Design, Modelling, and Experimental Validation of a Scalable Continuous-Flow Hydrothermal Liquefaction Pilot Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. General Design
2.1.1. Process System Design
2.1.2. Fluidic Design and Feed system
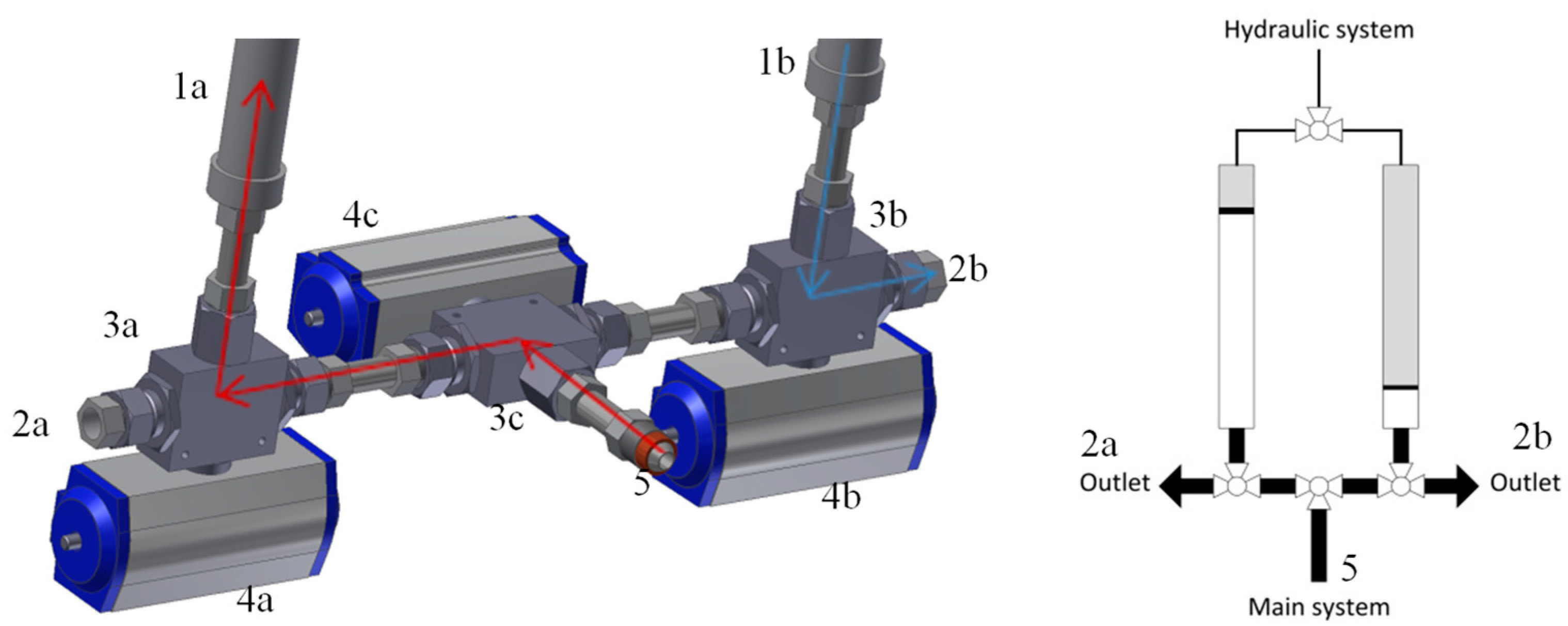
2.1.3. The Take-Off System
2.1.4. Oscillatory System
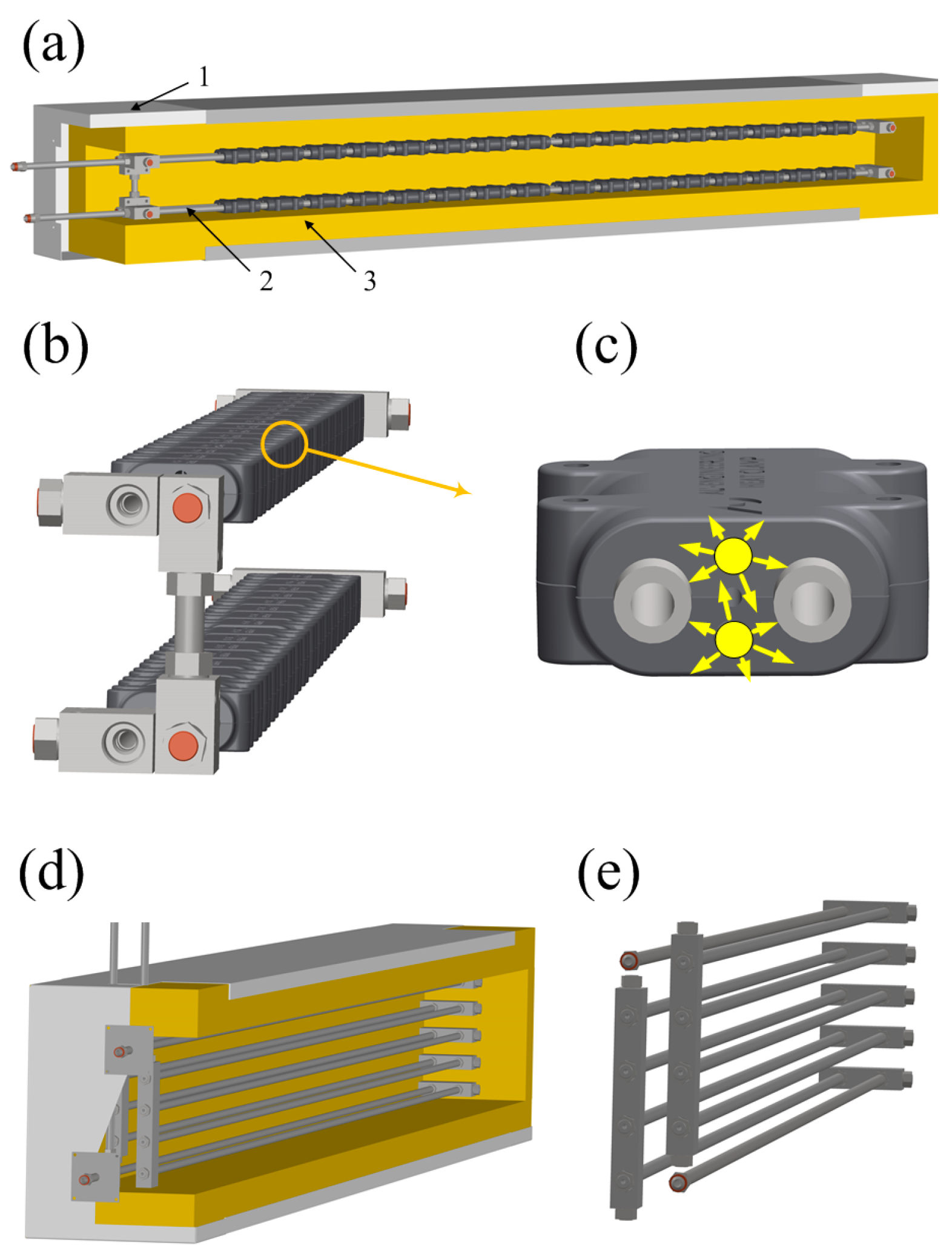
2.1.5. Thermal System
2.1.6. Clean in Place System (CIP)
2.1.7. Pressure Relief System
2.2. Mathematical Model
2.2.1. Modelling Approach and Assumptions
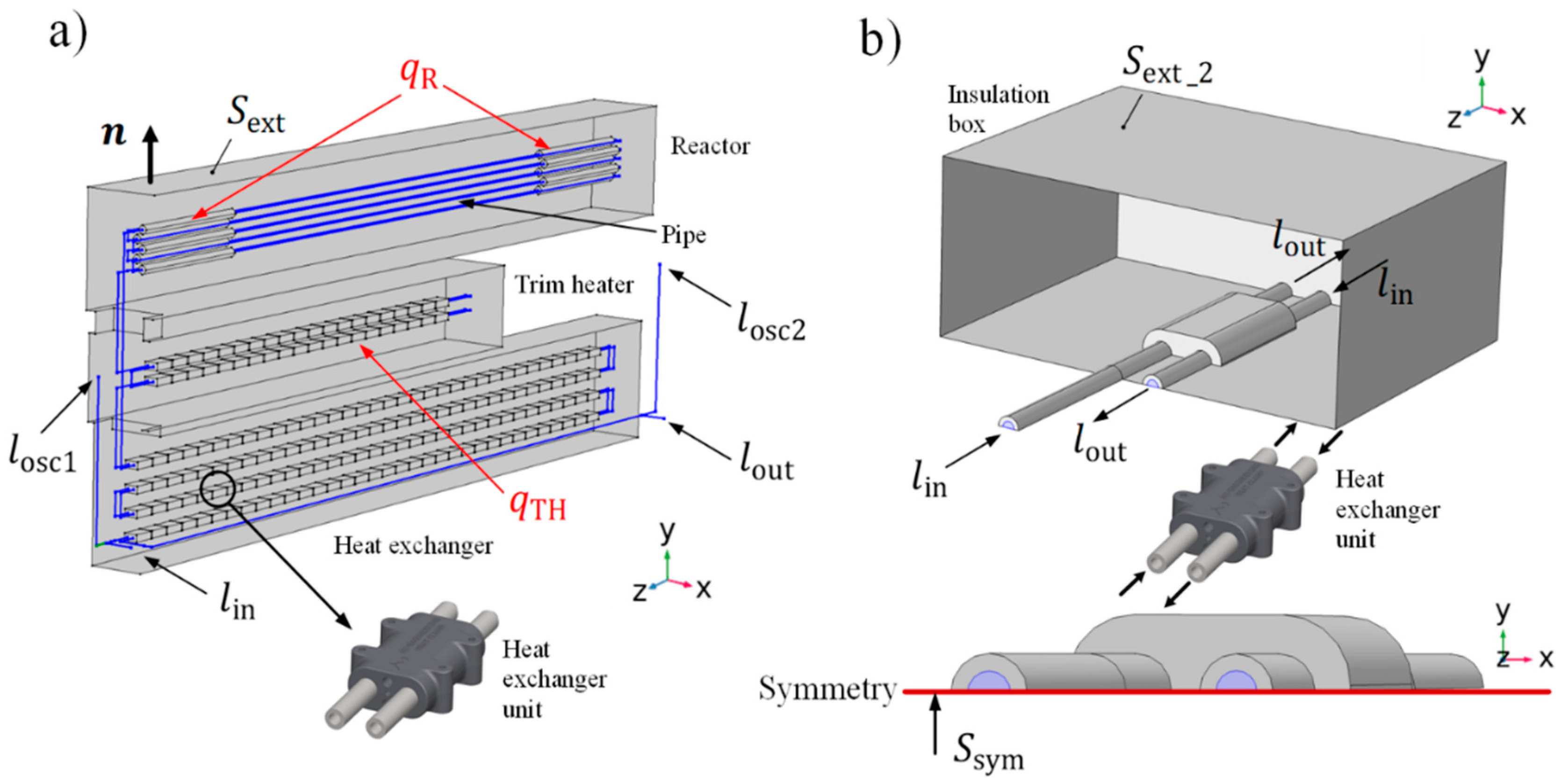
2.2.2. Macroscale Model: Fluid Flow and Heat Transfer (on the Pipe Domain)
2.2.3. Macroscale Model: Heat Transfer (on the Rest of the HTL System’s Domain)
2.2.4. Macroscale Model: The Coupling Interface between the Domains
2.2.5. Microscale Model
2.2.6. Numerical Solution
2.3. Experimental Characterization
2.3.1. Non-Newtonian Flow Characteristics of Biomass Slurries
2.3.2. HTL Plant Facility
2.3.3. Slurry Preparation
2.3.4. Flow Characterization Method
2.3.5. Output Response Characterization
2.3.6. Ion Concentration Analysis
3. Results
3.1. Non Newtonian Behavior of Biomass Slurries
3.2. Flow Characterization Verification
3.3. Temperature Distribution in the HTL System
3.4. The Effect of Forced Flow Oscillation on the Heat Transfer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.; García-Gutiérrez, P.; Dimitriou, I.; Elder, R.H.; Allen, R.W.; Azapagic, A. Utilising carbon dioxide for transport fuels: The economic and environmental sustainability of different Fischer-Tropsch process designs. Appl. Energy 2019, 253, 113560. [Google Scholar] [CrossRef]
- Madsen, R.B.; Anastasakis, K.; Biller, P.; Glasius, M. Rapid Determination of Water, Total Acid Number, and Phenolic Content in Bio-Crude from Hydrothermal Liquefaction of Biomass using FT-IR. Energy Fuels 2018, 32, 7660–7669. [Google Scholar] [CrossRef]
- Biller, P.; Johannsen, I.; dos Passos, J.S.; Ottosen, L.D.M. Primary sewage sludge filtration using biomass filter aids and subsequent hydrothermal co-liquefaction. Water Res. 2018, 130, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal Jr, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Barcock, R.A.; Balasubramanian, M.; Greenhill, J.V.; Siskin, M.; Olmstead, W.N. Aqueous High-Temperature Chemistry of Carbo- and Heterocycles. 20. Reactions of Some Benzenoid Hydrocarbons and Oxygen-Containing Derivatives in Supercritical Water at 460 °C. Energy Fuels 1994, 8, 487–497. [Google Scholar] [CrossRef]
- Gai, C.; Li, Y.; Peng, N.; Fan, A.; Liu, Z. Co-liquefaction of microalgae and lignocellulosic biomass in subcritical water. Bioresour. Technol. 2015, 185, 240–245. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Production of polyols via direct hydrolysis of kraft lignin: Effect of process parameters. Bioresour. Technol. 2013, 139, 13–20. [Google Scholar] [CrossRef]
- Li, H.Q.; Shao, Q.; Luo, H.; Xu, J. Polyurethane foams from alkaline lignin-based polyether polyol. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Yang, J.; Corscadden, K.; Niu, H.; Lin, J.; Astatkie, T. Advanced models for the prediction of product yield in hydrothermal liquefaction via a mixture design of biomass model components coupled with process variables. Appl. Energy 2019, 233, 906–915. [Google Scholar] [CrossRef]
- Li, Y.; Leow, S.; Fedders, A.C.; Sharma, B.K.; Guest, J.S.; Strathmann, T.J. Quantitative multiphase model for hydrothermal liquefaction of algal biomass. Green Chem. 2017, 19, 1163–1174. [Google Scholar] [CrossRef]
- Barnard, A.C.L.; Hunt, W.A.; Timlake, W.P.; Varley, E. A Theory of Fluid Flow in Compliant Tubes. Biophys. J. 1966, 6, 717–724. [Google Scholar] [CrossRef]
- Botto, L.; Preuss, K.; Robertson, L.X.; Xu, X.Y. Physical characterisation and yield stress of a concentrated Miscanthus suspension. Rheol. Acta 2014, 53, 805–815. [Google Scholar] [CrossRef]
- Duncan, J.C.; Shahravan, A.; Samaniuk, J.R.; Root, T.W.; Graham, M.D.; Klingenberg, D.J.; Scott, C.T.; Bourne, K.J.; Gleisner, R. Pressure-driven flow of lignocellulosic biomass: A compressible Bingham fluid. J. Rheol. 2018, 62, 801–815. [Google Scholar] [CrossRef]
- Mitsoulis, E. Flows of viscoplastic materials: Models and computations. Rheol. Rev. 2007, 43, 135–178. [Google Scholar]
- Lurie, M.V. Modeling of Oil Product and Gas Pipeline Transportation; Wiley: Hoboken, NJ, USA, 2009; pp. 1–214. [Google Scholar]
- Lienhard, J.H., IV; Lienhard, J.H., V. A Heat Transfer Textbook, 4th ed.; Phlogiston Press: Cambridge, MA, USA, 2018; p. 768. [Google Scholar]
- Sagaut, P.; Deck, S.; Terracol, M. Multiscale and Multiresolution Approaches in Turbulence; World Scientific: Singapore, 2006; pp. 1–341. [Google Scholar]
- Bazilevs, Y.; Calo, V.M.; Cottrell, J.A.; Hughes, T.J.R.; Reali, A.; Scovazzi, G. Variational multiscale residual-based turbulence modeling for large eddy simulation of incompressible flows. Comput. Methods Appl. Mech. Eng. 2007, 197, 173–201. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous Hydrothermal Liquefaction of Biomass in a Novel Pilot Plant with Heat Recovery and Hydraulic Oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- De Jong, S.; Hoefnagels, R.; Faaij, A.; Slade, R.; Mawhood, R.; Junginger, M. The feasibility of short-term production strategies for renewable jet fuels—A comprehensive techno-economic comparison. Biofuels Bioprod. Biorefin. 2015, 9, 778–800. [Google Scholar] [CrossRef]
- De Jong, S.; Antonissen, K.; Hoefnagels, R.; Lonza, L.; Wang, M.; Faaij, A.; Junginger, M. Life-cycle analysis of greenhouse gas emissions from renewable jet fuel production. Biotechnol. Biofuels 2017, 10, 64–71. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johannsen, I.; Kilsgaard, B.; Milkevych, V.; Moore, D. Design, Modelling, and Experimental Validation of a Scalable Continuous-Flow Hydrothermal Liquefaction Pilot Plant. Processes 2021, 9, 234. https://doi.org/10.3390/pr9020234
Johannsen I, Kilsgaard B, Milkevych V, Moore D. Design, Modelling, and Experimental Validation of a Scalable Continuous-Flow Hydrothermal Liquefaction Pilot Plant. Processes. 2021; 9(2):234. https://doi.org/10.3390/pr9020234
Chicago/Turabian StyleJohannsen, Ib, Björn Kilsgaard, Viktor Milkevych, and Dale Moore. 2021. "Design, Modelling, and Experimental Validation of a Scalable Continuous-Flow Hydrothermal Liquefaction Pilot Plant" Processes 9, no. 2: 234. https://doi.org/10.3390/pr9020234
APA StyleJohannsen, I., Kilsgaard, B., Milkevych, V., & Moore, D. (2021). Design, Modelling, and Experimental Validation of a Scalable Continuous-Flow Hydrothermal Liquefaction Pilot Plant. Processes, 9(2), 234. https://doi.org/10.3390/pr9020234





