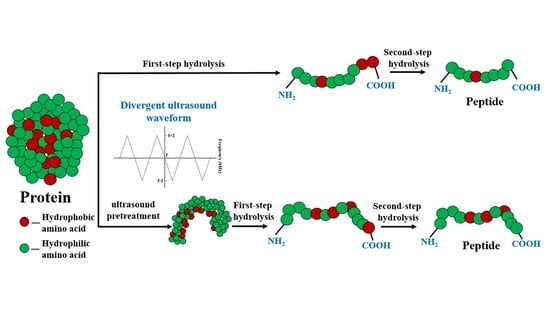
Ultrasound-Assisted Multi-Enzymatic System for the Preparation of ACE Inhibitory Peptides with Low Bitterness from Corn Gluten Meal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
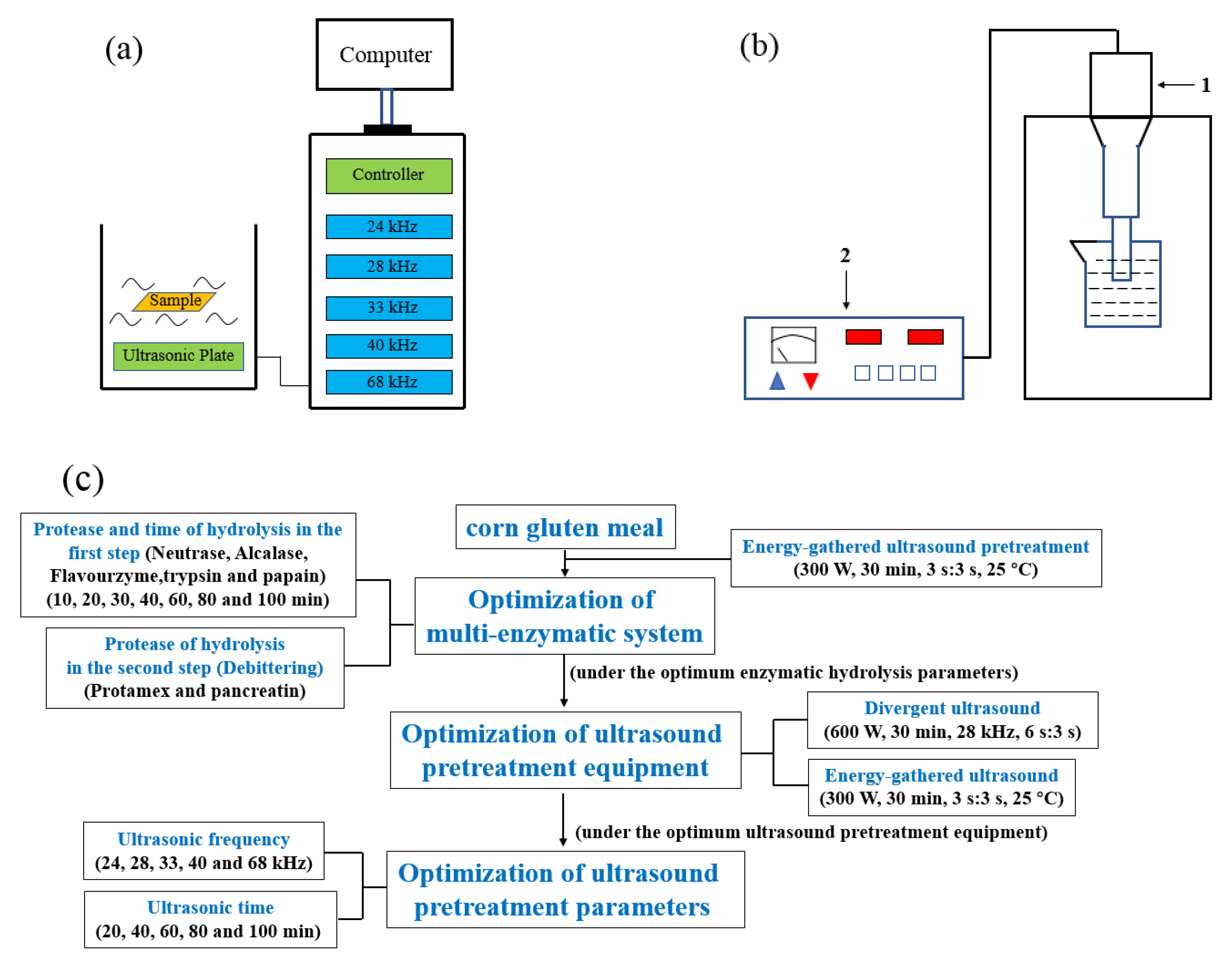
2.2. Energy-Gathered Ultrasound Pretreatment
2.3. Optimization of Enzymatic Hydrolysis Parameters
2.4. Optimization of Ultrasound Pretreatment Equipment
2.4.1. Divergent Ultrasound
2.4.2. Energy-Gathered Ultrasound
2.4.3. Positive Control
2.5. Optimization of Ultrasound Pretreatment Parameters
2.6. Determination of the Degree of Hydrolysis (DH)
2.7. Determination of ACE Inhibitory Activity
2.8. Sensory Evaluation of Bitterness
2.9. Characterization by Intrinsic Fluorescence Spectra
2.10. Hydrophobic Amino Acid Composition Analysis
2.11. Measurement of Molecular Weight Distribution of Hydrolysates
2.12. Statistical Analysis
3. Results and Discussion
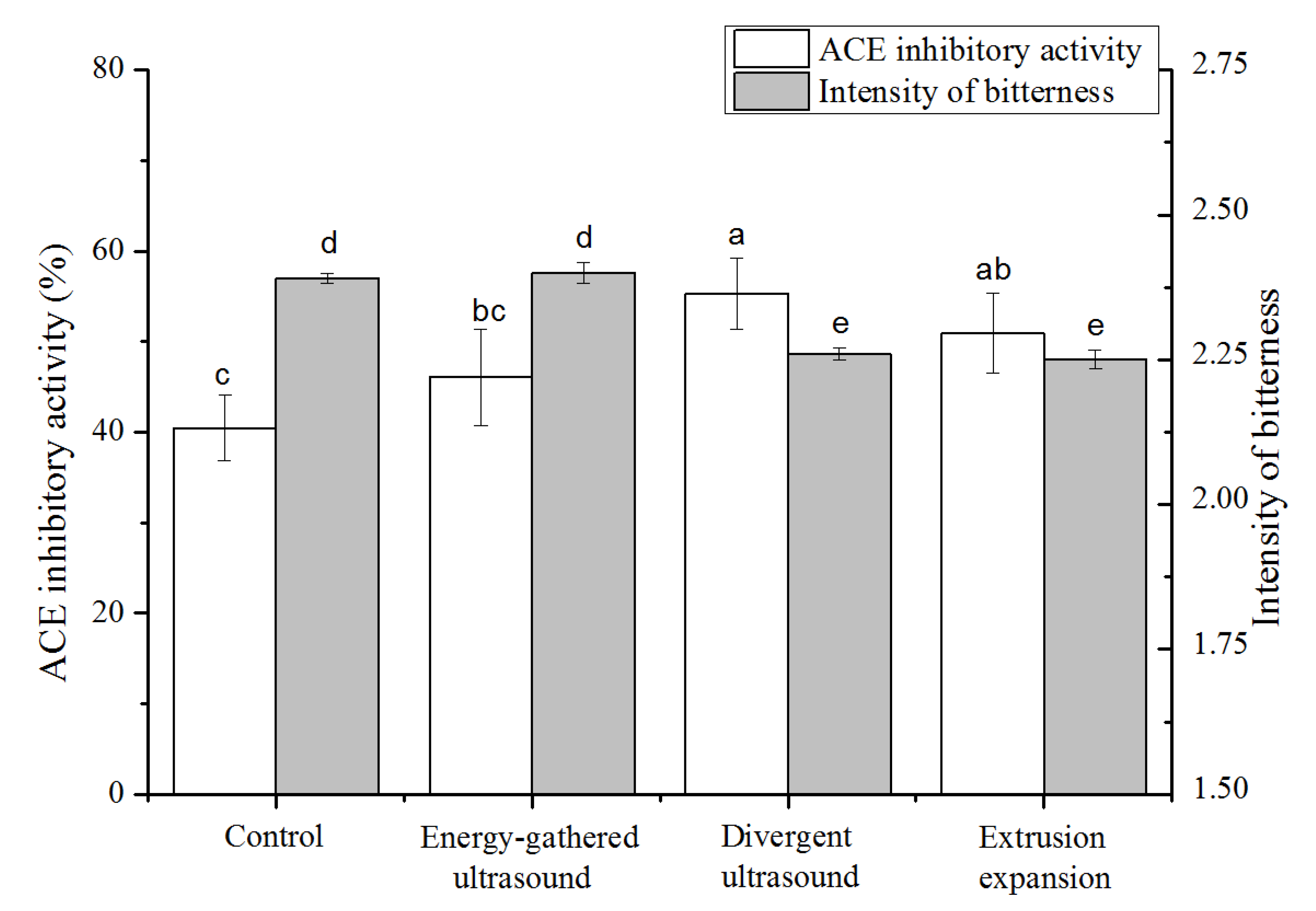
3.1. ACE Inhibitory Activity of CGMHs
3.2. Evaluation of the Bitterness Sensation and Intensity of CGMHs
3.3. Effects of Ultrasound Pretreatment on the Enzymolysis of Corn Gluten Meal
3.4. Effects of Ultrasonic Frequency and Ultrasonic Time on the Enzymolysis of Corn Gluten Meal
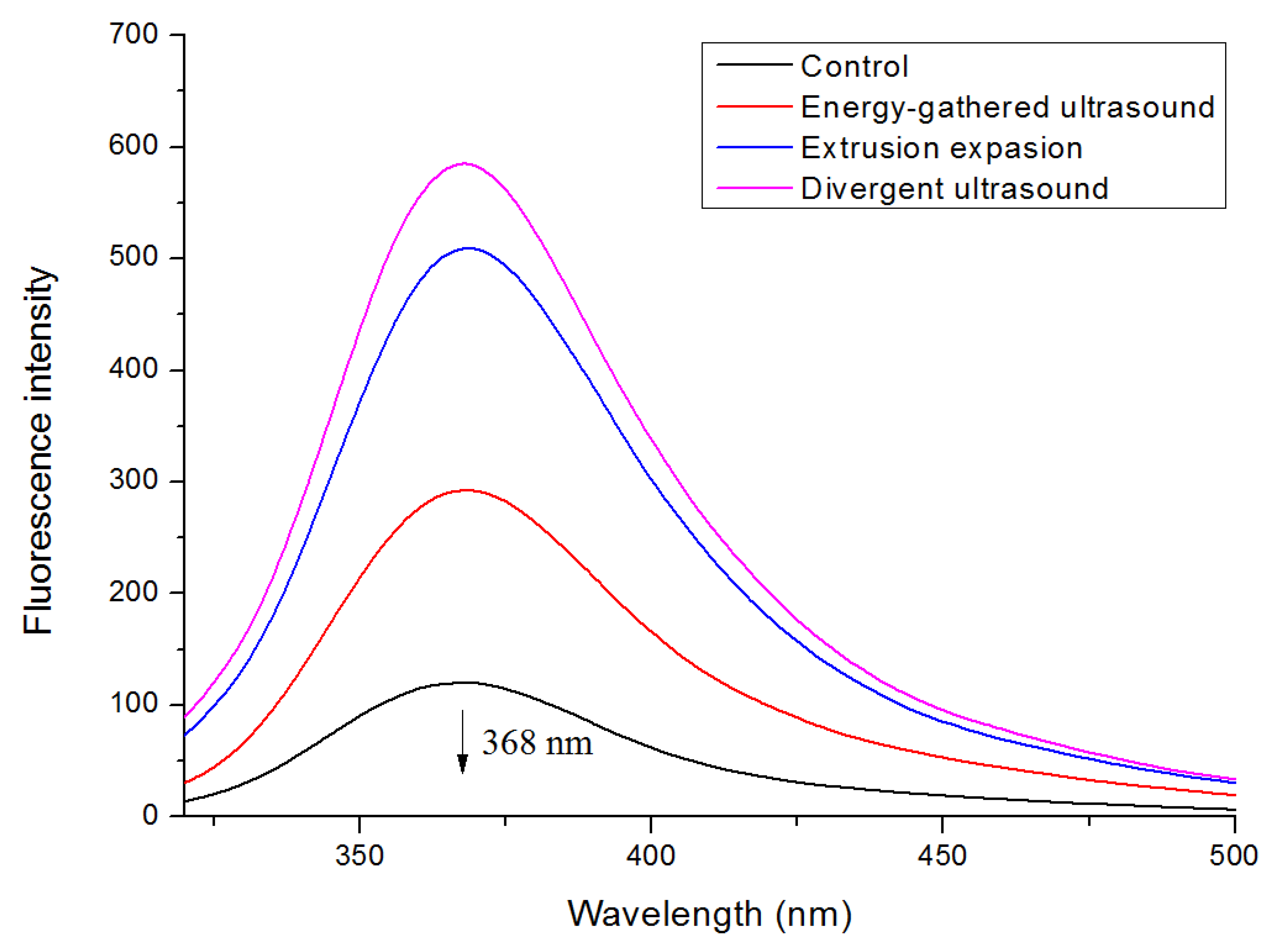
3.5. Intrinsic Fluorescence Spectra
3.6. Hydrophobic Amino Acid Composition of CGMHs
3.7. Molecular Weight Distribution of CGMHs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Men, K.; Ai, Q.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H. Effects of dietary corn gluten meal on growth, digestion and protein metabolism in relation to IGF-I gene expression of Japanese seabass, Lateolabrax japonicus. Aquaculture 2014, 428, 303–309. [Google Scholar] [CrossRef]
- Liying, W.; Long, D.; Ying, W.; Yan, Z.; Jingbo, L. Isolation and characterisation of in vitro and cellular free radical scavenging peptides from corn peptide fractions. Molecules 2015, 20, 3221–3237. [Google Scholar]
- Jia, J. Effect and Mechanism of Ultrasound on Enzymatic Preparation of ACE Inhibitory Peptides from Wheat Grm Protein; Jiangsu University: Zhenjiang, China, 2009. [Google Scholar]
- Ceren, D.; Aysun, Y.; Funda, K.G.; Hayrettin, D.; Beraat, O. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar]
- Wu, S.; Qi, W.; Su, R.; Li, T.; Lu, D.; He, Z. CoMFA and CoMSIA analysis of ACE-inhibitory, antimicrobial and bitter-tasting peptides. Eur. J. Med. Chem. 2014, 84, 100–106. [Google Scholar] [CrossRef]
- Lin, F.; Chen, L.; Liang, R.; Zhang, Z.; Wang, J.; Cai, M.; Li, Y. Pilot-scale production of low molecular weight peptides from corn wet milling byproducts and the antihypertensive effects in vivo and in vitro. Food Chem. 2011, 124, 801–807. [Google Scholar] [CrossRef]
- Huang, W.H.; Sun, J.; He, H. Antihypertensive effect of corn peptides, produced by a continuous production in enzymatic membrane reactor, in spontaneously hypertensive rats. Food Chem. 2011, 128, 968–973. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Artini, S.M.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef]
- Thielen, A.; Oueslati, M.; Hermosilla, R.; Krause, G.; Oksche, A.; Rosenthal, W.; Schülein, R. The hydrophobic amino acid residues in the membrane-proximal C tail of the G protein-coupled vasopressin V2 receptor are necessary for transport-competent receptor folding. FEBS Lett. 2005, 579, 5227–5235. [Google Scholar] [CrossRef] [Green Version]
- Cheung, I.W.Y.; Li-Chan, E.C.Y. Angiotensin-I-converting enzyme inhibitory activity and bitterness of enzymatically-produced hydrolysates of shrimp (Pandalopsis dispar) processing byproducts investigated by Taguchi design. Food Chem. 2010, 122, 1003–1012. [Google Scholar] [CrossRef]
- Pripp, A.H.; Ardö, Y. Modelling relationship between angiotensin-(I)-converting enzyme inhibition and the bitter taste of peptides. Food Chem. 2007, 102, 880–888. [Google Scholar] [CrossRef]
- Ishibashi, N.; Kouge, K.; Shinoda, I.; Kanehisa, H.; Okai, H. A Mechanism for Bitter Taste Sensibility in Peptides. Agric. Biol. Chem. 1988, 52, 819–827. [Google Scholar]
- Mane, S.; Damle, M.; Harikumar, P.; Jamdar, S.; Gade, W. Purification and characterization of aminopeptidase N from chicken intestine with potential application in debittering. Process Biochem. 2010, 45, 1011–1016. [Google Scholar] [CrossRef]
- Synowiecki, J.; Jagietka, R.; Shahidi, F. Preparation of hydrolysates from bovine red blood cells and their debittering following plastein reaction. Food Chem. 1996, 57, 435–439. [Google Scholar] [CrossRef]
- Lei, F.; Zhao, Q.; Sun-Waterhouse, D.; Zhao, M. Characterization of a salt-tolerant aminopeptidase from marine Bacillus licheniformis SWJS33 that improves hydrolysis and debittering efficiency for soy protein isolate. Food Chem. 2017, 214, 347–353. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Mora, L.; Jemil, I.; Jridi, M.; Toldra, F.; Nasri, M.; Nasri, R. Effect of ultrasound pretreatment and Maillard reaction on structure and antioxidant properties of ultrafiltrated smooth-hound viscera proteins-sucrose conjugates. Food Chem. 2017, 230, 507–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P. Study on Soybean Peptide Debitterizing and Physiological Function; University of Jinan: Jinan, China, 2014. [Google Scholar]
- Gallego, M.; Mora, L.; Toldrá, F. The relevance of dipeptides and tripeptides in the bioactivity and taste of dry-cured ham. Food Prod. Process. Nutr. 2019, 1, 1–14. [Google Scholar]
- Huang, S.; Li, Y.; Li, C.; Ruan, S.; Roknul, A.S.M.; Ou, Y.N.; Ye, X.; Wang, Y.; Ma, H. Effects of ultrasound-assisted sodium bisulfite pretreatment on the preparation of cholesterol-lowering peptide precursors from soybean protein. Int. J. Biol. Macromol. 2021, 183, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Ma, H.; Liu, B.; He, R.; Pan, Z.; Abano, E. Enzymolysis reaction kinetics and thermodynamics of defatted wheat germ protein with ultrasonic pretreatment. Ultrason. Sonochem. 2013, 20, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Joseph, L.; Bennett, J.A.; Jacobson, H.I.; Andersen, T.T. Design and synthesis of biologically active peptides: A ’tail’ of amino acids can modulate activity of synthetic cyclic peptides. Peptides 2011, 32, 2504–2510. [Google Scholar] [CrossRef] [Green Version]
- Guerard, F.; Decourcelle, N.; Sabourin, C.; Floch-Laizet, C.; Bourseau, P. Recent developments of marine ingredients for food and nutraceutical applications: A review. J. Sci. Halieut. Aquat. 2010, 2, 21–27. [Google Scholar]
- Zhao, M.M.; Guo, S.G.; Cui, C.; Liu, T.X. Enzymatic hydrolysis of porcine hemoglobin and debittering of hydrolysates. J. Jilin Univ. 2006, 7, 941–945. [Google Scholar]
- Adlernissen, J. Enzymic hydrolysis of food proteins. Can. Med. Assoc. J. 1986, 172, 1783–1785. [Google Scholar]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Minagawa, E.; Kaminogawa, S.; Tsukasaki, F.; Yamauchi, K. Debittering Mechanism in Bitter Peptides of Enzymatic Hydrolysates from Milk Casein by Aminopeptidase T. J. Food Sci. 2010, 54, 1225–1229. [Google Scholar] [CrossRef]
- Hua, Z.; Zhen-Yu, W.; Xin, Y.; Hai-Tian, Z.; Ying-Chun, Z.; Ai-Jun, D.; Jing, J.; Jing, W. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an Amino Acid Analyzer and ICP-MS with micro-wave digestion. Food Chem. 2014, 147, 189–194. [Google Scholar]
- Aissaoui, N.; Abidi, F.; Marzouki, M.N. ACE inhibitory and antioxidant activities of red scorpionfish (Scorpaena notata) protein hydrolysates. J. Food Sci. Technol. 2015, 52, 7092–7102. [Google Scholar] [CrossRef]
- Newman, J.; Egan, T.; Harbourne, N.; Oriordan, D.; Jacquier, J.C.; Osullivan, M. Correlation of sensory bitterness in dairy protein hydrolysates: Comparison of prediction models built using sensory, chromatographic and electronic tongue data. Talanta 2014, 126, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.J.; Whang, J.H.; Kim, Y.S.; Bae, S.H.; Noh, D.O. Preparation of angiotensin I converting enzyme inhibitor from corn gluten. Process Biochem. 2003, 38, 1239–1244. [Google Scholar] [CrossRef]
- Ko, S.C.; Lee, J.K.; Byun, H.G.; Lee, S.C.; Jeon, Y.J. Purification and characterization of angiotensin I-converting enzyme inhibitory peptide from enzymatic hydrolysates of Styela clava flesh tissue. Process Biochem. 2012, 47, 34–40. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Yang, H.; Liu, D. Preparation of ACE inhibitory peptide from corn gluten meal and pilot-scale production. Food Ferment. Ind. 2016, 42, 158–164. [Google Scholar]
- Tsai, J.-S.; Chen, J.-L.; Pan, B.S. ACE-inhibitory peptides identified from the muscle protein hydrolysate of hard clam (Meretrix lusoria). Process Biochem. 2008, 43, 743–747. [Google Scholar] [CrossRef]
- Tong, X.; Lian, Z.; Miao, L.; Qi, B.; Zhang, S.; Li, Y.; Wang, H.; Jiang, L. An innovative two-step enzyme-assisted aqueous extraction for the production of reduced bitterness soybean protein hydrolysates with high nutritional value. LWT 2020, 134, 110151. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Schweiggert-Weisz, U.; Brode, V.; Eisner, P. Enzyme assisted degradation of potential soy protein allergens with special emphasis on the technofunctionality and the avoidance of a bitter taste formation. LWT Food Sci. Technol. 2016, 68, 707–716. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Hansen, E.T.; Bredie, W.L.P.; Lametsch, R. Structural characteristics of low bitter and high umami protein hydrolysates prepared from bovine muscle and porcine plasma. Food Chem. 2018, 257, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E. Quantitative structure-activity relationship study of bitter di- and tri-peptides including relationship with angiotensin I-converting enzyme inhibitory activity. J. Peptide Sci. 2007, 13, 63–69. [Google Scholar] [CrossRef]
- Jin, J. Study on Preparation of High Bioavailability Corn Protein Based on Computer Simulation and Ultrasound Assisted Proteolysis; Jiangsu University: Zhenjiang, China, 2015. [Google Scholar]
- Ma, H.; Huang, L.; Jia, J.; He, R.; Luo, L.; Zhu, W. Effect of energy-gathered ultrasound on Alcalase. Ultrason. Sonochem. 2011, 18, 419–424. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, S.; Oladejo, A.O.; Wang, Y.; Huang, S.; Zhou, C.; Wang, Y.; Mao, L.; Zhang, Y.; et al. Effects of low power density multi-frequency ultrasound pretreatment on the enzymolysis and the structure characterization of defatted wheat germ protein. Ultrason. Sonochem. 2017, 38, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhi-He, H.U.; Zhao, Y. Using Electronic Tongue to Evaluate the Debittering Effect of Flavourzyme Treatment on Casein Hydrolysate with ACE Inhibitory Activity. Food Sci. 2013, 4, 220–224. [Google Scholar]
- Pan, M.; Xu, F.; Wu, Y.; Yao, M.; Xiao, X.; Zhang, N.; Ju, X.; Wang, L. Application of ultrasound-assisted physical mixing treatment improves in vitro protein digestibility of rapeseed napin. Ultrason. Sonochem. 2020, 67, 105136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Hu, J.; Yu, X.; Yagoub, A.E.A.; Zhang, Y.; Ma, H.; Gao, X.; Out, P.N.Y. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT 2017, 77, 488–496. [Google Scholar] [CrossRef]
- Ney, K.H. Prediction of bitterness of peptides from their amino acid composition. Z. Lebensm. Unters. Forsch. 1971, 147, 64–68. [Google Scholar] [CrossRef]



| Name of Amino Acids | HA Mass Fraction/% | HB Mass Fraction/% |
|---|---|---|
| Pro | 9.0 ± 0.03 a | 8.9 ± 0.06 a |
| Tyr | 5.2 ± 0.04 b | 5.4 ± 0.09 a |
| Phe | 5.6 ± 0.06 a | 5.6 ± 0.04 a |
| Leu | 16.8 ± 0.07 a | 13.6 ± 0.03 b |
| Ile | 4.1 ± 0.02 b | 4.6 ± 0.01 a |
| Val | 4.7 ± 0.03 b | 5.4 ± 0.02 a |
| Ala | 8.0 ± 0.07 a | 6.7 ± 0.06 b |
| Met | 2.3 ± 0.03 b | 2.8 ± 0.04 a |
| Total amino acids | 55.7 ± 0.04 a | 53.0 ± 0.04 b |
| Molecular Weight (Da) | >1500 | 1000–1500 | 500–1000 | <500 |
|---|---|---|---|---|
| HA Proportion (%) | 9.96 ± 1.32 b | 7.57 ± 1.89 b | 57.15 ± 1.16 a | 25.32 ± 2.67 a |
| HB Proportion (%) | 14.51 ± 1.19 a | 10.94 ± 2.34 a | 51.09 ± 2.53 b | 23.46 ± 1.58 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Li, Y.; Li, C.; Ruan, S.; Qu, W.; Ding, Y.; Ye, X.; Ma, H. Ultrasound-Assisted Multi-Enzymatic System for the Preparation of ACE Inhibitory Peptides with Low Bitterness from Corn Gluten Meal. Processes 2021, 9, 2170. https://doi.org/10.3390/pr9122170
Huang S, Li Y, Li C, Ruan S, Qu W, Ding Y, Ye X, Ma H. Ultrasound-Assisted Multi-Enzymatic System for the Preparation of ACE Inhibitory Peptides with Low Bitterness from Corn Gluten Meal. Processes. 2021; 9(12):2170. https://doi.org/10.3390/pr9122170
Chicago/Turabian StyleHuang, Shanfen, Yunliang Li, Chengliang Li, Siyu Ruan, Wenjuan Qu, Yanhua Ding, Xiaofei Ye, and Haile Ma. 2021. "Ultrasound-Assisted Multi-Enzymatic System for the Preparation of ACE Inhibitory Peptides with Low Bitterness from Corn Gluten Meal" Processes 9, no. 12: 2170. https://doi.org/10.3390/pr9122170
APA StyleHuang, S., Li, Y., Li, C., Ruan, S., Qu, W., Ding, Y., Ye, X., & Ma, H. (2021). Ultrasound-Assisted Multi-Enzymatic System for the Preparation of ACE Inhibitory Peptides with Low Bitterness from Corn Gluten Meal. Processes, 9(12), 2170. https://doi.org/10.3390/pr9122170