Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review
Abstract
1. Introduction
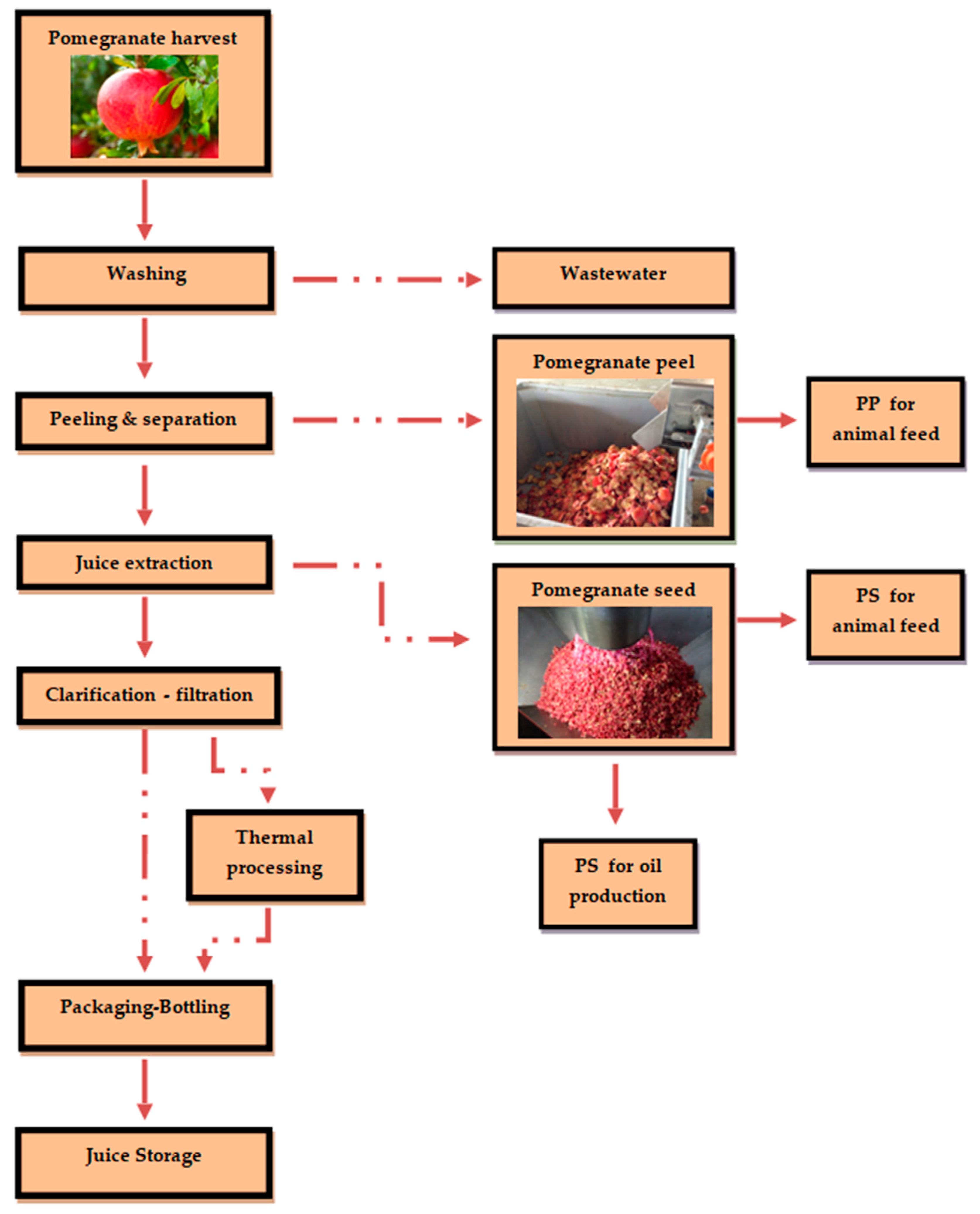
2. Pomegranate Juice Production and Wastes
3. Phenolic Profile of Solid Waste of Pomegranate Juice Process
4. Technologies and Extraction Methods Used for PP
4.1. Extraction Technique with Simple Stirring
4.2. Extraction by Applying Pressure
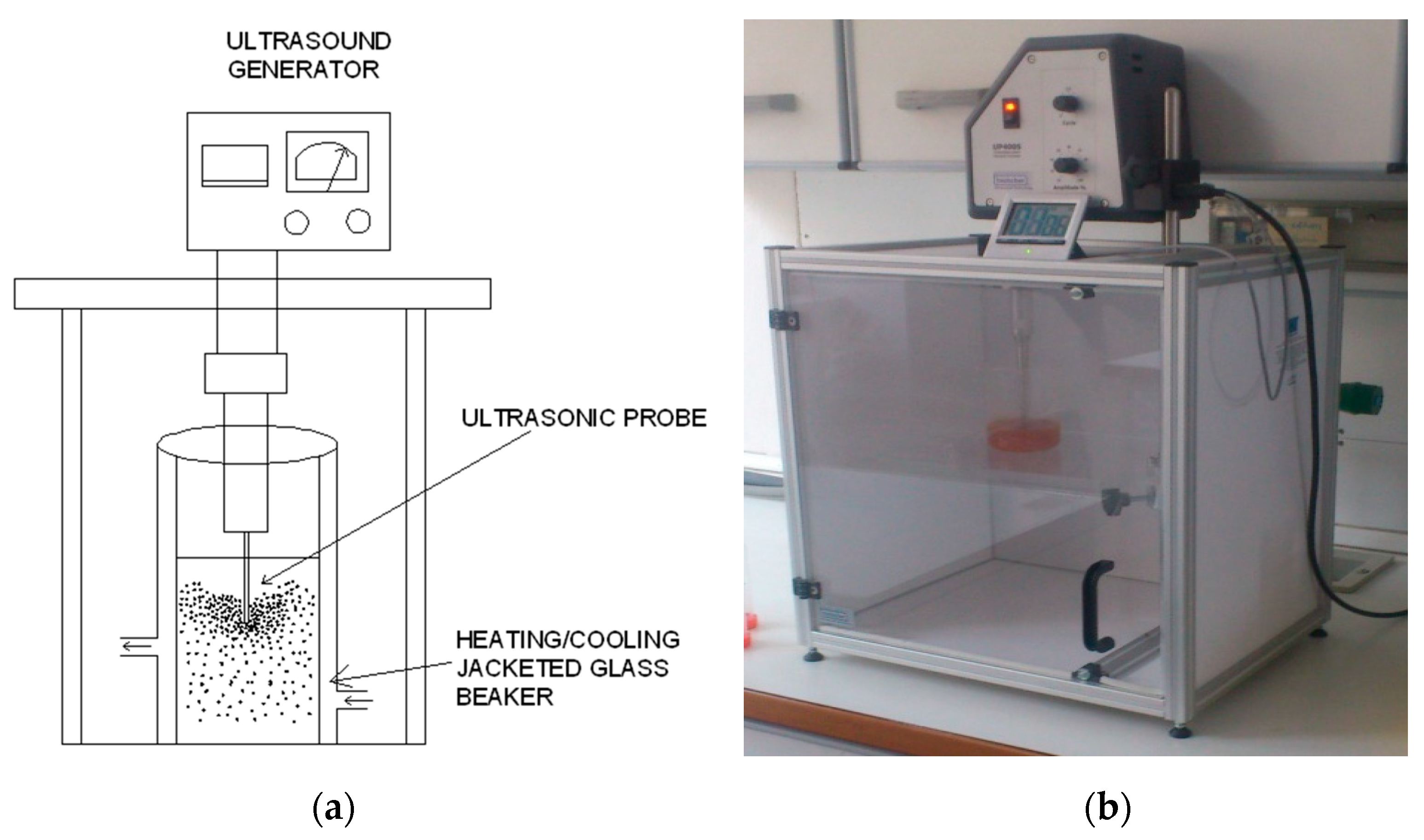
4.3. Ultrasound-Assisted Extraction (UAE)
4.4. Microwave Assistant Extraction (MAE)
4.5. Comparison of UAE, MAE and Conventional Extraction Methods
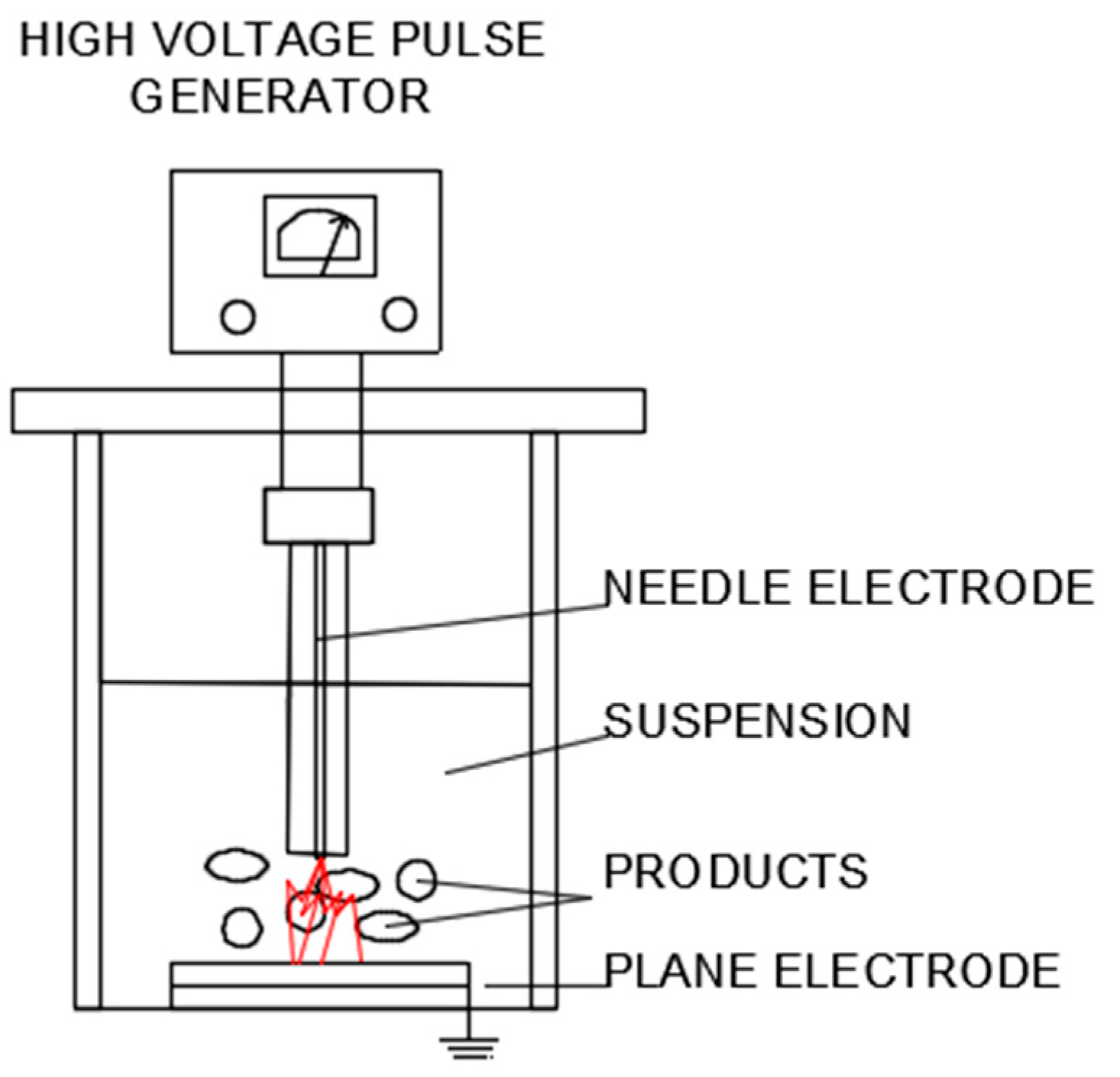
4.6. Pulsed Electric Fields(PEF) and High Voltage Electrical Discharge (HVED) Assisted Extraction
4.7. Non Conventional Extraction Solvents Used on PP
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choe, E.; Min, D.B. Chemistry and reactions of reactive oxygen species in foods. J. Food Sci. 2006, 70, R142–R159. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Melgarejo, P.; Valero, D. The pomegranate tree in the world: Its problems and uses. In Options méditerranéennes, Proceedings of the II International Symposium on the Pomegranate, Madrid, Spain, 19–21 October 2011; CIHEAM-IAMZ: Zaragoza, Spain, 2012; pp. 11–26. [Google Scholar]
- Melgarejo, P.; Núñez-Gómez, D.; Legua, P.; Martínez-Nicolás, J.J.; Almansa, M.S. Pomegranate (Punica granatum L.) a dry pericarp fruit with fleshy seeds. Trends Food Sci. Technol. 2020, 102, 232–236. [Google Scholar] [CrossRef]
- Holland, I.; Bar-Yaakov, A. The Pomegranate: New interest in an ancient fruit. Chron. Hortic. 2008, 48, 12–15. [Google Scholar]
- Da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- La Rue, J.H. Growing Pomegranates in California; UC Fruit and Nut Research Information center: Davis, CA, USA, 1980. [Google Scholar]
- Mars, M. La culture du grenadier (Punica granatum L.) et du figuier (Ficus parica L.) en Tunisia. In First Meeting CIHEAM Coop; Res. Network on underutilised Fruit Trees: Zaragoza, Spain, 1994; pp. 76–83. [Google Scholar]
- Frison, E.A.; Servinsky, J. Directory of European Institutions Holding Crop Genetic Resources Collections, 4th ed.; International Plant Genetic Resources Institute: Washington, DC, USA, 1995; Volume 1. [Google Scholar]
- Melgarejo, P.; Salazar, D.M. Tratado de fruticultura para zonas áridas y semiáridas. In Algarrobo, Granado y Jinjolero; Mundi-Prensa y AMV Ediciones: Madrid, Spain, 2003; Volume II, p. 430. [Google Scholar]
- Rajaei, H.; Yazdanpanah, P. Buds and leaves in Pomegranate (Punica granatum L.): Phenology in relation to structure and development. Flora: Morphology, distribution. Funct. Ecol. Plants 2015, 214, 61–69. [Google Scholar] [CrossRef]
- Seeram, N.P.; Schulman, R.N.; Heber, D. Pomegranates: Ancient Roots to Modern Medicine; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Priyadarshini, A.; Priyadarshini, A. Market Dimensions of the Fruit Juice Industry, Chapter 2. In Fruit Juices Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 15–32. [Google Scholar]
- Tarifa, M.C.; Lozano, J.E.; Brugnoni, L.I. Disinfection efficacy over yeast biofilms of juice processing industries. Food Res. Int. 2018, 105, 473–481. [Google Scholar] [CrossRef]
- Tüccar, G.; Uludamar, E. Emission and engine performance analysis of a diesel engine using hydrogen enriched pomegran-ate seed oil biodiesel. Int. J. Hydrog. Energy 2018, 43, 18014–18019. [Google Scholar] [CrossRef]
- Khanali, M.; Kokei, D.; Aghbashlo, M.; Nasab, F.K.; Hosseinzadeh-Bandbafha, H.; Tabatabaei, M. Energy flow modeling and life cycle assessment of apple juice production: Recommendations for renewable energies implementation and climate change mitigation. J. Clean. Prod. 2020, 246, 118997. [Google Scholar] [CrossRef]
- Anatolioti, V.; Leontopoulos, S.; Skoufogianni, G.; Skenderidis, P. A study on the potential use of energy crops as alternative cultivation in Greece. Issues of farmer’s attitudes. In Proceedings of the 4th International Conference on Food and Biosystems Engineering, Crete Island, Greece, 30 May–2 June 2019; FaBE: Wooster, OH, USA, 2019; pp. 410–445. [Google Scholar]
- Ali, M.; Diso, S.U.; Nas, F.S.; Nasir, A.S. Phytochemical screening and antibacterial activity of Citrus sinensis peel extracts on clinical isolates of Staphylococcus aureus and Salmonella typhi. J. Appl. Pharm. Sci. 2018, 1, 375–380. [Google Scholar]
- Gunaseelan, V. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Dimou, C.; Koutelidakis, A.Ε. From pomegranate processing by-products to innovative value added functional ingredients and bio-based products with several applications in food sector. Bao J. Biotech. 2017, 3, 1–8. [Google Scholar]
- Mekki, A.; Dhouib, A.; Sayadi, S. Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. J. Environ. Manag. 2007, 84, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Cristinzio, G.; Evidente, A.; Scognamiglio, F. Isolation, spectroscopy and selective phytotoxic effects of poly-phenols from vegetable waste waters. Phytochemistry 1992, 31, 4125–4128. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, J.; Ge, J. Dynamic game in agriculture and industry cross-sectoral water pollution governance in developing countries. Agric. Water Manag. 2021, 243, 106417. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Wine as a biological fluid: History, production and role in disease prevention. J. Clin. Lab. An. 1997, 11, 287–313. [Google Scholar] [CrossRef]
- Toguri, T.; Umemoto, N.; Kobayashi, O.; Ohtani, T. Activation of anthocyanin synthesis genes by white light in eggplant hypocotyl tissues, and identification of an inducible P-450 cDNA. Plant Mol. Biol. 1993, 23, 933–946. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Fakhri, S.; Elham, A. Ultrasound-Assisted osmotic treatment of model food impregnated with pomegranate peel phenolic compounds: Mass transfer, texture, and phenolic evaluations. Food Bioprocess Technol. 2018, 11, 1061–1074. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Malik, A.; Afaq, F.; Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Mukhtar, H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 14813–14818. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity potential of polyphenolic compounds in human health and their effectiveness against various food borne and plant pathogens. A review. Int. J. Food Biosys. Eng. 2017, 7, 1–19. [Google Scholar]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Petrotos, K.; Leontopoulos, S. Functional Properties of Goji Berry (Lycium barbarum) Fruit Extracts, Chapter 10. In Phytochemicals in Goji Berries; Ye, X., Jiang, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 181–224. [Google Scholar]
- Leontopoulos, S.; Skenderidis, P.; Vagelas, I.K. Potential use of polyphenolic compounds obtained from olive mill waste waters on plant pathogens and plant parasitic nematodes. In Progress in Biological Control; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 22, pp. 137–177. [Google Scholar]
- Endo, E.H.; Cortez, D.A.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.D. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Giavasis, I.; Petrotos, K.; Lampakis, D.; Leontopoulos, S.; Hadjichristodoulou, C.; Tsakalof, A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019, 13, 2017–2031. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Skoufogianni, G. Potential use of medicinal plants as biological crop protection agents. Biomed. J. Sci. Tech. Res. 2020, 25, 19320–19324. [Google Scholar] [CrossRef]
- Andrade, A. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate peel as suitable source of high-added value bioactives: Tailored functionalized meat products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011, 18, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, Z.; Ma, H.; Atungulu, G.G. Extract of phenolics from pomegranate peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Alonso-Salces, R.; Korta, E.; Barranco, A.; Berrueta, L.; Gallo, B.; Vicente, F. Pressurized liquid extraction for the determination of polyphenols in apple. J. Chromatogr. A 2001, 933, 37–43. [Google Scholar] [CrossRef]
- Li, B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A green deep eutectic solvent-based aqueous two-phase system for protein ex-tracting. Anal. Chim. Acta 2015, 864, 9–20. [Google Scholar] [CrossRef]
- More, P.R.; Arya, S.S. A novel, green cloud point extraction and separation of phenols and flavonoids from pomegranate peel: An optimization study using RCCD. J. Environ. Chem. Eng. 2019, 7, 103306. [Google Scholar] [CrossRef]
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Giavasis, I. Optimization of vacuum microwave-assisted extraction of pomegranate fruits peels by the evaluation of extracts’ phenolic content and antioxidant activity. Foods 2020, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: A review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef] [PubMed]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine, the bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Anjaly, P.; Mahendran, R. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar]
- Cheng, X.L.; Wan, J.Y.; Li, P.; Qi, L.W. Ultrasonic/microwave assisted extraction and diagnostic ion filtering strategy by liquid chromatography–quadrupole time-off light mass spectrometry for rapid characterisation of flavonoids in Spatholobus suberectus. J. Chromatogr. 2011, 1218, 5774–5786. [Google Scholar] [CrossRef]
- Wang, Y.C.; Ying, L.; Sun, D.; Xu, P. Supercritical carbon dioxide extraction of bioactive compounds from Ampelopsis grossed-entata stems: Process optimisation and antioxidant activity. Int. J. Mol. Sci. 2011, 12, 6856. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of microwave extraction. In Microwave-Assisted Extraction for Bioactive compounds; Food Engineerging Series; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–52. [Google Scholar]
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Qu, W.; Pan, Z.; Ma, H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010, 99, 16–23. [Google Scholar] [CrossRef]
- Negi, P.; Jayaprakasha, G.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Ranjbar, N.; Eikani, M.H.; Javanmard, M.; Golmohammad, F. Impact of instant controlled pressure drop on phenolic com-pounds extraction from pomegranate peel. Innov. Food Sci. Emerg. Technol. 2016, 37, 177–183. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Hayder, Z.; Elfalleh, W.; Othman, K.B.; Benabderrahim, M.A.; Hannachi, H. Modeling of polyphenols extraction from pomegranate by-product using rotatable central composite design of experiments. Acta Ecol. Sin. 2020. [Google Scholar] [CrossRef]
- Dimitrov, K.; Pradal, D.; Vauchel, P.; Baouche, B.; Nikov, I.; Dhulster, P. Modeling and optimization of extraction and energy consumption during Ultrasound-Assisted Extraction of antioxidant polyphenols from pomegranate peels. Environ. Prog. Sustain. Energy 2019, 38, 13148. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Xian-Zhe, Z.; Yin, F.; Liu, C.; Xu, X. Effect of process parameters of Microwave Assisted Extraction (MAE) on polysaccharides yield from pumpkin. J. Northeast. Agric. Univ. Engl. Ed. 2011, 18, 79–86. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-Assisted Extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process. Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative process of polyphenol recovery from pomegranate peels by combining green deep eutectic solvents and a new infrared technology. LWT 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. Trac. Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Ohnishi-Kameyama, M.; Ono, H.; Yoshida, M.; Jaganmohan, R.L. Phenolic constituents in the fruits of Cinnamomum zeylanicum and their antioxidant activity. J. Agric. Food Chem. 2006, 54, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Pekić, B.; Kovač, V.; Alonso, E.; Revilla, E. Study of the extraction of proanthocyanidins from grape seeds. Food Chem. 1998, 61, 201–206. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Chapter 13-Pressurized Liquid Extraction. In Handbooks in Separation Science; Poole, C.F.B.T.-L.-P.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. ISBN 978-0-12-816911-7. [Google Scholar]
- Arwa, M.; Mijangos, L.; Turner, C. Pressurized hot ethanol extraction of carotenoids from carrot by-products. Molecules 2012, 17, 1809–1818. [Google Scholar]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F.J. Screening of functional com-pounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Ramos, L.; Kristenson, E.; Brinkman, U. Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis. J. Chromatogr. A 2002, 975, 3–29. [Google Scholar] [CrossRef]
- Mason, T.; Paniwnyk, L.; Lorimer, J. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Mason, T.J.; Chemat, F.; Vinatoru, M. The Extraction of Natural Products using Ultrasound or Microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Vinatoru, M.; Toma, M.; Mason, T.J. Ultrasonically assisted extraction of bioactive principles from plants and their constituents, January 1999. In Advances in Sonochemistry, 5th ed.; JAI Press/Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Buldini, P.L.; Ricci, L.; Sharma, J.L. Recent applications of sample preparation techniques in food analysis. J. Chromatogr. A 2002, 975, 47–70. [Google Scholar] [CrossRef]
- Tiwari, H.C.; Singh, P.; Mishra, P.K.; Srivastava, P. Evaluation of various techniques for extraction of natural colorants from pomegranate rind-ultrasound and enzyme assisted extraction. Indian J. Fibre Text. Res. 2010, 35, 272–276. [Google Scholar]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green extraction from pomegranate marcs for the production of functional foods and cosmetics. Pharmaceutical 2016, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, B.; Li, L.; Zhu, Z. Microwave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J. Med. Plants Res. 2011, 5, 1004–1011. [Google Scholar]
- Huang, J.; He, W.; Yan, C.; Du, X.; Shi, X. Microwave assisted extraction of flavonoids from pomegranate peel and its antioxidant activity. BIO Web Conf. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano-Tixier, A.S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Application of Natural Deep Eutectic Solvents for Extraction of Polyphenolics from Olive Oil By-Products Using Microwaves. 2015. Available online: https://www.semanticscholar.org/paper/Application-of-natural-deep-eutectic-solvents-for-Chanioti-iamandoura/cb86464b064a6af288437c3d0ad358fa25a6a34f (accessed on 16 December 2020).
- Wu, P.; Gu, Y.; Zhao, R.; Liu, Y.; Wang, Y.; Lv, G.; Li, Z.; Bao, Y. Residual pomegranate affecting the nonspecific immunity of juvenile Darkbarbel catfish. Fish Shellfish Immunol. 2019, 95, 190–194. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Touya, G.; Reess, T.; Pécastaing, L.; Gibert, A.; Domens, P. Development of subsonic electrical discharges in water and measurements of the associated pressure waves. J. Phys. D Appl. Phys. 2006, 39, 5236–5244. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolicsin foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Abbott, P.A.; Capper, G.; Davies, L.D.; Munro, L.H.; Rasheed, K.R.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 19, 2010–2011. [Google Scholar] [CrossRef] [PubMed]
- Abbott, P.A.; Capper, G.; Davies, L.D.; Munro, L.H.; Rasheed, K.R.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, P.A.; Capper, G.; Davies, L.D.; Munro, L.H.; Rasheed, K.R.; Tambyrajah, V. Deep eutectic solvents formed between choline chloride and carboxylic Acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Ruß, C.; König, B. Low melting mixtures in organic synthesis-An alternative to ionic liquids? Green Chem. 2012, 14, 2969. [Google Scholar] [CrossRef]


| Extraction Technique | Time (min) | Solvents | Total Phenolics | Flavonoids | Tannins | Reference |
|---|---|---|---|---|---|---|
| Simple stirring | 60 | Water, methanol, ethanol, ethyl acetate acetone | 119–82.6 mg GAE/g DM | |||
| 240 | 249.4 ± 17.2 mg GAE/g DM | 59.1 ± 4.8 mg/g | 10.9 ± 0.5 mg/g | [37,44,45] | ||
| 2 | water | 229mg TAE/g DM | [60] | |||
| Soxhlet | 240 | ethyl acetate acetone, methanol, Water | 165 mg CE/g DM 520 mg CE/g DM 462 mg CE/g DM 48 mg CE/g DM | [61] | ||
| Pressure | 60 | Water, methanol | 45.65 mg GAE/g DM | [62,63] | ||
| Water | 264 mg TAE/g DM | 13 mg CE/g | Hydrolyzable tannins 262 mg TAE/g) Condensed tannins 9.5 mg CE/g) | |||
| UAE (continuous) UAE (pulsed) | 6 10 | Water Water | 148 mg GAE/g DM 145 mg GAE/g DM | |||
| UAE | Water/ethanol | 188.1 mg GAE/g DM | 62.6 mg RE/g DM) | 23.2 mg CE/g DM | [44,64,65,66] | |
| Water/ethanol | >200 mg GAE/g DM | |||||
| 10 | ethyl-acetate | 138.5 mg GAE/g DM | ||||
| MAE VMAAE | 1 10 | Extraction yield increased in thefollowing order of solvents: 70% methanol < 50% methanol < water < 70% ethanol < 50% Water | 24.64 mg GAE/g DM. Ethanol varied from 202.8 to 214.5 mg GAE/g DM 146 mg GAE/g | [44,67,68] [52] | ||
| Enzyme-assisted supercritical fluid extraction | 85 | 301.53 mg GAE/g | [69] | |||
| ΙRA | 90 | DES | 152 mg/g DM | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes 2021, 9, 236. https://doi.org/10.3390/pr9020236
Lampakis D, Skenderidis P, Leontopoulos S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes. 2021; 9(2):236. https://doi.org/10.3390/pr9020236
Chicago/Turabian StyleLampakis, Dimitrios, Prodromos Skenderidis, and Stefanos Leontopoulos. 2021. "Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review" Processes 9, no. 2: 236. https://doi.org/10.3390/pr9020236
APA StyleLampakis, D., Skenderidis, P., & Leontopoulos, S. (2021). Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes, 9(2), 236. https://doi.org/10.3390/pr9020236







