Stabilization of Anaerobic Co-Digestion Process via Constant the Digestate Solids Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
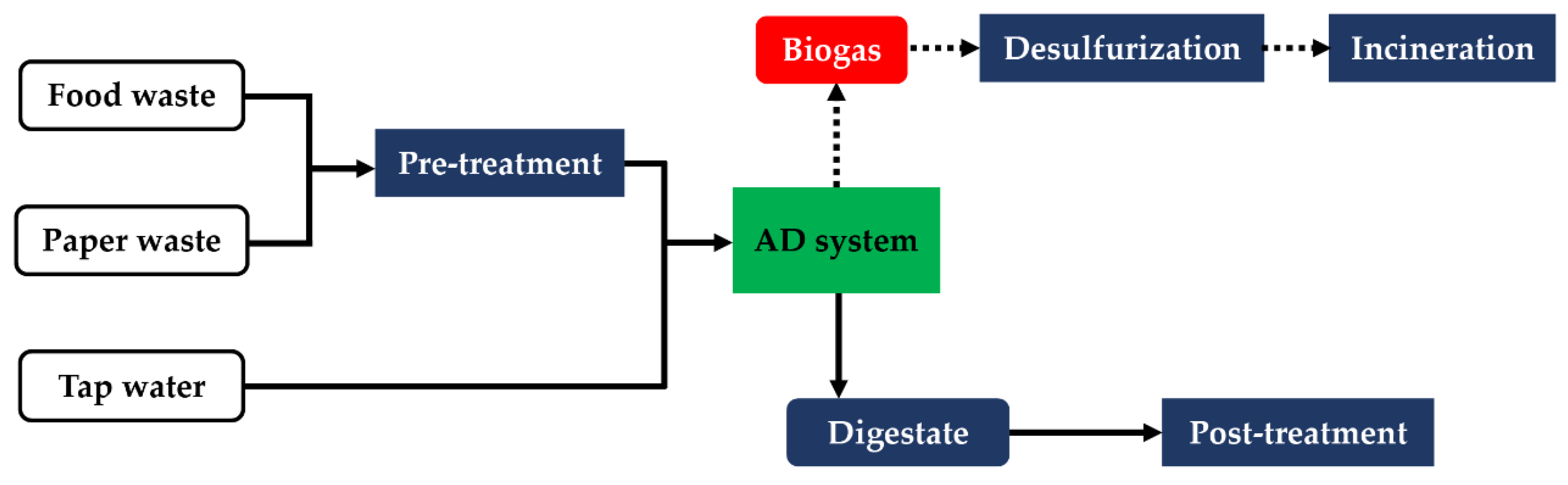
2.2. Experimental Set-up
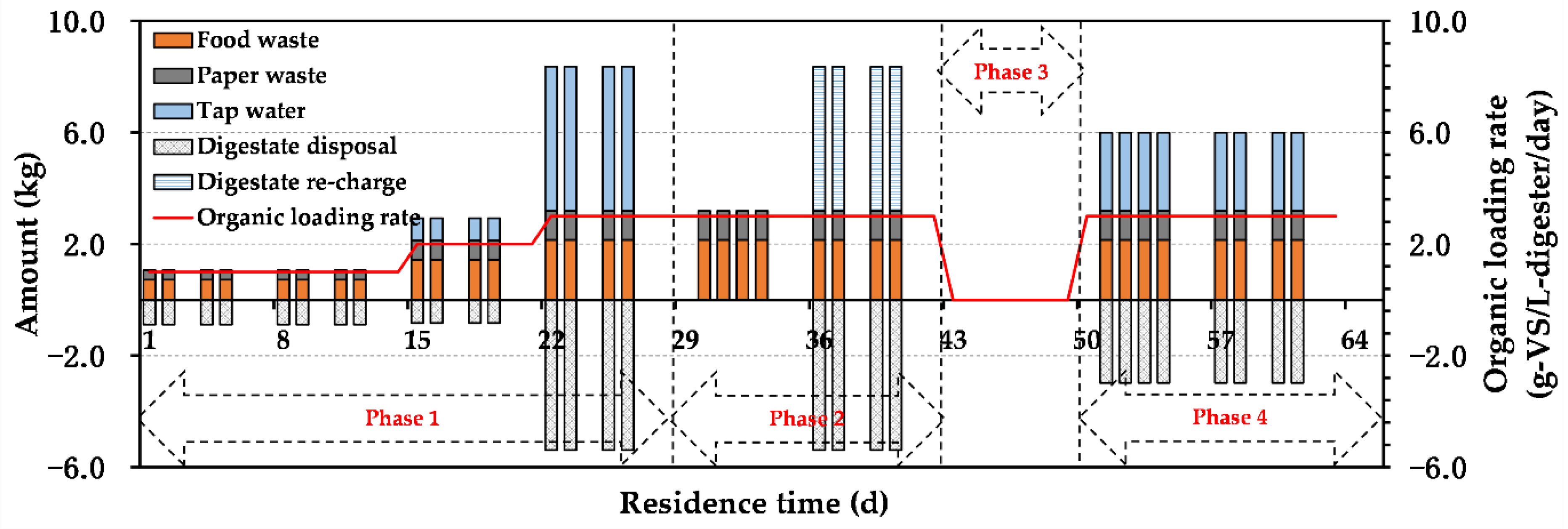
2.3. Constant Condition Derivation of Digestate TS Content
2.4. Analytical Methods
3. Results and Discussion
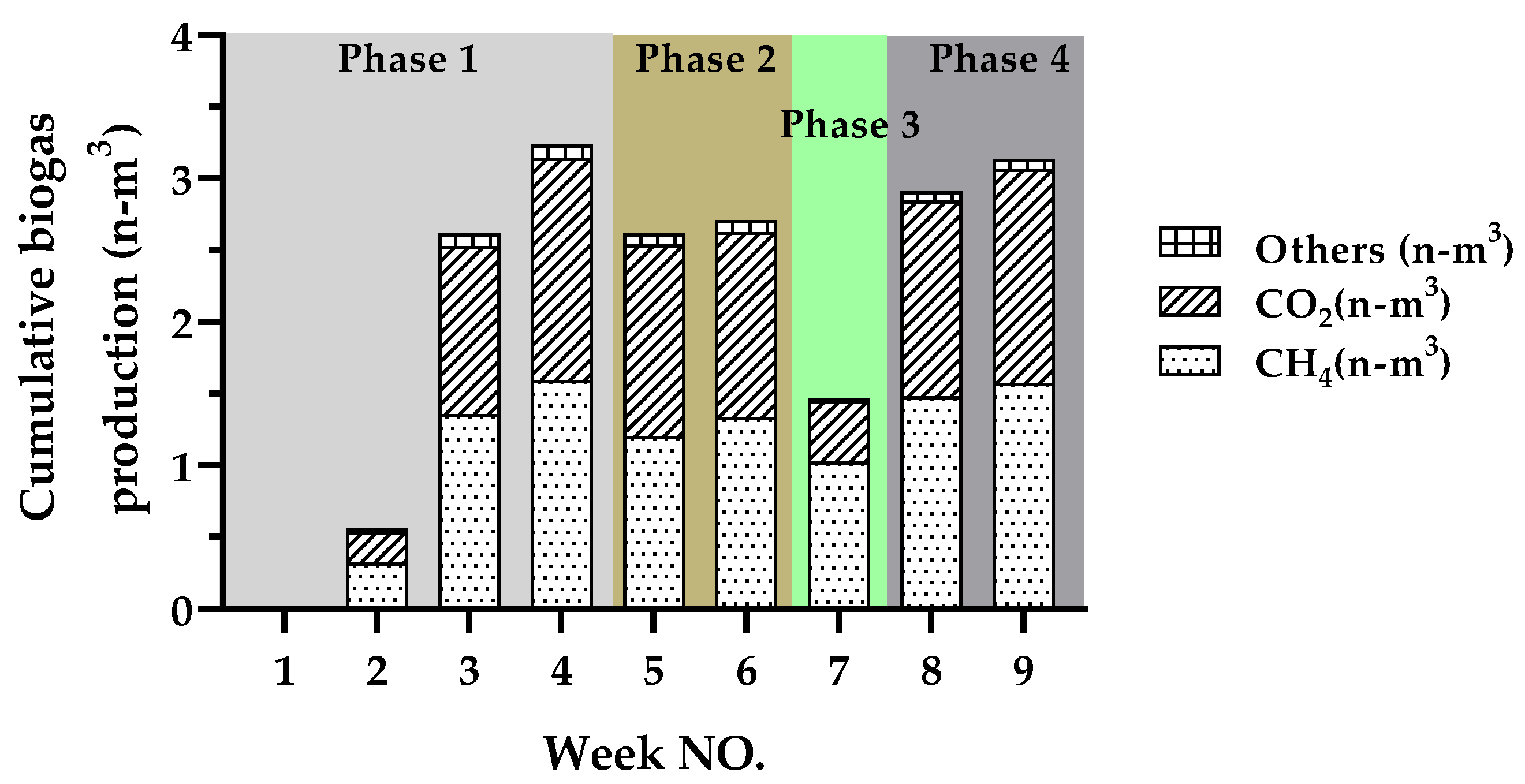
3.1. Cumulative Biogas Production and Methane Concentration
3.2. Anaerobic Digestion of Liquid Fraction
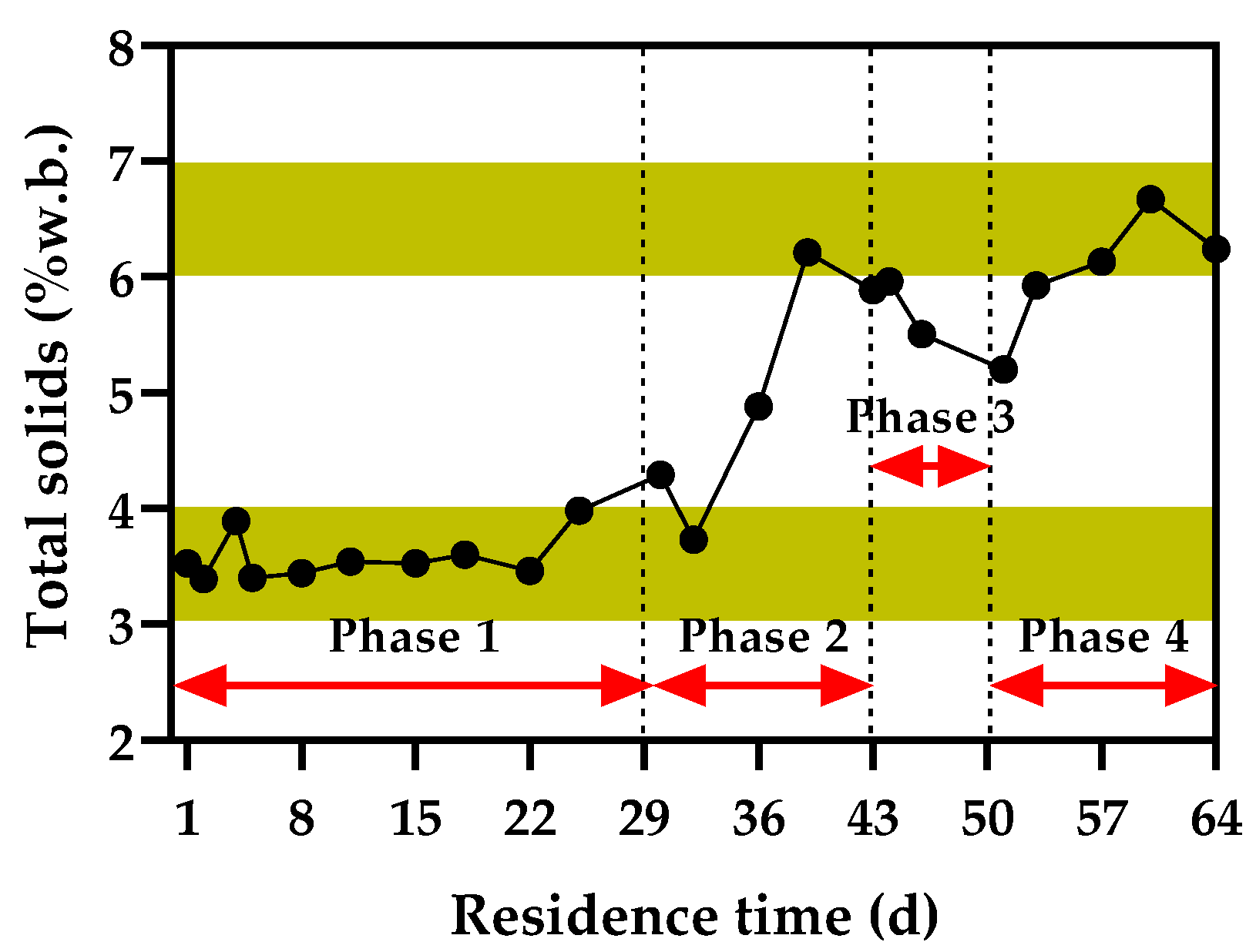
3.2.1. TS Content of Digestate during AD
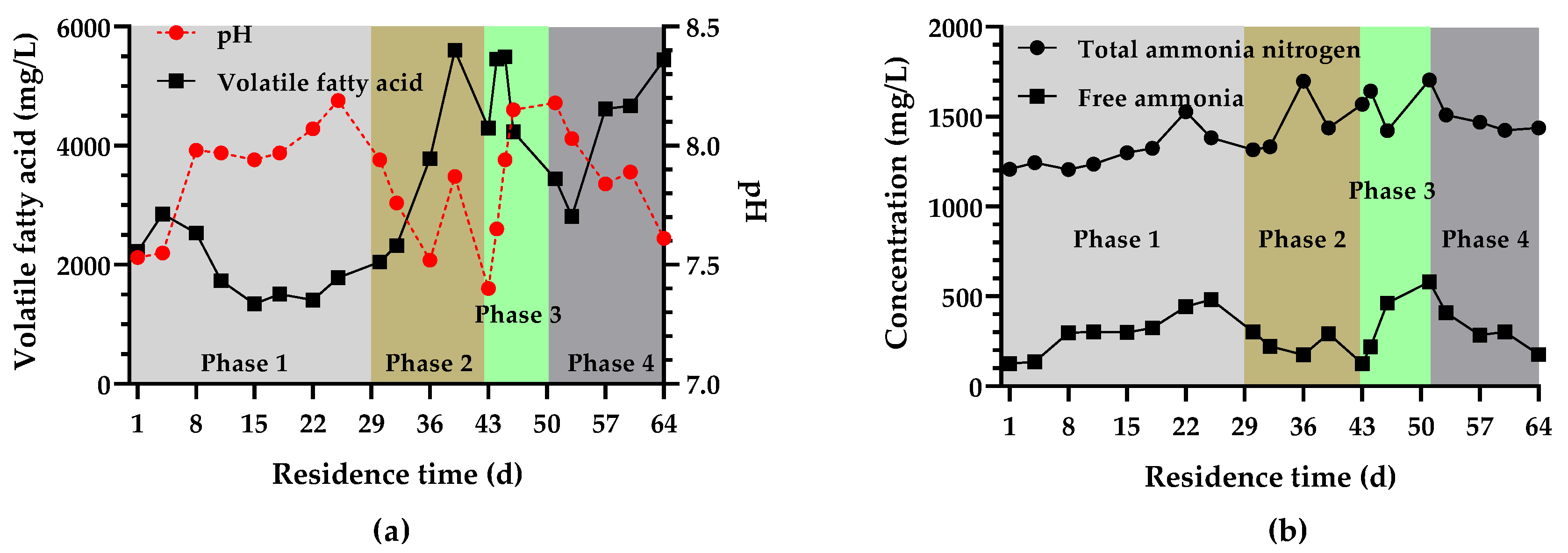
3.2.2. VFA, TAN Concentration, and pH
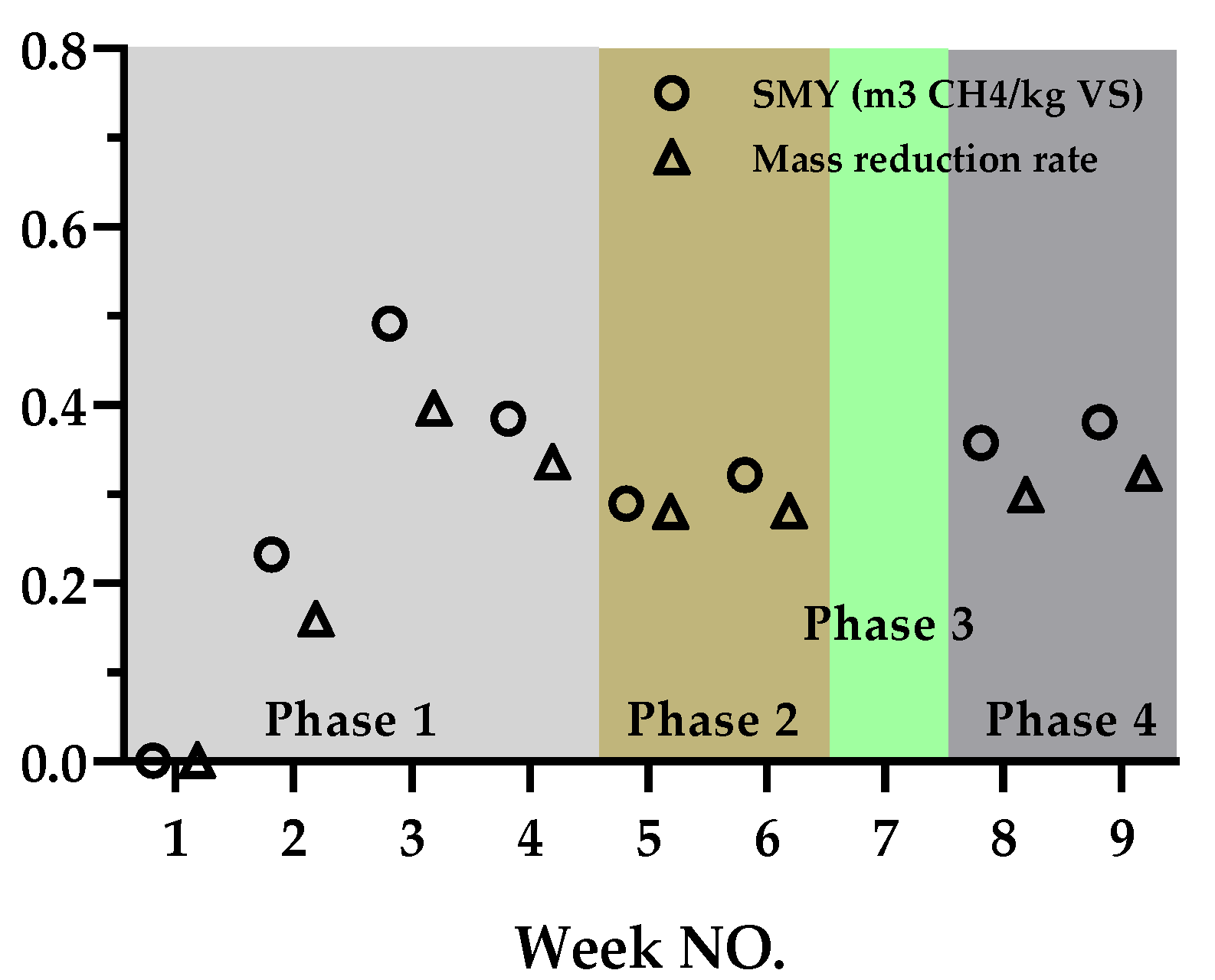
3.3. Mass Reduction Rate and SMY
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Bank. What A Waste 2.0. Available online: https://datatopics.worldbank.org/what-a-waste/index.html (accessed on 12 November 2020).
- Kaza, S.; Lisa, Y.; Perinaz, B.-T.; Frank, V.W. What a Waste 2.0: A global Snapshot of Solid Waste Management to 2050; Urban Development Series; License: Creative Commons Attribution CC BY 3.0 IGO; World Bank: Washington, DC, USA, 2018; p. 29. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- United Nations Development Program. Goal 7 Targets. Available online: https://www.undp.org/content/undp/en/home/sustainable-development-goals/goal-7-affordable-and-clean-energy/targets.html (accessed on 28 April 2020).
- Yoshida, K.; Kametani, K.; Shimizu, N. Adaptive identification of anaerobic digestion process for biogas production management systems. Bioprocess Biosyst. Eng. 2019, 43, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Pérez, M.; Romero-García, L.I. Dry thermophilic anaerobic digestion of the organic fraction of municipal solid wastes: Solid retention time optimization. Chem. Eng. J. 2014, 251, 435–440. [Google Scholar] [CrossRef]
- Banks, C.J.; Chesshire, M.; Heaven, S.; Arnold, R. Anaerobic digestion of source-segregated domestic food waste: Performance assessment by mass and energy balance. Bioresour. Technol. 2011, 102, 612–620. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Long-term anaerobic digestion of food waste stabilized by trace elements. Waste Manag. 2012, 32, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Hecht, C.; Griehl, C. Investigation of the accumulation of aromatic compounds during biogas production from kitchen waste. Bioresour. Technol. 2009, 100, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, G.; An, C.; Chen, X.; Zhang, P.; Xin, X.; Shen, J.; Agnew, J. Anaerobic digestion of livestock manure in cold regions: Technological advancements and global impacts. Renew. Sustain. Energy Rev. 2020, 119, 109494. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, S.-K.; Shin, H.-S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrog. Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Qiang, H.; Lang, D.-L.; Li, Y.-Y. High-solid mesophilic methane fermentation of food waste with an emphasis on Iron, Cobalt, and Nickel requirements. Bioresour. Technol. 2012, 103, 21–27. [Google Scholar] [CrossRef]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef]
- Grimberg, S.; Hilderbrandt, D.; Kinnunen, M.; Rogers, S. Anaerobic digestion of food waste through the operation of a mesophilic two-phase pilot scale digester–Assessment of variable loadings on system performance. Bioresour. Technol. 2015, 178, 226–229. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.-C.; Lee, J.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Three-stage anaerobic co-digestion of food waste and horse manure. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.-C.; Li, W.; Lim, J.W.; Dai, Y.; Tong, Y.W. Three-stage anaerobic digester for food waste. Appl. Energy 2017, 194, 287–295. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A.; Dhar, B.R. A critical review of conventional and emerging methods for improving process stability in thermophilic anaerobic digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Overcoming organic and nitrogen overload in thermophilic anaerobic digestion of pig slurry by coupling a microbial electrolysis cell. Bioresour. Technol. 2016, 216, 362–372. [Google Scholar] [CrossRef]
- Marone, A.; Carmona-Martínez, A.; Sire, Y.; Meudec, E.; Steyer, J.-P.; Bernet, N.; Trably, E. Bioelectrochemical treatment of table olive brine processing wastewater for biogas production and phenolic compounds removal. Water Res. 2016, 100, 316–325. [Google Scholar] [CrossRef]
- Pommier, S.; Chenu, D.; Quintard, M.; Lefebvre, X. A logistic model for the prediction of the influence of water on the solid waste methanization in landfills. Biotechnol. Bioeng. 2007, 97, 473–482. [Google Scholar] [CrossRef]
- Motte, J.-C.; Trably, E.; Escudié, R.; Hamelin, J.; Steyer, J.-P.; Bernet, N.; Delgenes, J.-P.; Dumas, C. Total solids content: A key parameter of metabolic pathways in dry anaerobic digestion. Biotechnol. Biofuels 2013, 6, 164. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Z.-W.; Tang, L.; Li, Y. A mass diffusion-based interpretation of the effect of total solids content on solid-state anaerobic digestion of cellulosic biomass. Bioresour. Technol. 2014, 167, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Bollon, J.; Le-Hyaric, R.; Benbelkacem, H.; Buffière, P. Development of a kinetic model for anaerobic dry digestion processes: Focus on acetate degradation and moisture content. Biochem. Eng. J. 2011, 56, 212–218. [Google Scholar] [CrossRef]
- Nakajima, S.; Shimizu, N.; Ishiwata, H.; Ito, T. The start-up of thermophilic anaerobic digestion of municipal solid waste. J. Jpn. Inst. Energy 2016, 95, 645–647. [Google Scholar] [CrossRef][Green Version]
- IEA Bioenergy. Nutrient Recovery by Biogas Digestate Processing. Available online: https://www.ieabioenergy.com/blog/publications/nutrient-recovery-by-biogas-digestate-processing/ (accessed on 7 January 2021).
- Kim, D.-Y.; Yang, J.; Shinde, S.S.; Mistry, B.M.; Kim, D.-Y.; Sung, J.-S.; Kadam, A.A. Paper waste extracted α-cellulose fibers super-magnetized and chitosan-functionalized for covalent laccase immobilization. Bioresour. Technol. 2018, 261, 420–427. [Google Scholar] [CrossRef]
- Byadgi, S.A.; Kalburgi, P. Production of bioethanol from waste newspaper. Procedia Environ. Sci. 2016, 35, 555–562. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Cheng, Y.; Chen, N.; Li, C.; Yang, Y. Process performance of high-solids batch anaerobic digestion of sewage sludge. Environ. Technol. 2014, 35, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Mshandete, A.; Murto, M.; Kivaisi, A.; Rubindamayugi, M.; Mattiasson, B. Influence of recirculation flow rate on the performance of anaerobic packed-bed bioreactors treating potato-waste leachate. Environ. Technol. 2004, 25, 929–936. [Google Scholar] [CrossRef]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef]
- Wu, D.; Lü, F.; Shao, L.; He, P. Effect of cycle digestion time and solid-liquid separation on digestate recirculated one-stage dry anaerobic digestion: Use of intact polar lipid analysis for microbes monitoring to enhance process evaluation. Renew. Energy 2017, 103, 38–48. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Sun, S.; Qiao, W.; Xiao, M. Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour. Technol. 2011, 102, 11177–11182. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and pH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]

| TS (%w.b.) | VS (%w.b.) | VFA (mg/L) | TAN (mg/L) | C (%d.b.) | N (%d.b.) | |
|---|---|---|---|---|---|---|
| Inoculum | 3.53 ± 0.03 | 2.51 ± 0.04 | 2.22 × 103 ± 64.1 | 12.1 × 103 ± 60.1 | nd | nd |
| FW | 19.7 ± 0.17 | 17.5 ± 0.16 | nd | nd | 30.0 | 3.00 |
| PW | 94.9 ± 0.05 | 82.0 ± 0.05 | nd | nd | 38.4 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, R.; Uchitani, K.; Yoshida, K.; Shimizu, N. Stabilization of Anaerobic Co-Digestion Process via Constant the Digestate Solids Content. Processes 2021, 9, 197. https://doi.org/10.3390/pr9020197
Na R, Uchitani K, Yoshida K, Shimizu N. Stabilization of Anaerobic Co-Digestion Process via Constant the Digestate Solids Content. Processes. 2021; 9(2):197. https://doi.org/10.3390/pr9020197
Chicago/Turabian StyleNa, Risu, Keisuke Uchitani, Kazuto Yoshida, and Naoto Shimizu. 2021. "Stabilization of Anaerobic Co-Digestion Process via Constant the Digestate Solids Content" Processes 9, no. 2: 197. https://doi.org/10.3390/pr9020197
APA StyleNa, R., Uchitani, K., Yoshida, K., & Shimizu, N. (2021). Stabilization of Anaerobic Co-Digestion Process via Constant the Digestate Solids Content. Processes, 9(2), 197. https://doi.org/10.3390/pr9020197




