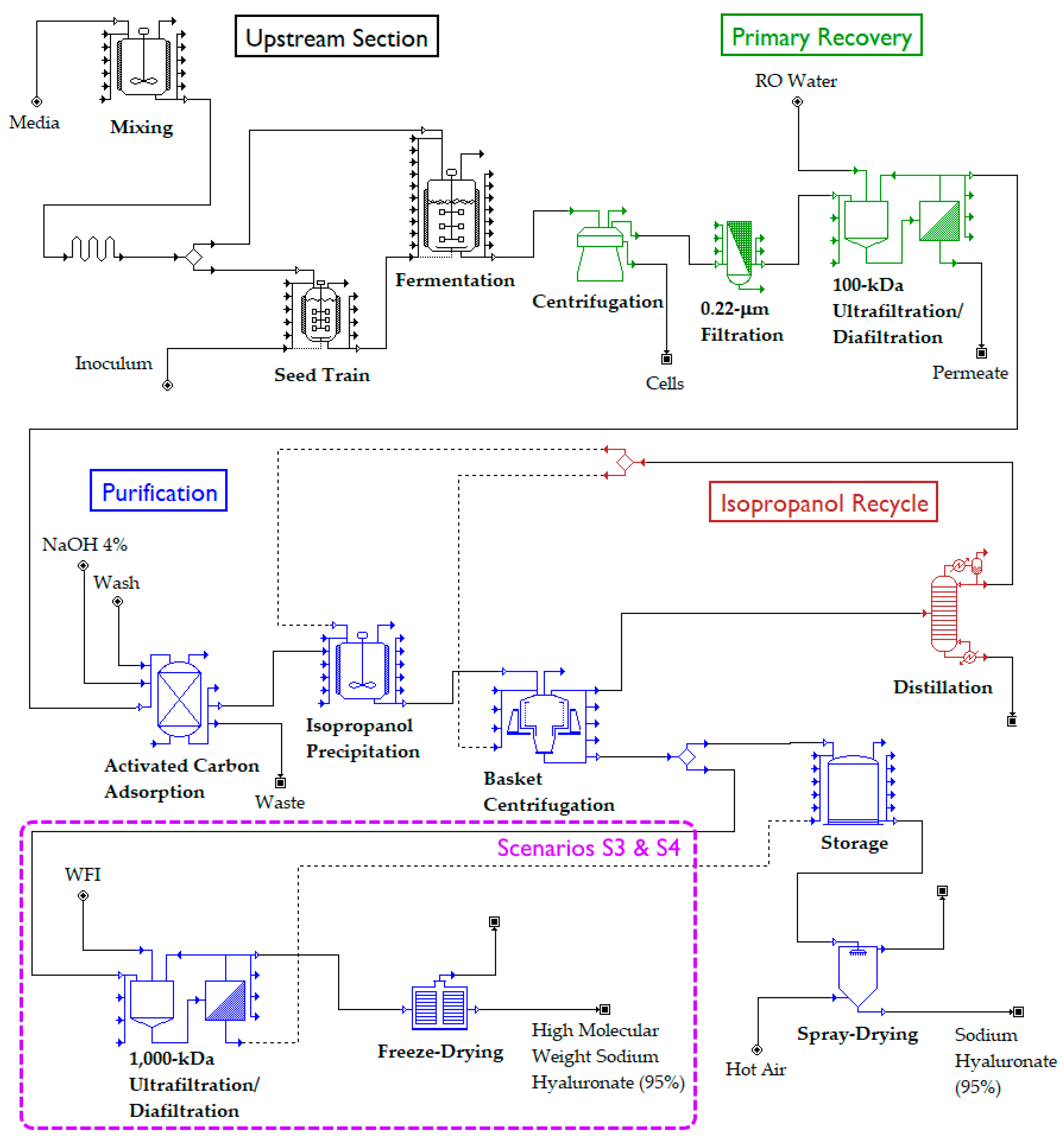
2.2. Flowsheet Sections
2.2.1. Upstream Section
- Sodium salts of HA, acetic acid, and lactic acid (sodium hyaluronate, sodium acetate, and sodium lactate, respectively);
- Biomass, cell debris, bacterial proteins, nucleic acids, and other impurities (amino acids, nucleotides, minerals, etc.);
- Residual glucose, yeast extract, and medium salts.
2.2.2. Primary Recovery Section
2.2.3. Purification Section
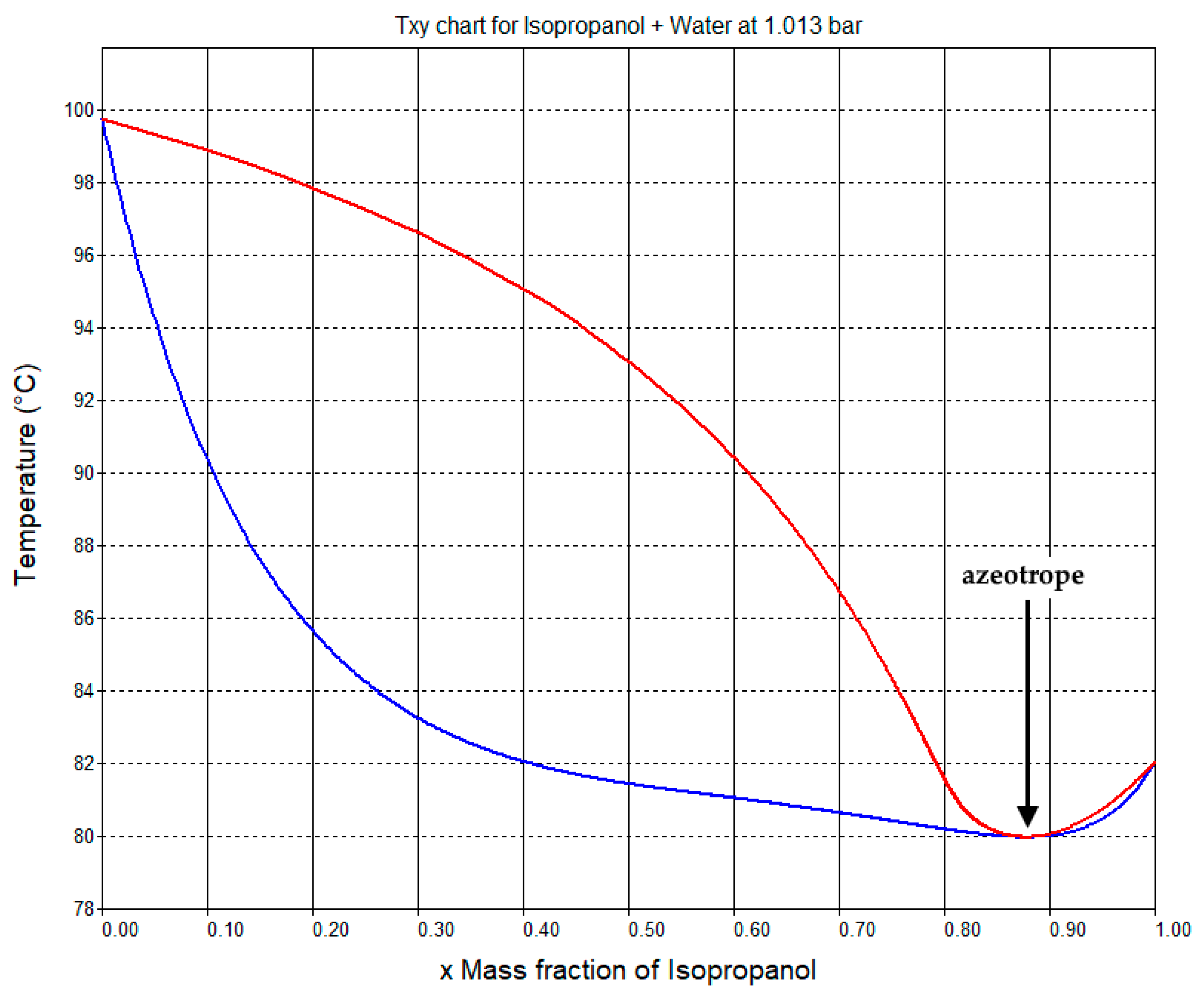
2.2.4. Isopropanol Recycle Section
2.3. Estimation of Operating and Capital Costs
3. Results
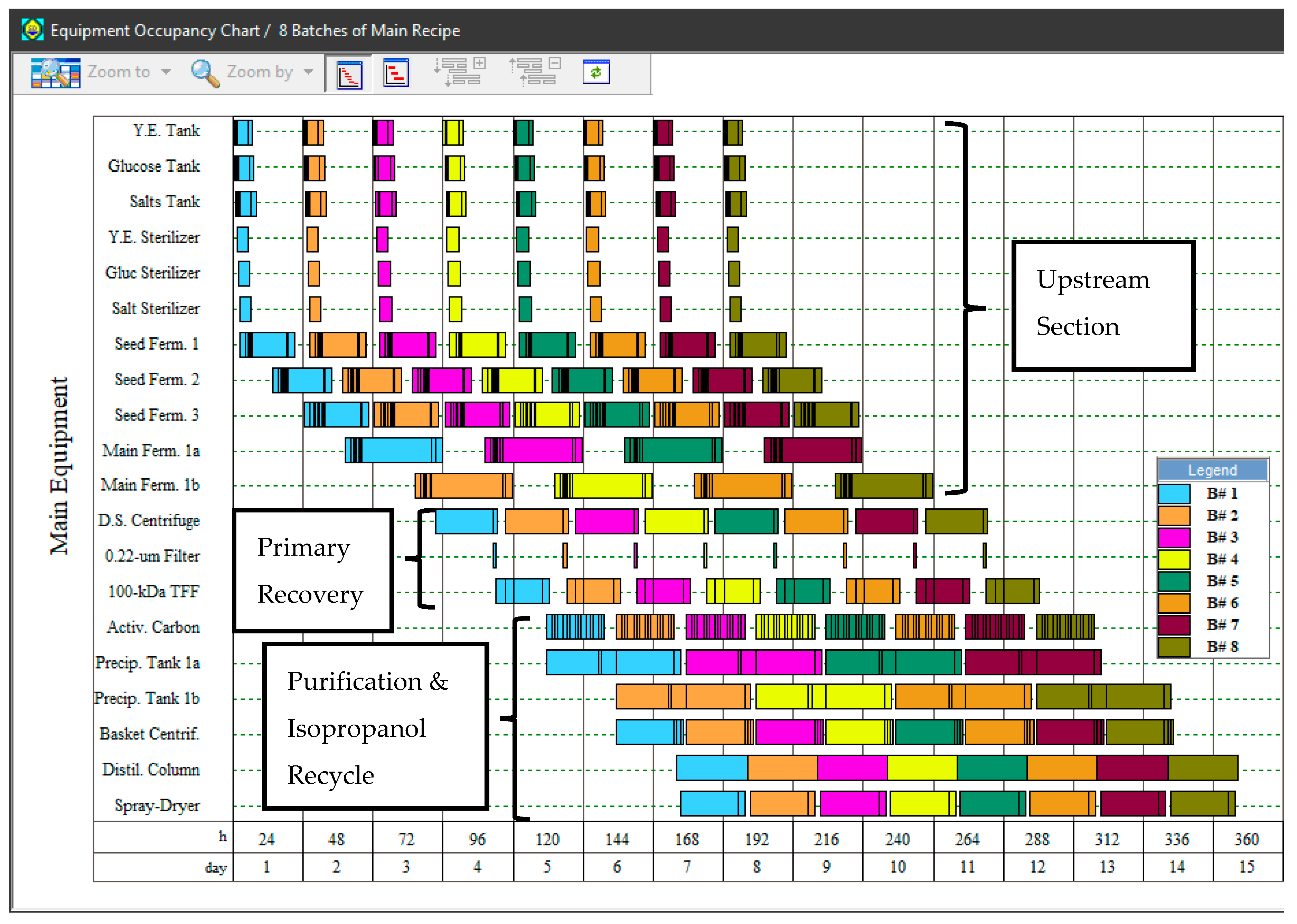
3.1. Process Simulation
3.2. Economic Evaluation
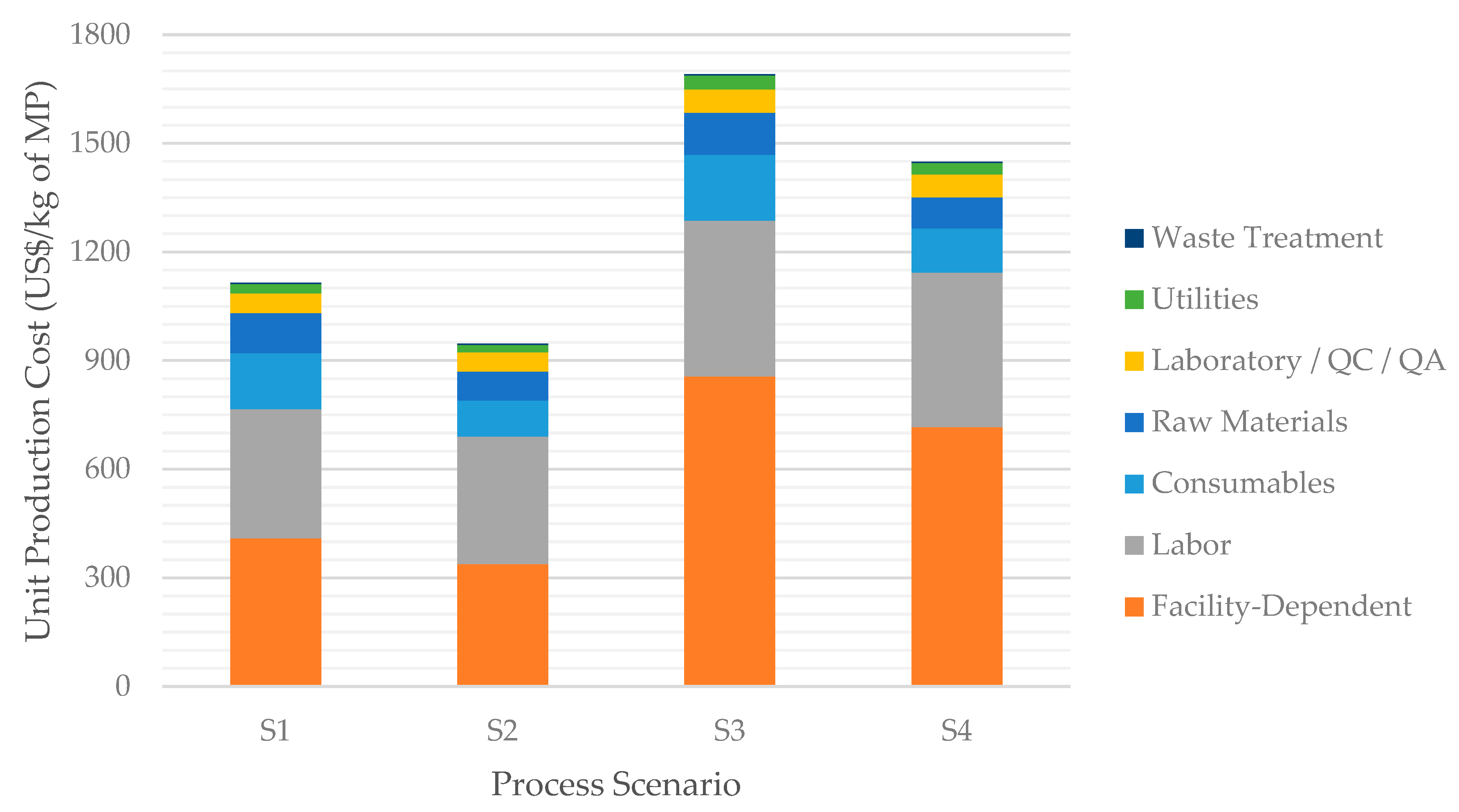
3.2.1. Comparison of Process Scenarios S1–S4
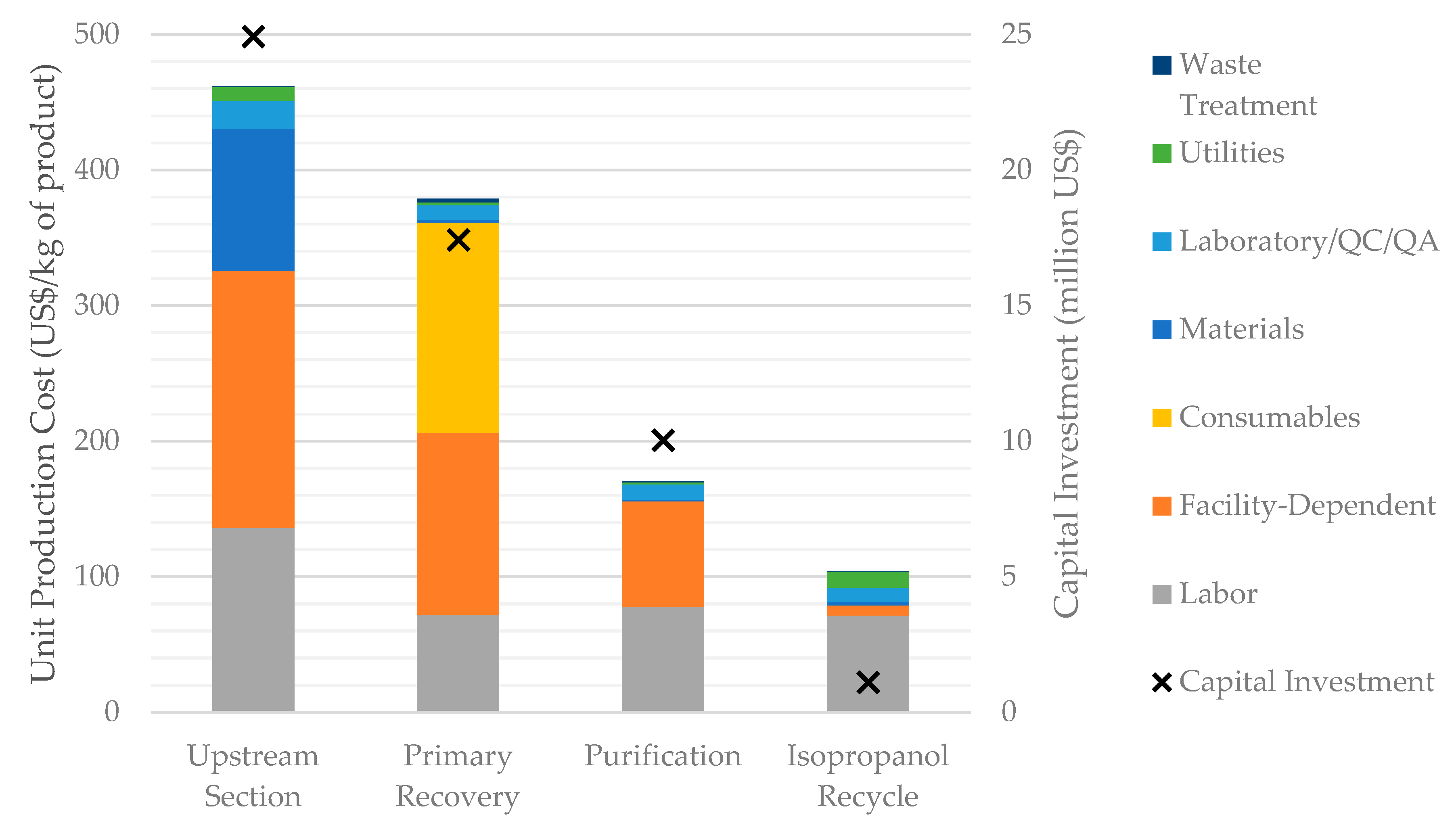
3.2.2. Breakdown of Production Costs by Process Section in Scenario S1
3.2.3. Effect of Production Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C. “The Tree”: Hyaluronan Research in the 20th Century. Hyaluronan Today 2002. Available online: https://www.glycoforum.gr.jp/article/06A1.html (accessed on 29 November 2020).
- Cavalcanti, A.D.D.; de Melo, B.A.G.; Ferreira, B.A.M.; Santana, M.H.A. Performance of the main downstream operations on hyaluronic acid purification. Process Biochem. 2020, 99, 160–170. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef]
- de Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.V.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016, 15, 119. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10, 1–9. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic Acid and Wound Healing. J. Pharm. Pharm. Sci. 2015, 18, 53. [Google Scholar] [CrossRef]
- Lin, F.-H.; Su, W.-Y.; Chen, Y.-C.; Chen, K.-H. Cross-Linked Oxidated Hyaluronic Acid for Use as a Vitreous Substitute. U.S. Patent 8,197,849 B2, 12 June 2012. [Google Scholar]
- Nicholls, M.A.; Fierlinger, A.; Niazi, F.; Bhandari, M. The Disease-Modifying Effects of Hyaluronan in the Osteoarthritic Disease State. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2017, 10, 117954411772361. [Google Scholar] [CrossRef]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Benitez, C.A.; Kohane, D.S. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 2007, 28, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Highley, C.B.; Bellas, E.; Ito, T.; Marini, R.; Langer, R.; Kohane, D.S. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials 2006, 27, 4698–4705. [Google Scholar] [CrossRef] [PubMed]
- Schulte, S.; Doss, S.S.; Jeeva, P.; Ananth, M.; Blank, L.M.; Jayaraman, G. Exploiting the diversity of streptococcal hyaluronan synthases for the production of molecular weight–tailored hyaluronan. Appl. Microbiol. Biotechnol. 2019, 103, 7567–7581. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, E.; Steen, J.A.; Nielsen, L.K. Insight into hyaluronic acid molecular weight control. Appl. Microbiol. Biotechnol. 2014, 98, 6947–6956. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.V.; Badle, S.S.; Ramachandran, K.B. Hyaluronic acid production and molecular weight improvement by redirection of carbon flux towards its biosynthesis pathway. Biochem. Eng. J. 2013, 80, 53–60. [Google Scholar] [CrossRef]
- Chen, W.Y.; Marcellin, E.; Steen, J.A.; Nielsen, L.K. The Role of Hyaluronic Acid Precursor Concentrations in Molecular Weight Control in Streptococcus zooepidemicus. Mol. Biotechnol. 2014, 56, 147–156. [Google Scholar] [CrossRef]
- Cheng, F.; Yu, H.; Stephanopoulos, G. Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid. Metab. Eng. 2019, 55, 276–289. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Corsa, V.; Carpanese, G. Process for the Purification of Hyaluronic Acid. U.S. Patent 2019/0023813 A1, 24 January 2019. [Google Scholar]
- Wang, Y.; Hu, L.; Huang, H.; Wang, H.; Zhang, T.; Chen, J.; Du, G.; Kang, Z. Eliminating the capsule-like layer to promote glucose uptake for hyaluronan production by engineered Corynebacterium glutamicum. Nat. Commun. 2020, 11, 3120. [Google Scholar] [CrossRef]
- Gomes, A.M.V.; Netto, J.H.C.M.; Carvalho, L.S.; Parachin, N.S. Heterologous Hyaluronic Acid Production in Kluyveromyces lactis. Microorganisms 2019, 7, 294. [Google Scholar] [CrossRef]
- Westbrook, A.W.; Ren, X.; Oh, J.; Moo-Young, M.; Chou, C.P. Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Metab. Eng. 2018, 47, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Hyaluronic Acid Market Size, Industry Analysis Report, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/hyaluronic-acid-market (accessed on 15 November 2020).
- Hyaluronic Acid Producers Benefit from Beauty Boom. Available online: http://www.chinadaily.com.cn/global/2019-08/07/content_37499383.htm (accessed on 15 November 2020).
- Statista. Total Sales Volume of the Hyaluronic Acid Raw Materials in China from 2014 to 2018. Available online: https://www.statista.com/statistics/1043602/china-hyaluronic-acid-raw-material-market-sales-volume/ (accessed on 15 November 2020).
- Santosh, Y.; Dharmendra, J.; Nataraj, V.; Velankar, H.; Kapat, A.; Rangaswamy, V. Process for Purification of High Molecular Weight Hyaluronic Acid. EP 2 216 412 B1, 3 October 2012. [Google Scholar]
- Pires, A.M.B. Estudos Metabólicos para Otimização de Condições Nutricionais e de Cultivo para Produção Microbiana de Ácido Hialurônico. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2009. [Google Scholar]
- Rangaswamy, V.; Jain, D. An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol. Lett. 2008, 30, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, M.; Ruggiero, A.; Volpe, F. Production of Highly Purified Sodium Hyaluronate (HANA) with Controlled Molecular Weight. U.S. Patent 9,347,079 B2, 24 May 2016. [Google Scholar]
- Altergon Altergon—Quality & Safety. Available online: https://www.altergon.com/hyaluronic-acid/quality-safety.php?PHPSESSID=plhl6eae87em1gjvbdlg89kr77 (accessed on 18 December 2020).
- US Bureau of Labor Statistics Hourly Mean Wage for Chemical Equipment Operators and Tenders in Chemical Manufacturing in the United States. Available online: https://beta.bls.gov/dataViewer/view/timeseries/OEUN000000032500051901103 (accessed on 14 December 2020).
- US International Trade Commission. US International Trade Commission Dataweb. Available online: https://dataweb.usitc.gov/trade/search/ (accessed on 18 November 2020).
- DACE—Dutch Association of Cost Engineers DACE Price Booklet. Available online: https://www.dacepricebooklet.com/ (accessed on 19 October 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3776, Isopropyl Alcohol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3776#section=Kovats-Retention-Index (accessed on 22 December 2020).
- Toemmeraas, K.; Bach, P. Spray Drying of High Molecular Weight Hyaluronic Acid. U.S. Patent Application 2014/0155347 A1, 5 June 2014. [Google Scholar]
- Vazquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microb. Cell Fact. 2010, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Puetz, J.; Wurm, F.M. Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing. Processes 2019, 7, 476. [Google Scholar] [CrossRef]

| Parameter | Specification |
|---|---|
| Production Scale | 20 MT of HA/yr |
| Annual Operating Time | 7920 h (330 days) |
| Process Cycle Time | 24 h |
| Production Fermentation Cycle Duration | 33 h |
| Concentration of HA (as sodium hyaluronate) in the broth 1 | 2.5 g/L |
| Concentration of biomass (DCW) in the broth 1 | 5.0 g/L |
| Contaminating peptides/proteins in the broth 1 | 21 g/L |
| % of Proteins in the Final Product | ≤0.1% 2 |
| Raw Material | Upstream Section (kg/kg MP 1) | Primary Recovery (kg/kg MP) | Purification (kg/kg MP) | Isopropanol Recycle (kg/kg MP) | Total (kg/kg MP) |
|---|---|---|---|---|---|
| Air | 1729.2 | 0.0 | 18.8 | 0.0 | 1748.0 |
| CIP-Acid | 13.5 | 30.0 | 15.5 | 0.0 | 59.0 |
| CIP-Caustic | 34.7 | 56.1 | 27.5 | 0.0 | 118.2 |
| Glucose | 12.3 | 0.0 | 0.0 | 0.0 | 12.3 |
| Isopropanol | 0.0 | 0.0 | 0.0 | 2.6 | 2.6 |
| NaOH (20% w/w) | 10.3 | 0.0 | 0.0 | 0.0 | 10.3 |
| NaOH (4% w/w) | 0.0 | 0.0 | 8.0 | 0.0 | 8.0 |
| RO Water | 427.4 | 2133.3 | 300.3 | 0.0 | 2861.0 |
| Salts | 4.8 | 0.0 | 0.0 | 0.0 | 4.8 |
| Water | 399.0 | 0.0 | 0.0 | 0.0 | 399.0 |
| Yeast Extract | 29.6 | 0.0 | 0.0 | 0.0 | 29.6 |
| S1 | S2 | S3 | S4 | |
|---|---|---|---|---|
| Total Capital Investment (million US$) | 53.5 | 44.3 | 107.0 | 89.6 |
| Unit Production Cost (US$/kg) | 1115 | 946 | 1691 | 1449 |
| Return on Investment (ROI) | 32.6% | 43.5% | 42.5% | 53.1% |
| Payback Time (years) | 3.07 | 2.30 | 2.35 | 1.88 |
| Net Present Value 1 (NPV) (million US$) | 92.4 | 115.3 | 276.5 | 308.7 |
| Product for Topical Use (kg) | 20,000 | 20,000 | 19,067 | 19,067 |
| Product for Injectable Use (kg) | 0 | 0 | 871 | 871 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation. Processes 2021, 9, 241. https://doi.org/10.3390/pr9020241
Ferreira RG, Azzoni AR, Santana MHA, Petrides D. Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation. Processes. 2021; 9(2):241. https://doi.org/10.3390/pr9020241
Chicago/Turabian StyleFerreira, Rafael G., Adriano R. Azzoni, Maria Helena Andrade Santana, and Demetri Petrides. 2021. "Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation" Processes 9, no. 2: 241. https://doi.org/10.3390/pr9020241
APA StyleFerreira, R. G., Azzoni, A. R., Santana, M. H. A., & Petrides, D. (2021). Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation. Processes, 9(2), 241. https://doi.org/10.3390/pr9020241









