A Review on Temperature Control of Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
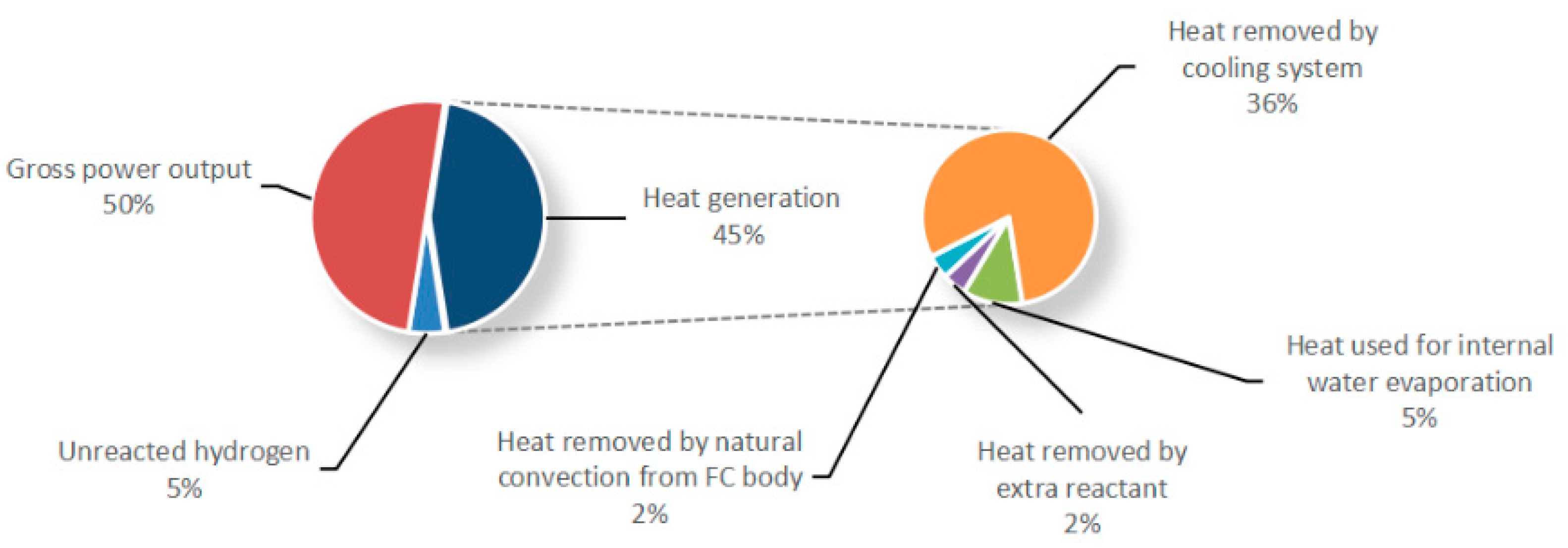
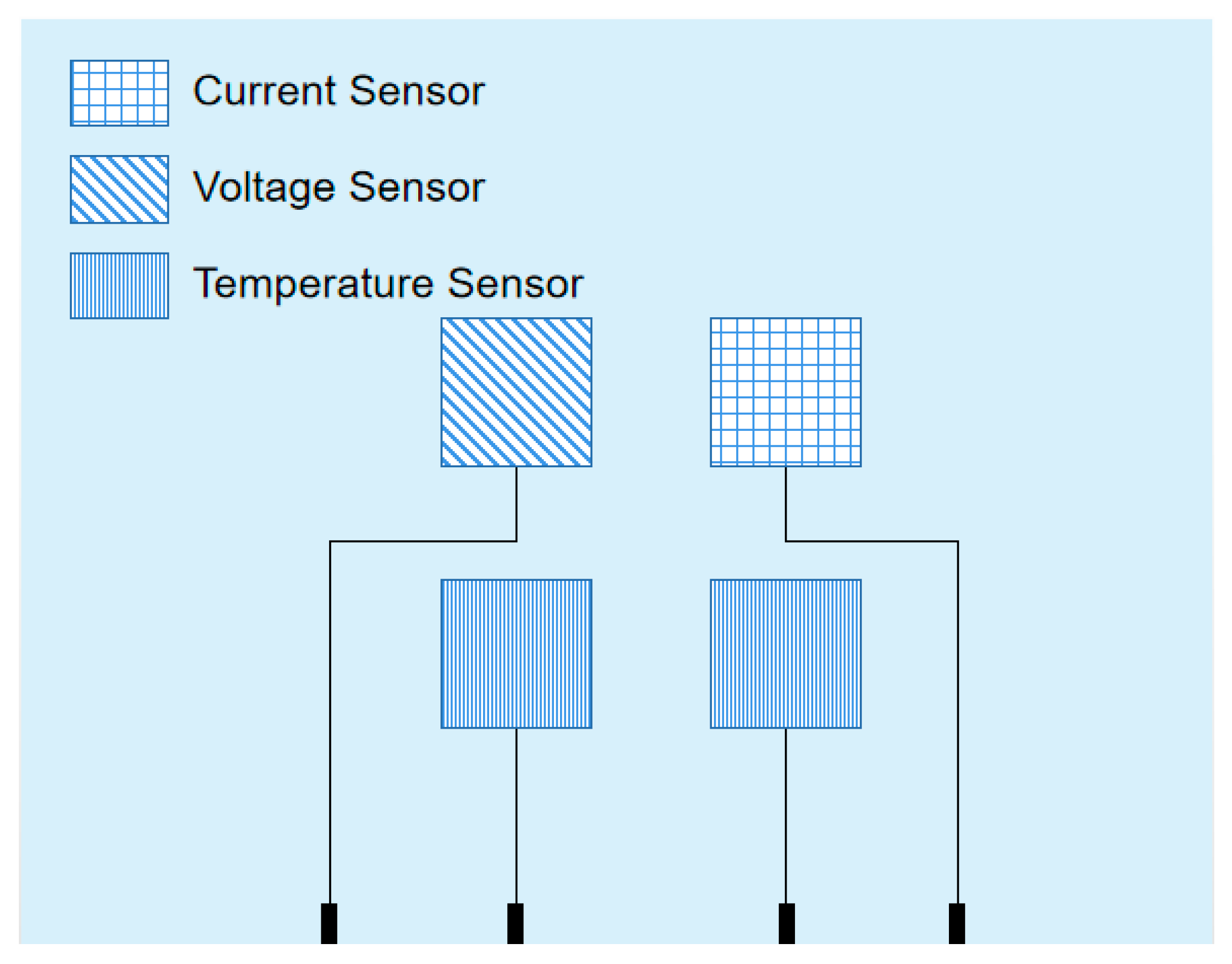
2. Research on Temperature Characteristics
3. Temperature Control Based on Battery Material Structure
3.1. Material-Based
3.1.1. Bipolar Plates
3.1.2. Gas Diffusion Layer
3.1.3. Proton Exchange Membrane
3.2. Structure-Based
3.2.1. Channel Structure of Reactants
3.2.2. Cooling Channel Structure
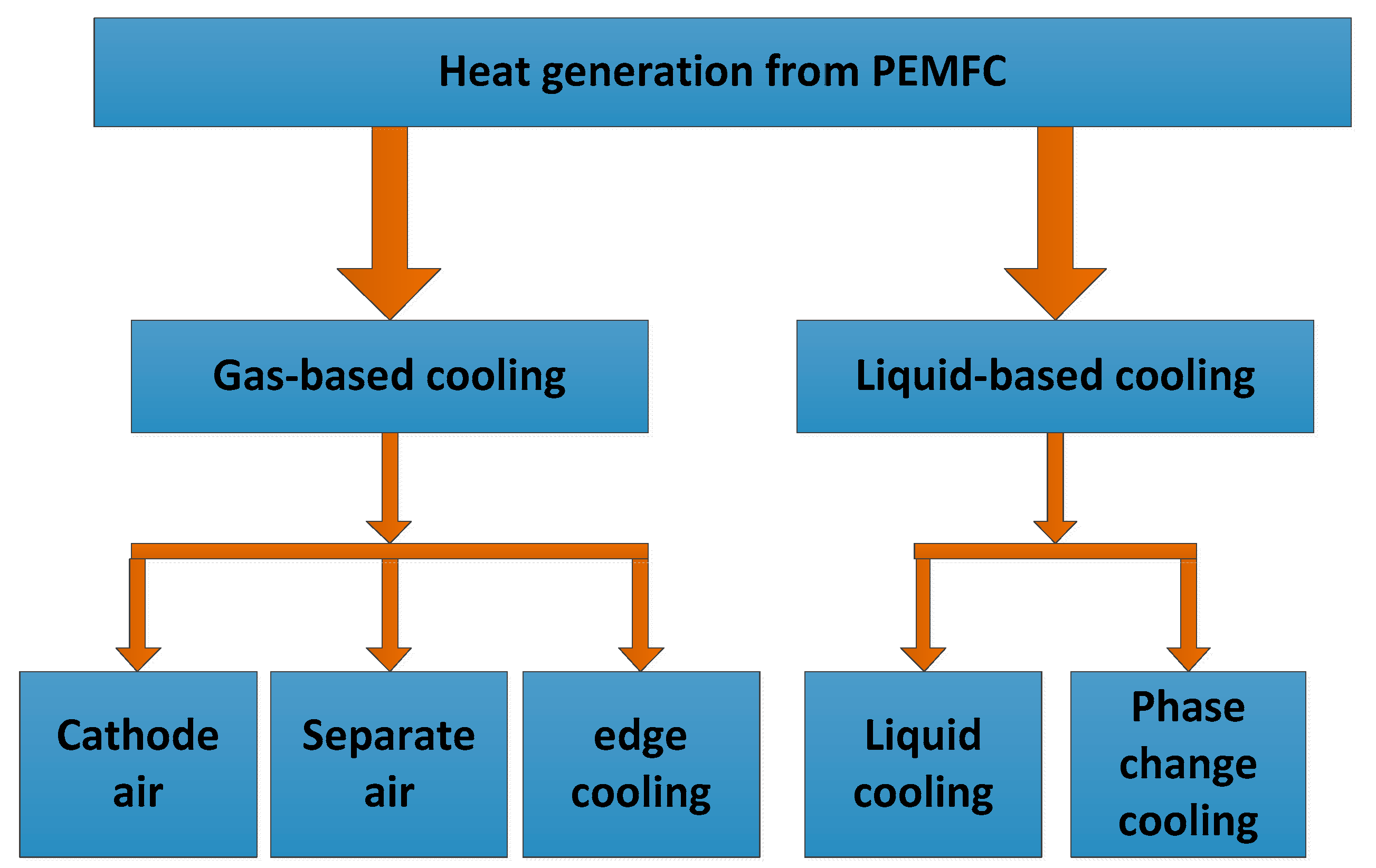
4. Temperature Control Based on Cooling Medium
4.1. Heat Dissipation of Gas Cooling Medium
4.1.1. Edge Cooling
4.1.2. Cathode Air
4.1.3. Separate Air
4.2. Liquid Cooling Medium Heat Dissipation
4.2.1. Liquid Cooling
4.2.2. Phase Change Cooling
5. Cold Start
6. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Kandlikar, S.G. A critical review of cooling techniques in proton exchange membrane fuel cell stacks. Int. J. Hydrog. Energy 2012, 37, 2412–2429. [Google Scholar] [CrossRef]
- Dicks, A.; Rand, D. Fuel Cell Systems Explained, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Rath, R.; Kumar, P.; Mohanty, S.; Nayak, S.K. Recent advances, unsolved deficiencies, and future perspectives of hydrogen fuel cells in transportation and portable sectors. Int. J. Energy Res. 2019, 43, 8931–8955. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Convers. Mgmt. 2020, 204, 112328. [Google Scholar] [CrossRef]
- Tiss, F.; Chouikh, R.; Guizani, A. Dynamic modeling of a PEM fuel cell with temperature effects. Int. J. Hydrog. Energy. 2013, 38, 8532–8541. [Google Scholar] [CrossRef]
- Shabani, B.; Andrews, J. An experimental investigation of a PEM fuel cell to supply both heat and power in a solar-hydrogen RAPS system. Int. J. Hydrog. Energy 2011, 36, 5442–5452. [Google Scholar] [CrossRef]
- Saygili, Y.; Eroglu, I.; Kincal, S. Model based temperature controller development for water cooled PEM fuel cell systems. Int. J. Hydrog. Energy 2015, 40, 615–622. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Fan, R.; Bao, H.; Wang, Y.; Huang, S.; Chin, C.S.; Li, C. Modelling and control of vehicle integrated thermal management system of PEM fuel cell vehicle. Energy 2020, 199, 117495. [Google Scholar] [CrossRef]
- Penga, Ž.; Pivac, I.; Barbir, F. Experimental validation of variable temperature flow field concept for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2017, 42, 26084–26093. [Google Scholar] [CrossRef]
- Hossain, M.S.; Shabani, B. Metal foams application to enhance cooling of open cathode polymer electrolyte membrane fuel cells. J. Power Sources 2015, 295, 275–291. [Google Scholar] [CrossRef]
- Islam, M.; Shabani, B.; Rosengarten, G.; Andrews, J. The potential of using nanofluids in PEM fuel cell cooling systems: A review. Renew. Sustain. Energy Rev. 2015, 48, 523–539. [Google Scholar] [CrossRef]
- Ramezanizadeh, M.; Nazari, M.A.; Ahmadi, M.H.; Chen, L. A review on the approaches applied for cooling fuel cells. Int. J. Heat Mass Transf. 2019, 139, 517–525. [Google Scholar] [CrossRef]
- Wu, J.; Galli, S.; Lagana, I.; Pozio, A.; Monteleone, G.; Yuan, X.Z.; Martin, J.; Wang, H. An Air-cooled Proton Exchange Membrane Fuel Cell with Combined Oxidant And Coolant Flow. J. Power Sources 2009, 188, 199–204. [Google Scholar] [CrossRef]
- Wu, H.-W. A review of recent development: Transport and performance modeling of PEM fuel cells. Appl. Energy 2016, 165, 81–106. [Google Scholar] [CrossRef]
- Shahsavari, S.; Desouza, A.; Bahrami, M.; Kjeang, E. Thermal analysis of air-cooled PEM fuel cells. Int. J. Hydrog. Energy 2012, 37, 18261–18271. [Google Scholar] [CrossRef]
- Macedo-Valencia, J.; Sierra, J.M.; Figueroa-Ramírez, S.J.; Díaz, S.E.; Meza, M. 3D CFD modeling of a PEM fuel cell stack. Int. J. Hydrog. Energy 2016, 41, 23425–23433. [Google Scholar] [CrossRef]
- Ou, K.; Yuan, W.W.; Choi, M.; Yang, S.; Kim, Y.B. Performance increase for an open-cathode PEM fuel cell with humidity and temperature control. Int. J. Hydrog. Energy 2017, 42, 29852–29862. [Google Scholar] [CrossRef]
- Luo, L.; Jian, Q.; Huang, B.; Huang, Z.; Zhao, J.; Cao, S. Experimental study on temperature characteristics of an air-cooled proton exchange membrane fuel cell stack. Renew. Energy 2019, 143, 1067–1078. [Google Scholar] [CrossRef]
- Jian, Q.; Huang, B.; Luo, L.; Zhao, J.; Cao, S.; Huang, Z. Experimental investigation of the thermal response of open-cathode proton exchange membrane fuel cell stack. Int. J. Hydrog. Energy 2018, 43, 13489–13500. [Google Scholar] [CrossRef]
- Zhao, J.; Jian, Q.; Luo, L.; Huang, B.; Cao, S.; Huang, Z. Dynamic behavior study on voltage and temperature of proton exchange membrane fuel cells. Appl. Eng. 2018, 145, 343–351. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chiang, Y.C.; Weng, F.B.; Li, S.C.; Wu, P.H.; Yueh, H.I. Flexible micro temperature, voltage and current sensors for local real-time microscopic diagnosis inside high temperature proton exchange membrane fuel cell stack. Renew. Energy 2017, 108, 126–131. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Fang, W.-Z.; Lin, H.; Tao, W.-Q. Thin film thermocouple fabrication and its application for real-time temperature measurement inside PEMFC. Int. J. Heat Mass Transf. 2019, 141, 1152–1158. [Google Scholar] [CrossRef]
- Inman, K.; Wang, X.; Sangeorzan, B. Design of an optical thermal sensor for proton exchange membrane fuel cell temperature measurement using phosphor thermometry. J. Power Sources 2010, 195, 4753–4757. [Google Scholar] [CrossRef]
- Siegel, C.; Mudi, G.B.; Heinzel, A. Solid-phase temperature measurements in a HTPEM fuel cell International. J. Hydrog. Energy 2011, 36, 12977–12990. [Google Scholar] [CrossRef]
- Jung, C.Y.; Shim, H.S.; Koo, S.M.; Lee, S.H.; Yi, S.C. Investigations of the temperature distribution in proton exchange membrane fuel cells. Appl. Energy 2012, 93, 733–741. [Google Scholar] [CrossRef]
- Ye, D.; Gauthier, E.; Benziger, J.B.; Pan, M. Bulk and contact resistances of gas diffusion layers in proton exchange membrane fuel cells. J. Power Sources 2014, 256, 449–456. [Google Scholar] [CrossRef]
- Netwall, C.J.; Gould, B.D.; Rodgers, J.A.; Nasello, N.J.; Swider-Lyons, K.E. Decreasing contact resistance in proton-exchange membrane fuel cells with metal bipolar plates. J. Power Sources 2013, 227, 137–144. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Lin, Y.-S.; Lu, C.-H. Performance of a proton exchange membrane fuel cell stack with thermally conductive pyrolytic graphite sheets for thermal management. J. Power Sources 2009, 189, 1100–1105. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Lin, Y.-S.; Lu, C.-H.; Luo, T.-W. Thermal management of a proton exchange membrane fuel cell stack with pyrolytic graphite sheets and fans combined. Int. J Hydrog. Energy 2011, 36, 6082–6089. [Google Scholar] [CrossRef]
- Tian, R.; Sun, J. Corrosion resistance and interfacial contact resistance of TiN coated 316L bipolar plates for proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2011, 36, 6788–6794. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Leslie, T.; El-Hassan, Z.; Thomposon, J.; Olabi, A.G. Technical evaluation of proton exchange membrane (PEM) fuel cell performance—A review of the effects of bipolar plates coating. Renew. Sustain. Energy Rev. 2019, 113, 109286.1–109286.15. [Google Scholar] [CrossRef]
- Jannat, S.; Rashtchi, H.; Atapour, M.; Golozar, M.A.; Elmkhah, H.; Zhiani, M. Preparation and performance of nanometric Ti/TiN multi-layer physical vapor deposited coating on 316L stainless steel as bipolar plate for proton exchange membrane fuel cells. J. Power Sources 2019, 435, 226818. [Google Scholar] [CrossRef]
- Chanda, U.K.; Behera, A.; Roy, S.; Pati, S. Evaluation of Ni-Cr-P coatings electrodeposited on low carbon steel bipolar plates for polymer electrolyte membrane fuel cell. Int. J. Hydrog. Energy 2018, 43, 23430–23440. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Lu, Z.; Wang, L.; Li, W. Preparation and performances of electrically conductive Nb-doped TiO_2 coatings for 316 stainless steel bipolar plates of proton-exchange membrane fuel cells. Corros. Sci. 2018, 142, 249–257. [Google Scholar] [CrossRef]
- Wang, J.; Min, L.; Fang, F.; Zhang, W.; Wang, Y. Electrodeposition of graphene nano-thick coating for highly enhanced performance of titanium bipolar plates in fuel cells. Int. J. Hydrog. Energy 2019, 44, 16909–16917. [Google Scholar] [CrossRef]
- Lyons, K.S.; Gould, B.D. Lightweight Titanium Metal Bipolar Plates for PEM Fuel Cells. Mater. Sci. Forum 2017, 879, 613–618. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L.; Guo, L.; Tuan, W.H. Corrosion behavior of TiN-coated stainless steel as bipolar plate for proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2010, 35, 3721–3726. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Fleury, E.; Han, S.-H. Surface properties of Ti(N, C, O) thin coating layers deposited by PA-MOCVD for bipolar plates used PEMFC. Met. Mater. Int. 2011, 17, 457–462. [Google Scholar] [CrossRef]
- Lee, W.; Yun, E.; Lee, H.; Hong, S.W.; Kwon, S. Ultrathin effective TiN protective films prepared by plasma-enhanced atomic layer deposition for high performance metallic bipolar plates of polymer electrolyte membrane fuel cells. Appl. Surf. Sci. 2020, 519, 146215. [Google Scholar] [CrossRef]
- Longrie, D.; Deduytsche, D.; Haemers, J.; Smet, P.F.; Driesen, K.; Detavernier, C. Thermal and plasma-enhanced atomic layer deposition of TiN using TDMAT and NH3 on particles agitated in a rotary reactor. Acs Appl. Mater. Interfaces 2014, 6, 7316–7324. [Google Scholar] [CrossRef]
- Lee, W.; Yun, E.; Lee, H.; Hong, S.W.; Kwon, S. Dataset for TiN thin films prepared by plasma-enhanced atomic layer deposition using tetrakis (dimethylamino) titanium (TDMAT) and titanium tetrachloride (TiCl4) precursor. Data Brief 2020, 31, 105777, in press. [Google Scholar] [CrossRef]
- Padula, N.A. Development of Multilayer Titanium Nitride-Based Coatings as Corrosion Resistant Films for Stainless Steel Bipolar Plates in PEM Fuel Cells; State University of New York at Albany: Albany, NY, USA, 2017. [Google Scholar]
- Zhang, H.; Lin, G.; Hou, M.; Hu, L.; Han, Z.; Fu, Y.; Shao, Z.; Yi, B. CrN/Cr multilayer coating on 316L stainless steel as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2012, 198, 176–181. [Google Scholar] [CrossRef]
- Li, R.; Cai, Y.; Wippermann, K.; Lehnert, W. CrN/Cr coating-modified 316L stainless steel bipolar plates for high temperature polymer electrolyte fuel cells. J. Power Sources 2019, 434, 226718. [Google Scholar] [CrossRef]
- Kim, L.H.; Kim, K.; Park, S.; Jeong, Y.J.; Kim, H.; Chung, D.S.; Kim, S.H.; Park, C.E. Al2O3/TiO2 nanolaminate thin film encapsulation for organic thin film transistors via plasma-enhanced atomic layer deposition. Acs Appl. Mater. Interfaces 2014, 6, 6731–6738. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; Yan, Y.; Cooper, J.R.; Zhang, Y.; Gibbs, Z.M.; Cavanagh, A.S.; Yang, R.G.; Lee, Y.C.; George, S.M. Al2O3 and TiO2 atomic layer deposition on copper for water corrosion resistance. Acs Appl. Mater. Interfaces 2011, 3, 4593–4601. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Liu, H.; Wang, S.; Fang, H.; Gao, H.; Gao, K.; Sun, J.; Yan, J. Polylaminate TaN/Ta coating modified ferritic stainless steel bipolar plate for high temperature proton exchange membrane fuel cell. J. Power Sources 2018, 399, 343–349. [Google Scholar] [CrossRef]
- Mendizabal, L.; Oedegaard, A.; Kongstein, O.E.; Lædre, S.; Walmsley, J.; Barriga, J.; Gonzalez, J.J. TaNx coatings deposited by HPPMS on SS316L bipolar plates for polymer electrolyte membrane fuel cells: Correlation between corrosion current, contact resistance and barrier oxide film formation. Int. J. Hydrog. Energy 2017, 42, 3259–3270. [Google Scholar] [CrossRef]
- Rajaei, V.; Rashtchi, H.; Raeissi, K.; Shamanian, M. The study of Ni-based nano-crystalline and amorphous alloy coatings on AISI 304 stainless steel for PEM fuel cell bipolar plate application. Int. J. Hydrog. Energy 2017, 42, 14264–14278. [Google Scholar] [CrossRef]
- Taherian, R. A review of composite and metallic bipolar plates in proton exchange membrane fuel cell: Materials, fabrication, and material selection. J. Power Sources 2014, 265, 370–390. [Google Scholar] [CrossRef]
- Yeetsorn, R.; Fowler, M.W.; Tzoganakis, C. A review of thermoplastic composites for bipolar plate materials in PEM fuel cells. In Nanocomposites with Unique Properties and Applications in Medicine and Industry; IntechOpen: London, UK, 2011. [Google Scholar]
- Mohd Radzuan, N.A.; Sulong, A.B.; Hui, D.; Verma, A. Electrical Conductivity Performance of Predicted Modified Fibre Contact Model for Multi-Filler Polymer Composite. Polymers 2019, 11, 1425. [Google Scholar] [CrossRef]
- Naji, A.; Krause, B.; Pötschke, P.; Ameli, A. Hybrid conductive filler/polycarbonate composites with enhanced electrical and thermal conductivities for bipolar plate applications. Polym. Compos. 2019, 40, 3189–3198. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, M.; Lee, D.G. Conductive particles embedded carbon composite bipolar plates for proton exchange membrane fuel cells. Compos. Struct. 2014, 108, 757–766. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, M.; Yu, Y.H. Development of carbon/PEEK composite bipolar plates with nano-conductive particles for High-Temperature PEM fuel cells (HT-PEMFCs). Compos. Struct. 2014, 118, 519–527. [Google Scholar] [CrossRef]
- Syahrial, A.Z.; Indriyana, B.; Albar, M.E.; Rohman, S. Role of Multiwall Carbon Nano Tubes (MWCNT) on Electrical Conductivity of Polymer Composite as Alternative Materials for Bipolar Plate Fuel Cell. Mater. Sci. Forum 2013, 737, 183–190. [Google Scholar]
- Banerjee, S.; Pattnayek, S.; Kumar, R.; Kar, K.K. Impact of Graphite on Thermomechanical, Mechanical, Thermal, Electrical Properties, and Thermal Conductivity of HDPE/Copper Composites. Fuel Cells 2020, 20, 116–130. [Google Scholar] [CrossRef]
- Park, H.; Woo, J.S.; Park, S. Poly (phenylene sulfide)-graphite composites for bipolar plates with preferred morphological orientation. Korean J. Chem. Eng. 2019, 36, 2133–2142. [Google Scholar] [CrossRef]
- Radzuan, N.A.M.; Sulong, A.B.; Somalu, M.R.; Abdullah, A.T.; Husaini, T.; Rosli, R.E.; Majlana, E.H.; Rosli, M.I. Fibre orientation effect on polypropylene/milled carbon fiber composites in the presence of carbon nanotubes or graphene as a secondary filler: Application on PEM fuel cell bipolar plate. Int. J. Hydrog. Energy 2019, 44, 30618–30626. [Google Scholar] [CrossRef]
- Park, H.; Woo, J.S.; Kim, S.H.; Park, K.S.; Park, S.H.; Park, S.Y. High-Performance Fluorinated Ethylene-Propylene/Graphite Composites Interconnected with Single-Walled Carbon Nanotubes. Macromol. Res. 2019, 27, 1161–1166. [Google Scholar] [CrossRef]
- Qiu, D.; Yi, P.; Peng, L.; Lai, X. Assembly design of proton exchange membrane fuel cell stack with stamped metallic bipolar plates. Int. J. Hydrog. Energ. 2015, 40, 11559–11568. [Google Scholar] [CrossRef]
- Meidanshahi, V.; Karimi, G. Dynamic modeling, optimization and control of power density in a PEM fuel cell. Appl. Energ. 2012, 93, 98–105. [Google Scholar] [CrossRef]
- Mason, T.J.; Millichamp, J.; Neville, T.P.; El-kharouf, A.; Pollet, B.G.; Brett, D.J.L. Effect of clamping pressure on ohmic resistance and compression of gas diffusion layers for polymer electrolyte fuel cells. J. Power Sources 2012, 219, 52–59. [Google Scholar] [CrossRef]
- Zamel, N.; Li, X.; Shen, J. Numerical estimation of the effective electrical conductivity in carbon paper diffusion media. Appl. Energ. 2012, 93, 39–44. [Google Scholar] [CrossRef]
- Qiu, D.; Janßen, H.; Peng, L.; Irmscher, P.; Lai, X.; Lehnert, W. Electrical resistance and microstructure of typical gas diffusion layers for proton exchange membrane fuel cell under compression. Appl. Energy 2018, 231, 127–137. [Google Scholar] [CrossRef]
- Lin, J.H.; Chen, W.H.; Su, Y.J.; Ko, T.H. Effect of gas diffusion layer compression on the performance in a proton exchange membrane fuel cell. Fuel 2008, 87, 2420–2424. [Google Scholar] [CrossRef]
- Ito, H.; Heo, Y.; Ishida, M.; Nakano, A.; Someya, S.; Munakata, T. Application of a self-supporting microporous layer to gas diffusion layers of proton exchange membrane fuel cells. J. Power Sources 2017, 342, 393–404. [Google Scholar] [CrossRef]
- Hasanpour, S.; Ahadi, M.; Bahrami, M.; Djilali, N.; Akbari, M. Woven gas diffusion layers for polymer electrolyte membrane fuel cells: Liquid water transport and conductivity trade-offs. J. Power Sources 2018, 403, 192–198. [Google Scholar] [CrossRef]
- Kong, I.M.; Choi, J.W.; Kim, S.I.; Lee, E.S.; Kim, M.S. Experimental study on the self-humidification effect in proton exchange membrane fuel cells containing double gas diffusion backing layer. Appl. Energ. 2015, 145, 345–353. [Google Scholar] [CrossRef]
- Zenyuk, I.V.; Parkinson, D.Y.; Connolly, L.G.; Weber, A.Z. Gas-diffusion-layer structural properties under compression via X-ray tomography. J. Power Sources 2016, 328, 364–376. [Google Scholar] [CrossRef]
- Yang., S.Y.; Guo., Q.S.; Li., L.J. Research progress of the sulfonated poly (ether ether ketone) s membranes for proton exchange membrane fuel cell. Chem. Ind. Eng. Prog. 2016, 35, 2850–2860. [Google Scholar]
- Yan, X.; Zheng, W.; Ruan, X.; Pan, Y.; Wu, X.; He, G. The control and optimization of macro/micro-structure of ion conductive membranes for energy conversion and storage. Chin. J. Chem. Eng. 2016, 24, 558–571. [Google Scholar] [CrossRef]
- Danilczuk, M.; Perkowski, A.J.; Schlick, S. Ranking the stability of perfluorinated membranes used in fuel cells to attack by hydroxyl radicals and the effect of Ce (III). A competitive kinetics approach based on spintrapping ESR. Macromolecules 2010, 43, 73352–73358. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.T.; Lee, J.H. Polymer mem-branes for high temperature proton exchange mem-brane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 6813–6843. [Google Scholar] [CrossRef]
- Song, T.; Chen, Z.; He, H.; Liu, Y.; Liu, Y.; Ramakrishna, S. Orthogonal design study on factors affecting the diameter of perfiuorinated sulfonic acidnanofibers during electrospinning. J. Appl. Polym. Sci. 2015, 132, 41755. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, G.M.; Li, J.Q.; Gao, C.M. Synthesis and characterization of partially fluorinated poly(fluorenyl ether ketone)s with different degrees of sulfonation as proton exchange membranes. Polymer Bulletin 2011, 66, 925–937. [Google Scholar] [CrossRef]
- Chun, J.H.; Kim, S.G.; Lee, J.Y.; Hyeon, D.H.; Chun, B.H.; Kim, S.H.; Park, K.T. Crosslinked sulfonated poly(arylene ether sulfone)/silica hybrid membranes for high temperature proton exchange membrane fuel cells. Renew. Energy 2013, 51, 22–28. [Google Scholar] [CrossRef]
- Hazarika, M.; Jana, T. Novel proton exchange membrane for fuel cell developed from blends of polybenzimidazole with fluorinated polymer. Eur. Polym. J. 2013, 49, 1564–1576. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Ozden, A.; Ercelik, M.; Devrim, Y.; Colpan, C.O.; Hamdullahpur, F. Evaluation of sulfonated polysulfone/zirconium hydrogen phosphate composite membranes for direct methanol fuel cells. Electrochim. Acta 2017, 256, 196–210. [Google Scholar] [CrossRef]
- Xie, H.; Tao, D.; Xiang, X.; Ou, Y.; Bai, X.; Wang, L. Synthesis and properties of highly branched star-shaped sulfonated block poly (arylene ether)s as proton exchange membranes. J. Membr. Sci. 2015, 473, 226–236. [Google Scholar] [CrossRef]
- Sun, H.; Tang, B.; Wu, P. Two-dimensional zeolitic imidazolate framework/carbon nanotube hybrid networks modified proton exchange membranes for improving transport properties. Acs Appl. Mater. Interfaces 2017, 9, 35075–35085. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Z.; Hu, M.; Hou, J.; Wu, L.; Xu, T. Perylene-based sulfonated aliphatic polyimides for fuel cell applications: Performance enhancement by stacking of polymer chains. J. Membr. Sci. 2018, 547, 43–50. [Google Scholar] [CrossRef]
- Matian, M.; Marquis, A.; Brandon, N. Model based design and test of cooling plates for an air-cooled polymer electrolyte fuel cell stack. Int. J. Hydrog. Energy 2011, 36, 6051–6066. [Google Scholar] [CrossRef]
- Guo, H.; Wang, M.H.; Liu, J.X.; Nie, Z.H.; Ye, F.; Ma, C.F. Temperature distribution on anodic surface of membrane electrode assembly in proton exchange membrane fuel cell with interdigitated flow bed. J. Power Sources 2015, 273, 775–783. [Google Scholar] [CrossRef]
- Guo, H.; Wang, M.H.; Ye, F.; Ma, C.F. Experimental study of temperature distribution on anodic surface of MEA inside a PEMFC with parallel channels flow bed. Int. J. Hydrog. Energy 2012, 37, 13155–13160. [Google Scholar] [CrossRef]
- Li, S.A.; Yuan, J.L.; Xie, G.N.; Sunden, B. Numerical investigation of transport phenomena in high temperature proton exchange membrane fuel cells with different flow field designs. Numer. Heat Transf. A 2017, 72, 807–820. [Google Scholar] [CrossRef]
- Han, C.L.; Chen, Z.Q. Numerical simulation for the effect of vaporization intensity in membrane on the performance of PEM fuel cell. Numer. Heat Transf. A 2018, 73, 177–194. [Google Scholar] [CrossRef]
- Singdeo, D.; Dey, T.; Gaikwad, S.; Andreasen, S.J.; Ghosh, P.C. A new modified serpentine flow field for application in high temperature polymer electrolyte fuel cell. Appl. Energy 2017, 195, 13–22. [Google Scholar] [CrossRef]
- Li, S.A.; Sunden, B. Three-dimensional modeling and investigation of high temperature proton exchange membrane fuel cells with metal foams as flow distributor. Int. J. Hydrog. Energy 2017, 42, 27323–27333. [Google Scholar] [CrossRef]
- Afshari, E.; Mosharaf-Dehkordi, M.; Rajabian, H. An investigation of the PEM fuel cells performance with partially restricted cathode flow channels and metal foam as a flow distributor. Energy 2017, 118, 705–715. [Google Scholar] [CrossRef]
- Tseng, C.J.; Heush, Y.J.; Chiang, C.J.; Lee, Y.H.; Lee, K.R. Application of metal foams to high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 16196–16204. [Google Scholar] [CrossRef]
- Baik, K.D.; Lee, E.H.; Yoon, H.; Kim, J.Y.; Yang, S.H. Effect of multi-hole flow field structure on the performance of H_2/O_2 polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2019, 44, 25894–25904. [Google Scholar] [CrossRef]
- Li, S.; Sundén, B. Numerical study on thermal performance of non-uniform flow channel designs for cooling plates of PEM fuel cells. Numeric. Heat Transf. Part A Appl. 2018, 74, 917–930. [Google Scholar] [CrossRef]
- Afshari, E.; Ziaei-Rad, M.; Dehkordi, M.M. Numerical investigation on a novel zigzag-shaped flow channel design for cooling plates of PEM fuel cells. J. Energy Inst. 2017, 90, 752–763. [Google Scholar] [CrossRef]
- Yu, S.H.; Sohn, S.; Nam, J.H.; Kim, C. Numerical study to examine the performance of multi-pass serpentine flow-fields for cooling plates in polymer electrolyte membrane fuel cells. J. Power Sources 2009, 194, 697–703. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Sasmito, A.P.; Mujumdar, A.S. Numerical investigation of laminar heat transfer performance of various cooling channel designs. Appl. Therm. Eng. 2011, 31, 1293–1304. [Google Scholar] [CrossRef]
- Ravishankar, S.; Prakash, K.A. Numerical studies on thermal performance of novel cooling plate designs in polymer electrolyte membrane fuel cell stacks. Appl. Therm. Eng. 2014, 66, 239–251. [Google Scholar] [CrossRef]
- Afshari, E.; Ziaei-Rad, M.; Shariati, Z. A study on using metal foam as coolant fluid distributor in the polymer electrolyte membrane fuel cell. Int. J. Hydrog. Energy 2016, 41, 1902–1912. [Google Scholar] [CrossRef]
- Noorkami, M.; Robinson, J.B.; Meyer, Q.; Obeisun, O.A.; Fraga, E.S.; Reisch, T.; Shearing, P.R.; Brett, D.J. Effect of temperature uncertainty on polymer electrolyte fuel cell performance. Int. J. Hydrog. Energy 2014, 39, 1439–1448. [Google Scholar] [CrossRef]
- De las Heras, A.; Vivas, F.J.; Segura, F.; Redondo, M.J.; Andújar, J.M. Air-cooled fuel cells: Keys to design and build the oxidant/cooling system. Renew. Energy 2018, 125, 1–20. [Google Scholar] [CrossRef]
- Oro, M.V.; Bazzo, E. Flat heat pipes for potential application in fuel cell cooling. Appl. Eng. 2015, 90, 848–857. [Google Scholar] [CrossRef]
- Tolj, I.; Penga, Ž.; Vukičević, D.; Barbir, F. Thermal management of edge-cooled 1 kW portable proton exchange membrane fuel cell stack. Appl. Energy 2020, 257, 114038. [Google Scholar] [CrossRef]
- Sasmito, A.P.; Shamim, T.; Birgersson, E.; Mujumdar, A.S. Computational Study of Edge Cooling for Open-Cathode Polymer Electrolyte Fuel Cell Stacks. J. Fuel Cell Sci. Technol. 2012, 9, 1–8. [Google Scholar] [CrossRef]
- Han, C.; Chen, Z. Study on electrochemical and mass transfer coupling characteristics of proton exchange membrane (PEM) fuel cell based on a fin-like electrode surface. Int. J. Hydrog. Energy 2018, 43, 8026–8039. [Google Scholar] [CrossRef]
- Sasmito, A.P.; Shamim, T.; Birgersson, E. Numerical Investigation of Water and Temperature Distributions for Open-Cathode Polymer Electrolyte Fuel Cell Stack with Edge Cooling. J. Fuel Cell Sci. Technol. 2013, 10, 061003. [Google Scholar] [CrossRef]
- Mohamed, W.A.N.W.; Atan, R. Experimental thermal analysis on air cooling for closed-cathode Polymer Electrolyte Membrane fuel cells. Int. J. Hydrog. Energy 2015, 40, 10605–10626. [Google Scholar] [CrossRef]
- Meyer, Q.; Himeur, A.; Ashton, S.; Curnick, O.; Clague, R.; Reisch, T.; Adcock, P.; Shearing, P.R.; Brett, D.J.L. System-level electro-thermal optimisation of air-cooled open-cathode polymer electrolyte fuel cells: Air blower parasitic load and schemes for dynamic operation. Int. J. Hydrog. Energy 2015, 40, 16760–16766. [Google Scholar] [CrossRef]
- Strahl, S.; Costa-Castello, R. Model-based analysis for the thermal management of open-cathode proton exchange membrane fuel cell systems concerning efficiency and stability. J. Process Control. 2016, 47, 201–212. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high- temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrog. Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Sasmito, A.; Lum, K.; Birgersson, E.; Mujumdar, A. Computational study of forced air-convection in open-cathode polymer electrolyte fuel cell stacks. J. Power Sources 2010, 195, 5550–5563. [Google Scholar] [CrossRef]
- Bvumbe, T.J.; Bujlo, P.; Tolj, I.; Mouton, K.; Swart, G.; Pasupathi, S.; Pollet, B.G. Review on management, mechanisms and modelling of thermal processes in PEMFC. Hydrog. Fuel Cells 2016, 1, 1–20. [Google Scholar] [CrossRef]
- Yuan, W.W.; Ou, K.; Kim, Y.B. Thermal management for an air coolant system of a proton exchange membrane fuel cell using heat distribution optimization. Appl. Eng. 2020, 167, 114715. [Google Scholar] [CrossRef]
- Cheng, S.; Fang, C.; Xu, L.; Li, J.; Ouyang, M. Model-based temperature regulation of a pem fuel cell system on a city bus. Int. J. Hydrog. Energy 2015, 40, 13566–13575. [Google Scholar] [CrossRef]
- Clement, J.; Wang, X. Experimental investigation of pulsating heat pipe performance with regard to fuel cell cooling application. Appl. Eng. 2013, 50, 268–274. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Electrical and Thermal Conductivities of 50/50 Water-ethylene Glycol Based TiO2 Nanofluids to be Used as Coolants in PEM. Fuel Cells Energy Procedia 2017, 110, 101–108. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ganji, D.D. Numerical modeling of magnetohydrodynamic CuO—Water transportation inside a porous cavity considering shape factor effect. Colloids Surf. A Phys. Eng. Asp. 2017, 529, 705–714. [Google Scholar] [CrossRef]
- Snoussi, L.; Ouerfelli, N.; Chesneau, X.; Chamkha, A.J.; Belgacem, F.B.M.; Guizani, A. Natural convection heat transfer in a nanofluid filled U-shaped enclosures: Numerical investigations. Heat Transf. Eng. 2017, 39, 1–11. [Google Scholar] [CrossRef]
- Hajmohammadi, M.R.; Tork, M.H.H.M.A. Effects of the magnetic field on the cylindrical Couette flow and heat transfer of a nanofluid. Phys. A Stat. Mech. Appl. 2019, 523, 234–245. [Google Scholar] [CrossRef]
- Haridas, D.; Rajput, N.S.; Srivastava, A. Interferometric study of heat transfer characteristics of Al2O3 and SiO2-based dilute nanofluids under simultaneously developing flow regime in compact channels. Int. J. Heat Mass Transf. 2015, 88, 713–727. [Google Scholar] [CrossRef]
- Kumar, N.; Sonawane, S.S. Experimental study of thermal conductivity and convective heat transfer enhancement using CuO and TiO2 nanoparticles. Int. J. Heat Mass Transf. 2016, 76, 98–107. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Nanofluids to improve the performance of PEM fuel cell cooling systems: A theoretical approach. Appl. Energy 2016, 178, 660–671. [Google Scholar] [CrossRef]
- Zakaria, I.A.; Mohamed, W.A.N.W.; Zailan, M.B.; Azmi, W.H. Experimental analysis of SiO2-Distilled water nanofluids in a Polymer Electrolyte Membrane fuel cell parallel channel cooling plate. Int. J. Hydrog. Energy 2019, 44, 25850–25862. [Google Scholar] [CrossRef]
- Islam, R.; Shabani, B. Prediction of electrical conductivity of TiO 2 water and ethylene glycol-based nanofluids for cooling application in low temperature PEM fuel cells. Energy Procedia 2019, 160, 550–557. [Google Scholar] [CrossRef]
- Zakaria, I.; Azmi, W.H.; Mohamed, W.A.N.W.; Mamat, R.; Najafi, G. Experimental investigation of thermal conductivity and electrical conductivity of Al2O3 nanofluid in water-ethylene glycol mixture for proton exchange membrane fuel cell application. Int. Commun. Heat Mass Transf. 2015, 61, 61–68. [Google Scholar] [CrossRef]
- Islam, R.; Shabani, B.; Andrews, J.; Rosengarten, G. Experimental investigation of using ZnO nanofluids as coolants in a PEM fuel cell. Int. J. Hydrog. Energy 2017, 42, 19272–19286. [Google Scholar] [CrossRef]
- Fly, A.; Thring, R.H. A comparison of evaporative and liquid cooling methods for fuel cell vehicles. Int. J. Hydrog. Energy 2016, 41, 14217–14229. [Google Scholar] [CrossRef]
- Song, T.-W.; Choi, K.-H.; Kim, J.-R.; Jung, S.Y. Pumpless thermal management of water-cooled high-temperature proton exchange membrane fuel cells. J. Power Sources 2011, 196, 4671–4679. [Google Scholar] [CrossRef]
- Ramezanizadeh, M.; Nazari, M.A.; Ahmadi, M.H.; Chau, K. Experimental and numerical analysis of a nanofluidic thermosyphon heat exchanger. Eng. Appl. Comput. Fluid Mech. 2019, 13, 40–47. [Google Scholar] [CrossRef]
- Alizadeh, H.; Ghasempour, R.; Shafii, M.B.; Ahmadi, M.H.; Yan, W.-M.; Nazari, M.A. Numerical simulation of PV cooling by using single turn pulsating heat pipe. Int. J. Heat Mass Transf. 2018, 127, 203–208. [Google Scholar] [CrossRef]
- Shirzadi, N.; Roshandel, R.; Shafii, M.B. Integration of miniature heat pipes into a proton exchange membrane fuel cell for cooling applications. Heat Transf. Eng. 2017, 38, 1595–1605. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, M.S. An experimental study on the cathode humidification and evaporative cooling of polymer electrolyte membrane fuel cells using direct water injection method at high current densities. Appl. Eng. 2016, 99, 635–644. [Google Scholar] [CrossRef]
- Schultze, M.; Horn, J. A control oriented simulation model of an evaporation cooled polymer electrolyte membrane fuel cell system. In Proceedings of the 18th International Federation of Automatic Control World Congress, Milan, Italy, 28 August–2 September 2011; pp. 14790–14795. [Google Scholar]
- Fly, A.; Thring, R.H. Temperature regulation in an evaporatively cooled proton exchange membrane fuel cell stack. Int. J. Hydrog. Energy 2015, 40, 11976–11982. [Google Scholar] [CrossRef]
- Luo, Y.; Jiao, K. Cold start of proton exchange membrane fuel cell. Prog. Energy Combust. 2018, 64, 29–61. [Google Scholar] [CrossRef]
- Tajiri, K.; Wang, C.Y. Ishothermal Cold Start of Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2006, 154, B147–152. [Google Scholar] [CrossRef]
- Yamada, K.; Suzuki, K.; Tabe, Y.; Chikahisa, T. Analysis of ice distribution in cathode catalyst layer and shutdown mechanism at PEFC cold start. Ecs Trans. 2013, 58, 463–471. [Google Scholar] [CrossRef]
- Amamou, A.; Kelouwani, S.; Boulon, L.; Agbossou, K. A comprehensive review of solutions and strategies for cold start of automotive proton exchange membrane fuel cells. Ieee Access 2016, 4, 4989–5002. [Google Scholar] [CrossRef]
- Tabe, Y.; Saito, M.; Fukui, K.; Chikahisa, T. Cold start characteristics and freezing mechanism dependence on start-up temperature in a polymer electrolyte membrane fuel cell. J. Power Sources 2012, 208, 366–373. [Google Scholar] [CrossRef]
- Pan, W.; Li, P. Thermal stability analysis of cold start processes in PEM fuel cells. Appl. Energy 2020, 261, 114430. [Google Scholar] [CrossRef]
- Dursch, T.J.; Trigub, G.J.; Lujan, R.; Liu, J.F.; Mukundan, R.; Radke, C.J.; Weber, A.Z. Ice-crystallization kinetics in the catalyst layer of a proton-exchange-membrane fuel cell. J. Electrochem. Soc. 2014, 16, F199–F207. [Google Scholar] [CrossRef]
- Huo, S.; Jiao, K.; Park, J.W. On the water transport behavior and phase transition mechanisms in cold start operation of PEM fuel cell. Appl. Energy 2019, 233–234, 776–788. [Google Scholar] [CrossRef]
- Ko, J.; Kim, W.-G.; Lim, Y.-D.; Ju, H. Improving the cold-start capability of polymer electrolyte fuel cells (PEFCs) by using a dual-function micro-porous layer (MPL): Numerical simulations. Int. J. Hydrog. Energy 2013, 38, 652–659. [Google Scholar] [CrossRef]
- Ko, J.; Ju, H. Effects of cathode catalyst layer design parameters on cold start behavior of polymer electrolyte fuel cells (PEFCs). Int. J. Hydrog. Energy 2013, 38, 682–691. [Google Scholar] [CrossRef]
- Xie, X.; Wang, R.; Jiao, K.; Zhang, G.; Zhou, J.; Du, Q. Investigation of the effect of micro-porous layer on PEM fuel cell cold start operation. Renew. Energy 2018, 117, 125–134. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Q.; Huo, S.; Jiao, K. Effect of membrane electrode assembly design on the cold start process of proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2017, 42, 25372–25387. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, G.; Zhou, J.; Jiao, K. Experimental and theoretical analysis of ionomer/carbon ratio effect on PEM fuel cell cold start operation. Int. J. Hydrog. Energy 2017, 42, 12521–12530. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.I.; Lee, N.W.; Kim, M.S. Study on a purge method using pressure reduction for effective water removal in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2015, 40, 9473–9484. [Google Scholar] [CrossRef]
- Wei, L.; Dafalla, A.M.; Jiang, F. Effects of reactants/coolant non-uniform inflow on the cold start performance of PEMFC stack. Int. J. Hydrog. Energy 2020, 45, 13469–13482. [Google Scholar] [CrossRef]
- Gwak, G.; Ko, J.; Ju, H. Numerical investigation of cold-start behavior of polymer-electrolyte fuel-cells from subzero to normal operating temperatures–effects of cell boundary and operating conditions. Int. J. Hydrog. Energy 2014, 39, 21927–21937. [Google Scholar] [CrossRef]
- Dafalla, A.M.; Wei, L.; Liao, Z.; Jiang, F. Numerical Investigation of Clamping System Effects on the Cold Start Polymer Electrolyte Fuel Cell Performance. In Proceedings of the International Heat Transfer Conference 16, Beijing, China, 10–15 August 2018. [Google Scholar]
- Dafalla, A.M.; Wei, L.; Liao, Z.H.; Jiang, F.M. Effects of Clamping Pressure on Cold Start Behavior of Polymer Electrolyte Fuel Cells. Fuel Cells 2019, 19, 221–230. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, M.; Wu, S.; Tongsh, C.; Sun, X.; Wang, B.; Park, J.W.; Jiao, K. Investigation of mechanical vibration effect on proton exchange membrane fuel cell cold start. Int. J. Hydrog. Energy 2020, 45, 14528–14538. [Google Scholar] [CrossRef]
- Yamada, K. Fuel cell device and related control method. U.S. Patent 7537850, 26 May 2009. [Google Scholar]
- Harris, D.I. Rapid light-off catalytic combustor for fuel cell vehicle. U.S. Patent 7960064, 14 June 2011. [Google Scholar]
- Knorr, F.; Sanchez, D.G.; Schirmer, J.; Gazdzicki, P.; Friedrich, K.A. Methanol as antifreeze agent for cold start of automotive polymer electrolyte membrane fuel cells. Appl. Energy 2019, 238, 1–10. [Google Scholar] [CrossRef]
- Gwak, G.; Ju, H. A rapid start-up strategy for polymer electrolyte fuel cells at subzero temperatures based on control of the operating current density. Int. J. Hydrog. Energy 2015, 40, 11989–11997. [Google Scholar] [CrossRef]
- Amamou, A.; Kandidayeni, M.; Boulon, L.; Kelouwani, S. Real time adaptive efficient cold start strategy for proton exchange membrane fuel cells. Appl. Energy 2018, 216, 21–30. [Google Scholar] [CrossRef]
- Reddy, E.H.; Jayanti, S. Thermal management strategies for a 1 k We stack of a high temperature proton exchange membrane fuel cell. Appl. Eng. 2012, 48, 465–475. [Google Scholar]
- Jiao, K.; Alaefour, I.E.; Karimi, G.; Li, X. Simultaneous measurement of current and temperature distributions in a proton exchange membrane fuel cell during cold start processes. Electrochim. Acta 2011, 56, 2967–2982. [Google Scholar] [CrossRef]
- Hasan, M.I. Numerical investigation of counter flow microchannel heat exchanger with MEPCM suspension. Appl. Therm. Eng. 2011, 31, 1068–1075. [Google Scholar] [CrossRef]
- Sasmito, A.P.; Shamim, T.; Mujumdar, A.S. Passive thermal management for PEM fuel cell stack under cold weather condition using phase change materials (PCM). Appl. Therm. Eng. 2013, 58, 615–625. [Google Scholar] [CrossRef]
- Li, L.; Wang, S. Cold-start method for proton-exchange membrane fuel cells based on locally heating the cathode. Appl. Energy 2019, 254, 113716. [Google Scholar] [CrossRef]
- Zhigang, Z.; Chong, Y.; Zhangrong, H.; Hui, W.; Sui, P.C.; Ned, D.; Mu, P. Experimental study on different preheating methods for the cold-start of PEMFC stacks. Energy 2018, 162, 1029–1040. [Google Scholar]
- Zhou, Y.; Luo, Y.; Yu, S.; Jiao, K. Modeling of cold start processes and performance optimization for proton exchange membrane fuel cell stacks. J. Power Sources 2014, 247, 738–748. [Google Scholar] [CrossRef]

| Cooling Method | Application Power Range | System Complexity | Difficulty in Maintaining Temperature Uniformity | Cooling Channel |
|---|---|---|---|---|
| Air-cooling | <2 kw | Lower | Higher | Higher |
| Liquid-cooling | >5 kw | Higher | Lower | Lower |
| Property | Graphite Bipolar Plate | Metal Bipolar Plates | Composite Bipolar Plate |
|---|---|---|---|
| mechanical strength | Low | High | High |
| Conductive intensity | High | High | Medium |
| thermal diffusivity | High | Medium | Low |
| Chemical stability (corrosion resistance) | High | Low | High |
| volume | High | Medium | Low |
| weight | High | High | Low |
| processing difficulty | High | Low | High |
| production cycle | High | Low | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, Z.; Sun, Y.; Yang, S.; Deng, C. A Review on Temperature Control of Proton Exchange Membrane Fuel Cells. Processes 2021, 9, 235. https://doi.org/10.3390/pr9020235
Li Q, Liu Z, Sun Y, Yang S, Deng C. A Review on Temperature Control of Proton Exchange Membrane Fuel Cells. Processes. 2021; 9(2):235. https://doi.org/10.3390/pr9020235
Chicago/Turabian StyleLi, Qinghe, Zhiqiang Liu, Yi Sun, Sheng Yang, and Chengwei Deng. 2021. "A Review on Temperature Control of Proton Exchange Membrane Fuel Cells" Processes 9, no. 2: 235. https://doi.org/10.3390/pr9020235
APA StyleLi, Q., Liu, Z., Sun, Y., Yang, S., & Deng, C. (2021). A Review on Temperature Control of Proton Exchange Membrane Fuel Cells. Processes, 9(2), 235. https://doi.org/10.3390/pr9020235







