Leuconostoc mesenteroides and Pediococcus pentosaceus Non-Alcoholic Pearl Millet Beverage Enriched with Moringa oleifera Leaf Powder: Nutritional and Sensory Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Raw Materials and Equipment
2.2. Production of Pearl Millet Flour
2.3. Preparation of Moringa Leaf Powder Extract
2.4. Production of Plain Non-Alcoholic Pearl Millet Beverage and Moringa Supplemented Non-Alcoholic Pearl Millet Beverage
2.5. Production of Traditional Non-Alcoholic Pearl Millet Beverage
2.6. Enumeration of Lactic Acid Bacteria in Pearl Millet Extract during Fermentation of Plain and Moringa-Supplemented Non-Alcoholic Pearl Millet Beverages
2.7. Physicochemical Analysis of Pearl Millet Extract (PME) during Fermentation
2.8. Determination of Soluble Sugars in Pearl Millet Extract during Fermentation
2.9. Proximate Analyses of Plain, Moringa-Supplemented and Traditional Non-Alcoholic Pearl Millet Beverages
2.10. Colour Measurement of Plain, Moringa-Supplemented, and Traditional Non-Alcoholic Pearl Millet Beverages
2.11. Extraction and Identification of Volatile Compounds in the Non-Alcoholic Pearl Millet Beverages
2.12. Sensory Evaluation of the Non-Alcoholic Pearl Millet Beverages
2.13. Data Analysis
3. Results
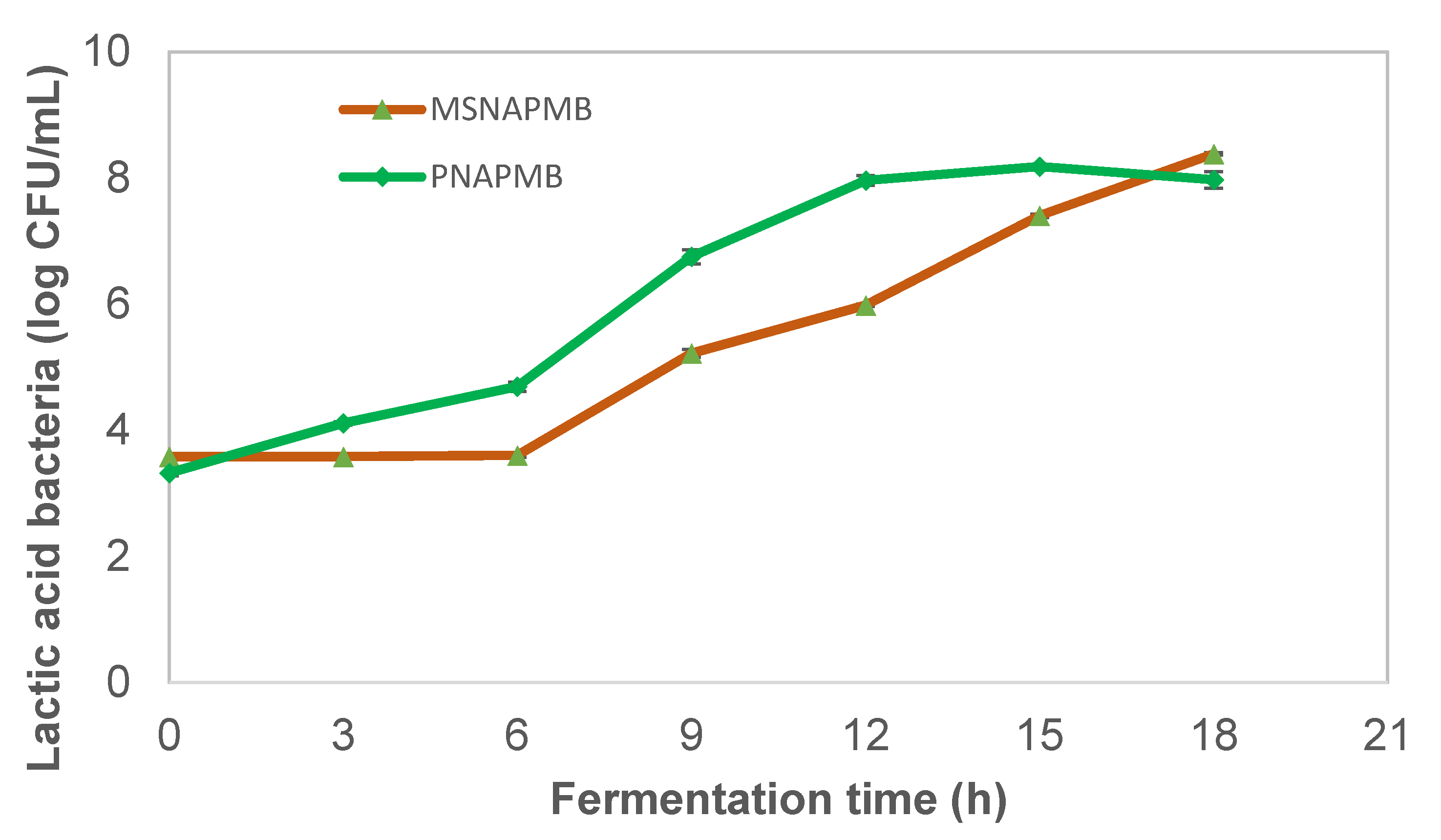
3.1. Effect of Fermentation Time on the Viability of Lactic Acid Bacteria in Pearl Millet Extract during Fermentation
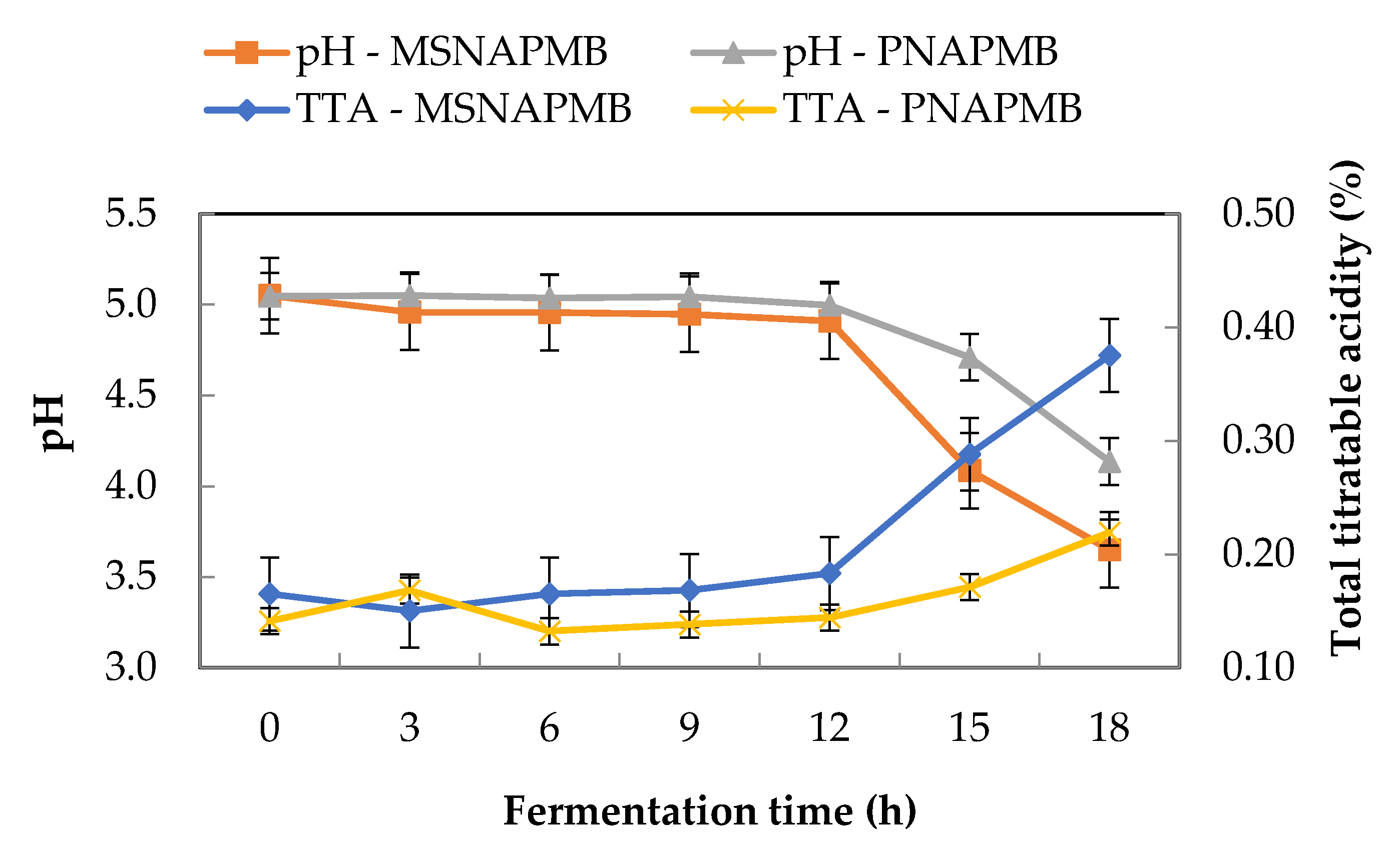
3.2. Effect of Fermentation Time on the pH and Total Titratable Acidity of Pearl Millet Extract during Fermentation
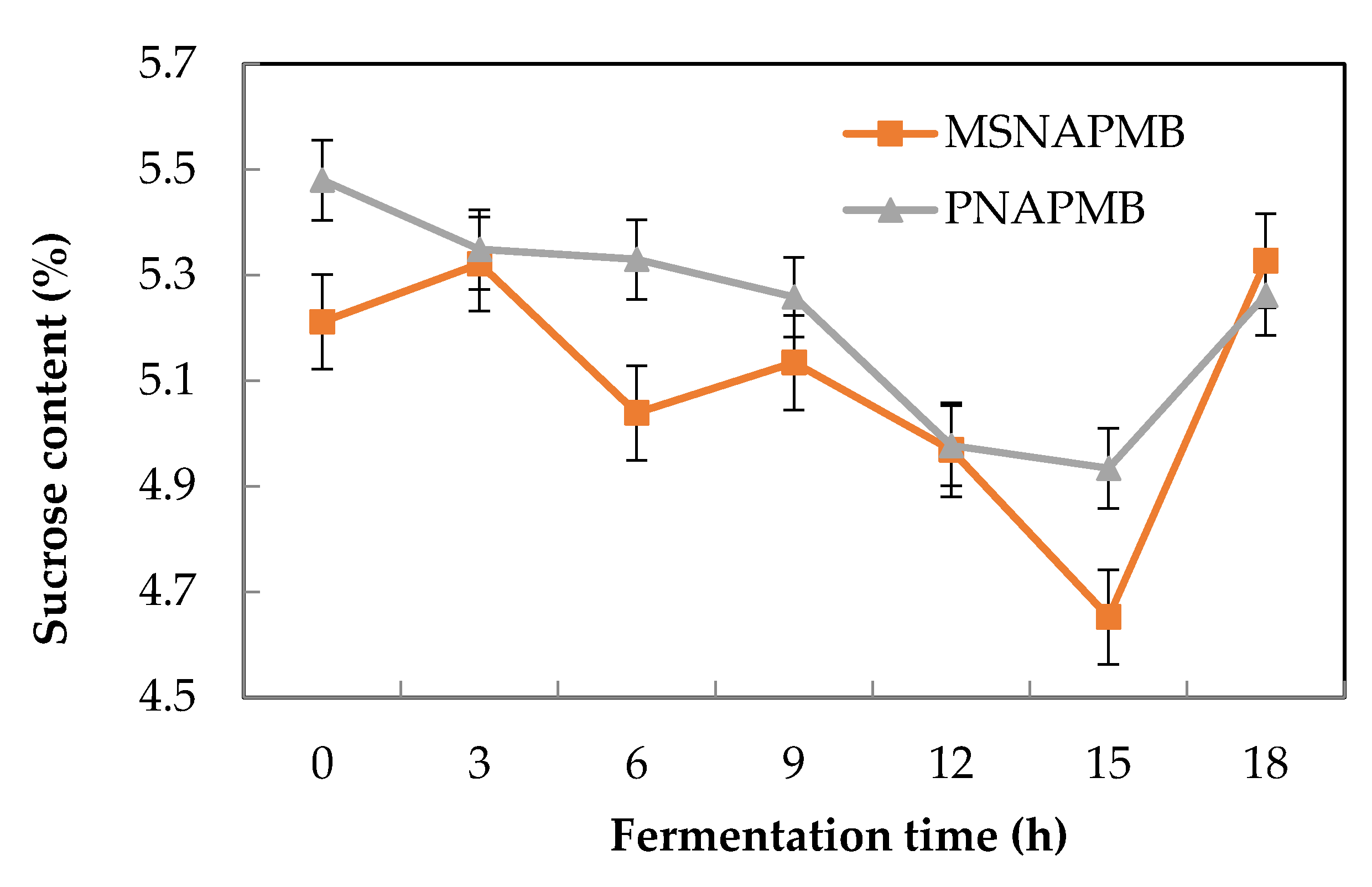
3.3. Effect of Fermentation Time on the Sugar Content of the Pearl Millet Extract during Fermentation
3.4. Proximate Composition of Plain, Moringa-Supplemented, and Traditional Non-Alcoholic Pearl Millet Beverages
3.5. Colour Characteristics of Pearl Millet Non-Alcoholic Beverages
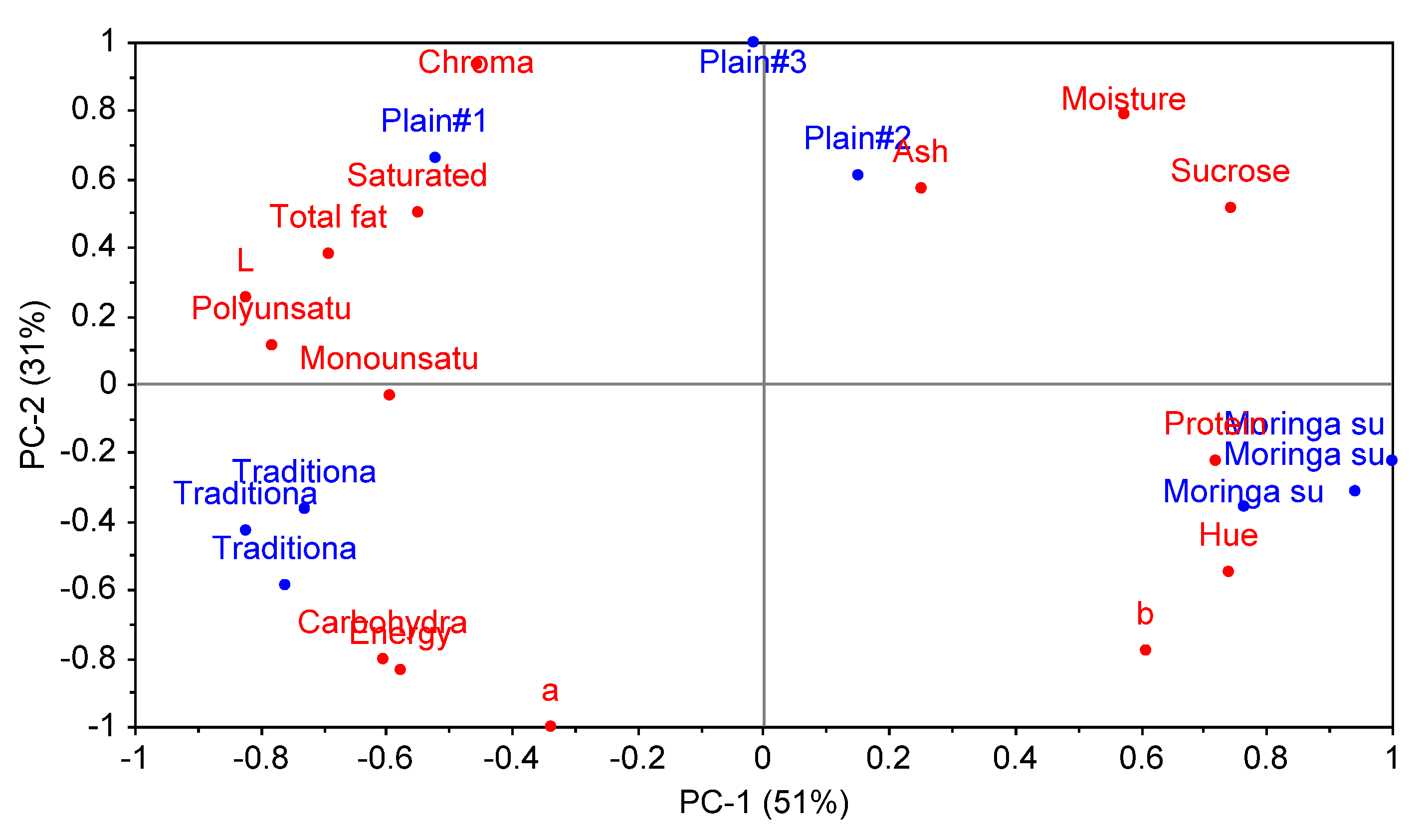
3.6. Characterisation of Chemical Composition and Colour of Non-Alcoholic Cereal Beverages Using Principal Component Analysis (PCA)
3.7. Volatile Compounds in the Non-Alcoholic Pearl Millet Beverages
3.8. Sensory Characteristics of the Non-Alcoholic Pearl Millet Beverages
3.9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef]
- Vasudha, S.; Mishra, H.N. Non Dairy Probiotic Beverages. Int. Food Res. J. 2013, 20, 7–15. [Google Scholar]
- Basinskiene, L.; Juodeikiene, G.; Vidmantiene, D.; Tenkanen, M.; Makaravicius, T.; Bartkiene, E. Non-Alcoholic Beverages from Fermented Cereals with Increased Oligosaccharide Content. Food Technol. Biotechnol. 2016, 54, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Terna, G.; Ayo, J.A. Innovations in the Traditional Kunun Zaki Production Process. Pak. J. Nutr. 2002, 1, 202–205. [Google Scholar] [CrossRef][Green Version]
- Oranusi, S.U.; Umoh, V.J.; Kwaga, J.K.P. Hazards and critical control points of kunun-zaki, a non-alcoholic beverage in Northern Nigeria. Food Microbiol. 2003, 20, 127–132. [Google Scholar] [CrossRef]
- Onaolapo, I.O.; Busari, T. The Effect of Pasteurization on Microbial Growth and Sensory Qualities of Sekete—A fermented maize beverage. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 51–54. [Google Scholar]
- Nkama, I.; Agarry, O.O.; Akoma, O. Sensory and nutritional quality characteristics of powdered “Kunun-zaki”: A Nigerian fermented cereal beverage. Afr. J. Food Sci. 2010, 4, 364–370. [Google Scholar]
- Uvere, P.O.; Amazikwu, U.C.J. Processing and evaluation of instant kunun zaki from millet-cowpea malt and millet-soybean malt. Afr. J. Food Sci. 2011, 5, 761–768. [Google Scholar] [CrossRef]
- Adelekan, A.O.; Alamu, A.E.; Arisa, N.U.; Adebayo, Y.O.; Dosa, A.S. Nutritional, Microbiological and Sensory Characteristics of Malted Soy-Kunu Zaki: An Improved Traditional Beverage. Adv. Microbiol. 2013, 3, 389–397. [Google Scholar] [CrossRef]
- Ayo, J.A.; Ayo, V.A.; Yelmi, B.; Onuoha, G.; Ikani, M.O. Effect of preservatives on microbiological qualities of kunu zaki. Int. J. Agric. Sci. Res. 2013, 2, 124–130. [Google Scholar]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The potential of Moringa oleifera in food formulation: A promising source of functional compounds with health-promoting properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Pieterse, P.J.; Mothapo, P.N.; Phiri, E.E. Moringa oleifera in South Africa: A review on its production, growing conditions and consumption as a food source. S. Afr. J. Sci. 2021, 117, 8689. [Google Scholar] [CrossRef]
- Olusanya, R.; Kolanisi, U.; van Onselen, A.; Ngobese, N.; Siwela, M. Nutritional composition and consumer acceptability of Moringa oleifera leaf powder (MOLP)-supplemented mahewu. S. Afr. J. Bot. 2020, 129, 175–180. [Google Scholar] [CrossRef]
- Jideani, V.; Ratau, M.; Okudoh, V. Non-Alcoholic Pearl Millet Beverage Innovation with Own Bioburden: Leuconostoc mesenteroides, Pediococcus pentosaceus and Enterococcus gallinarum. Foods 2021, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, K. Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. Afr. J. Biotechnol. 2007, 6, 1469–1478. [Google Scholar] [CrossRef]
- Kivanç, M.; Yilmaz, M. Isolation and identification of lactic acid bacteria from boza, and their microbial activity against several reporter strains. Turk. J. Biol. 2011, 35, 313–324. [Google Scholar] [CrossRef]
- Omemu, A.M. Fermentation dynamics during production of ogi, a Nigerian fermented cereal porridge. Rep. Opin. 2011, 3, 8–17. [Google Scholar]
- Nwachukwu, E.; Achi, O.K.; Ijeoma, I.O. Lactic acid bacteria in fermentation of cereals for the production of indigenous Nigerian foods. Afr. J. Food Sci. Technol. 2010, 1, 21–26. [Google Scholar]
- Temitope, O.S.; Taiyese, O.B. Quality assessment of “oti-oka” like beverage produced from pearl millet. J. Appl. Biosci. 2012, 51, 3608–3617. [Google Scholar]
- Osuntogun, B.; Aboaba, O.O. Microbiological and Physico-chemical Evaluation of Some Non-alcoholic Beverages. Pak. J. Nutr. 2004, 3, 188–192. [Google Scholar]
- Li, B.W.; Andrews, K.W.; Pehrsson, P.R. Individual Sugars, Soluble, and Insoluble Dietary Fiber Contents of 70 High Consumption Foods. J. Food Compos. Anal. 2002, 15, 715–723. [Google Scholar] [CrossRef]
- Azlan Azizan, K.; Haizun, N.; Ghani, A.; Firdaus Nawawi, M. GC-MS based metabolomics and multivariate statistical analysis of Wedelia trilobata extracts for the identification of potential phytochemical properties. Plant Omics 2015, 8, 16438. [Google Scholar]
- Simango, C. Lactic acid fermentation of sour porridge and mahewu, a non-alcoholic fermented cereal beverage. JASSA J. Appl. Sci. S. Afr. 2002, 8, 89–98. [Google Scholar] [CrossRef]
- Hekmat, S.; Morgan, K.; Soltani, M.; Gough, R. Sensory Evaluation of Locally-grown Fruit Purees and Inulin Fibre on Probiotic Yogurt in Mwanza, Tanzania and the Microbial Analysis of Probiotic Yogurt Fortified with Moringa oleifera. J. Health Popul. Nutr. 2015, 33, 60–67. [Google Scholar] [PubMed]
- Magala, M.; Kohajdová, Z.; Karovičová, J.; Hojerová, J.; Greifová, M. Application of lactic acid bacteria for production of fermented beverages based on rice flour. Czech J. Food Sci. 2015, 33, 458–463. [Google Scholar] [CrossRef]
- Wilson, P.; David, T.; Sam, B. Microbial and Biochemical Changes Occurring During Production of Traditional Rwandese Banana Beer “Urwagwa”. Ferment. Technol. 2012, 1, 104. [Google Scholar] [CrossRef]
- Nour, A.A.M.; Ibrahim, M.A.E.M. Effect of Supplementation with Moringa Leaves Powder (MLP) and Fermentation on Chemical Composition, Total Minerals Contents and Sensory Characteristics of Sorghum Flour. Int. J. Sci. Res. 2013, 5, 672–677. [Google Scholar]
- Garba, H.; Shettima, Y.M.; Mustapha, B.U.A.; Putaya, H.A.N. Proximate composition and phytochemical screening of Moringa oleifera leaves. Appl. Res. J. 2015, 1, 470–472. [Google Scholar]
- Olosunde, O.O.; Abiodun, O.A.; Amanyunose, A.A.; Adepeju, A.B. Sensory and Nutritional Characteristics of Kununzaki Enriched with Moringa (Moringa oleifera) Seed Flour. Am. J. Exp. Agric. 2014, 4, 1027–1035. [Google Scholar] [CrossRef]
- Funmilayo, V.; Abioye, V.F.; Mo, A. Proximate Composition and Sensory Properties of Moringa Fortified Maize-Ogi. J. Nutr. Food Sci. 2015, 12, 1. [Google Scholar] [CrossRef]
- Singh, E.; Nayyar, S. Potential Functional Implications of Finger Millet (Eleusine coracana) in Health and Disease. Int. J. Home Sci. 2016, 2, 151–155. [Google Scholar] [CrossRef]
- Sumarmono, J.; Sulistyowati, M. Soenarto Fatty Acids Profiles of Fresh Milk, Yogurt and Concentrated Yogurt from Peranakan Etawah Goat Milk. Procedia Food Sci. 2015, 3, 216–222. [Google Scholar] [CrossRef]
- Ovando-Martinez, M.; Daglioglu, O.; Guner, K.; Gecgel, U.; Simsek, S. Analysis of the Fatty Acids and Phenolic Compounds in a Cereal-Based Fermented Food (Tarhana). Food Nutr. Sci. 2014, 5, 1177–1184. [Google Scholar] [CrossRef]
- Bora, P. Nutritional Properties of Different Millet Types and their Selected Products. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2013. [Google Scholar]
- Pal, A.; Das, D.; Panda, P.; Rath, M.; Sharma, T. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacogn. Res. 2015, 7, 110–113. [Google Scholar] [CrossRef]
- Thomas, E.; Aneesh, T.P.; Thomas, D.G.; Anandan, R. View of Gc-Ms Analysis of Phytochemical Compounds Present in The Rhizomes of Nervilia aragoana GAUD. Asian J. Pharm. Clin. Res. 2013, 6, 68–74. [Google Scholar]
- Kohajdová, Z.; Karovičová, J. Fermentation of cereals for specific purpose. J. Food Nutr. Res. 2007, 46, 51–57. [Google Scholar]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology Practical Applications and Procedures, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Stats SA Mid-Year Population Estimates. 2016. Available online: https://www.statssa.gov.za/publications/P0302/P03022016.pdf (accessed on 24 October 2021).
- Murevanhema, Y.; Jideani, V. Production and Characterization of Milk Produced from Bambara Groundnut (Vigna subterranea) Varieties. J. Food Process. Preserv. 2015, 39, 1485–1498. [Google Scholar] [CrossRef]

| Nutrient | Proximate Composition * (%) | ||
|---|---|---|---|
| PNAPMB | MSNAPMB | TNAPMB | |
| Moisture | 91.74 ± 0.10 a | 91.03 ± 0.04 b | 87.59 ± 0.06 c |
| Ash | 2.00 ± 1.55 a | 1.56 ± 0.67 a | 1.18 ± 0.49 a |
| Protein | 1.62 ± 1.62 a | 2.17 ± 0.02 a | 1.50 ± 1.17 b |
| Total fats | 0.92 ± 0.01 a | 0.65 ± 0.01 a | 1.54 ± 0.09 a |
| Saturated fats | 0.23 ± 0.00 a | 0.16 ± 0.02 a | 0.48 ± 0.01 a |
| Palmitic acid (C16) | 0.19 ± 0.01 | 0.14 ± 0.00 | 0.41 ± 0.03 |
| Stearic acid (C18) | 0.05 ± 0.00 | 0.12 ± 0.03 | 0.07 ± 0.02 |
| Monounsaturated fats | 0.24 ± 0.00 a | 0.17 ± 0.01 a | 0.45 ± 0.03 a |
| Oleic acid (C18: 1n9c) | 0.237 ± 0.00 | 0.17 ± 0.01 | 0.45 ± 0.03 |
| Polyunsaturated fats | 0.45 ± 0.00 a | 0.32 ± 0.01 b | 0.61 ± 0.05 a |
| Linolelaidic acid (C18: 2n6t) | 0.45 ± 0.00 | 0.32 ± 0.01 | 0.61 ± 0.05 |
| Total sugars | 5.06 ± 0.03 | 5.31 ± 0.02 | 6.11 ± 0.06 |
| Sucrose | 5.06 ± 0.03 a | 5.31 ± 0.02 b | 3.78 ± 0.08 a |
| Glucose | 0.00 | 0.00 | 2.05 ± 0.03 |
| Fructose | 0.00 | 0.00 | 0.28 ± 0.02 |
| Carbohydrates | 4.31 ± 1.42 a | 5.03 ± 0.66 a | 9.41 ± 0.39 b |
| Energy (kJ/100 mL) | 113.23 ± 25.36 a | 130.23 ± 12.61 a | 197.48 ± 8.07 b |
| Attribute | PNAPMB | MSNAPMB | TNAPMB |
|---|---|---|---|
| L * | 58.44 ± 0.05 a | 52.70 ± 0.07 b | 59.67 ± 0.02 c |
| a * | −1.03 ± 0.03 a | 0.64 ± 0.03 b | 2.45 ± 0.19 c |
| b * | 15.11 ± 0.02 a | 27.48 ± 0.03 b | 19.71 ± 0.05 c |
| Chroma (C *) | 15.14 ± 0.02 a | 27.49 ± 0.03 b | 19.86 ± 0.08 c |
| Hue (h *) | 93.91 ± 0.13 a | 88.66 ± 0.06 b | 82.91 ± 0.52 c |
| ∆E | 5.91 | 10.60 |
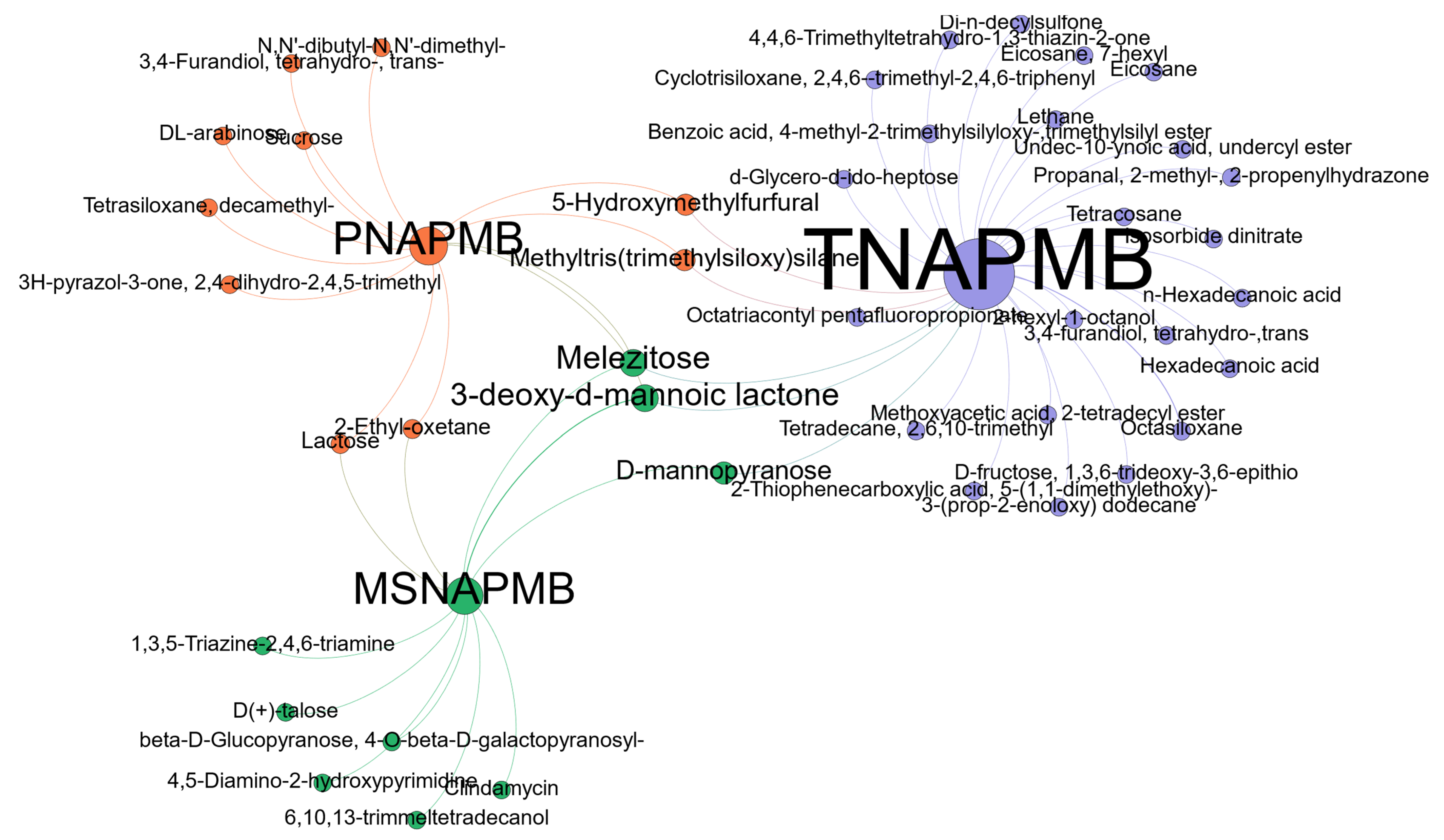
| Compound | Chemical Formula | Retention Time (min) | Molecular Weight | Category |
|---|---|---|---|---|
| 3H-pyrazol-3-one, 2,4-dihydro-2,4,5-trimethyl | C6H10N2O | 10.78 | 126.079 | alcohol |
| DL-arabinose | C5H10O5 | 12.80 | 150.053 | sugar |
| Melezitose | C18H32O16 | 15.37 | 504.169 | sugar |
| Lactose | C12H22O11 | 21.18 | 342.116 | sugar |
| 3-deoxy-d-mannoic lactone | C6H10O5 | 24.47 | 162.053 | Cyclic ester |
| 3,4-Furandiol, tetrahydro-, trans- | C4H8O3 | 12.76 | 104.047 | |
| 5-Hydroxymethylfurfural | C6H6O3 | 15.33 | 126.032 | Aldehyde |
| N,N’-dibutyl-N,N’-dimethyl- | 20.79 | 200.189 | ||
| 2-Ethyl-oxetane | C5H10O | 21.16 | 86.073 | |
| Sucrose | 32.25 | 342.116 | ||
| Tetrasiloxane, decamethyl- | C10H30O3Si4 | 42.18 | 310.127 | Organosilicon |
| Methyltris(trimethylsiloxy)silane | C10H30O3Si4 | 42.33 | 310.127 | Organosilicon |
| Clindamycin | C18H33ClO5S | 10.81 | 424.18 | |
| D-mannopyranose | C6H12O6 | 12.74 | 180.063 | |
| D(+)-talose | C6H12O6 | 12.80 | 180.063 | sugar |
| 6,10,13-trimmeltetradecanol | C17H36O | 18.55 | 256.277 | fatty alcohol |
| 4,5-Diamino-2-hydroxypyrimidine | C4H6N4O | 10.78 | 126.054 | |
| 1,3,5-Triazine-2,4,6-triamine | C3H6N6 | 16.20 | 126.065 | |
| beta-D-Glucopyranose, 4-O-beta-D-galactopyranosyl- | C12H22O11 | 32.24 | 342.116 | Sugar |
| 3,4-furandiol, tetrahydro-,trans | C4H8O3 | 13.08 | 104.047 | alcohol |
| Isosorbide dinitrate | C4H8N2O8 | 14.36 | 236.028 | |
| D-fructose, 1,3,6-trideoxy-3,6-epithio | C6H10O3S | 20.54 | 162.035 | sugar |
| Hexadecanoic acid | C16H33O2 | 29.51 | 256.24 | saturated fatty acid |
| 3-(prop-2-enoloxy) dodecane | C11H14O | 31.51 | 240.209 | alkeny |
| Tetradecane, 2,6,10-trimethyl | C17H36 | 33.21 | 336.303 | |
| Undec-10-ynoic acid, undercyl ester | C22H40O2 | 32.23 | 240.282 | Ester |
| Methoxyacetic acid, 2-tetradecyl ester | C17H34O3 | 33.21 | 286.251 | Ester |
| Octatriacontyl pentafluoropropionate | C41H77F5O2 | 34.39 | 697.0 | |
| 2-hexyl-1-octanol | C14H30O | 34.64 | 214.23 | fatty alcohol |
| Eicosane | C20H42 | 35.09 | 282.329 | alkane |
| Eicosane, 7-hexyl | C26H54 | 36.01 | 366.423 | |
| Octasiloxane | C16H50O7Si8 | 36.38 | 578.171 | |
| Di-n-decylsulfone | C20H42O2S | 36.77 | 346.291 | |
| Benzoic acid, 4-methyl-2-trimethylsilyloxy-,trimethylsilyl ester | C14H24O3Si2 | 38.06 | 296.126 | |
| Cyclotrisiloxane, 2,4,6--trimethyl-2,4,6-triphenyl | C21H24O3Si3 | 40.45 | 408.103 | |
| 4,4,6-Trimethyltetrahydro-1,3-thiazin-2-one | C7H13NOS | 12.72 | 159.072 | |
| 2-Thiophenecarboxylic acid, 5-(1,1-dimethylethoxy)- | C9H12O3S | 13.00 | 200.051 | Carboxylic acid |
| Propanal, 2-methyl-, 2-propenylhydrazone | C7H14N2 | 16.35 | 126.116 | N-alkylated hydrazones |
| Lethane | 20.59 | 203.098 | ||
| 3-Deoxy-d-mannoic lactone | C6H10O5 | 23.85 | 162.053 | |
| d-Glycero-d-ido-heptose | C7H14O7 | 26.10 | 210.074 | |
| n-Hexadecanoic acid | C16H32O | 29.52 | 256.24 | Fatty acid |
| Tetracosane | C24H50 | 34.39 | 338.391 | Alkane |
| Item | Frequency (Percentage) * |
|---|---|
| Gender | |
| Male | 38 (24) |
| Female | 106 (71) |
| No response | 8 (5) |
| Race | |
| Black | 126 (85) |
| Coloured | 15 (10) |
| White | 3 (2) |
| Other | 3 (2) |
| No response | 4 (1) |
| Status | |
| Staff | 24 (16) |
| Student | 123 (82) |
| No response | 3 (2) |
| Nationality | |
| National | 78 (52) |
| International | 36 (24) |
| No response | 36 (24) |
| Age group | |
| Less than or equal to 29 years | 115 (77) |
| 30–39 years | 15 (10) |
| 40 and above | 15 (10) |
| No response | 5 (3) |
| Non-Alcoholic Pearl Millet Beverage 1 | |||
|---|---|---|---|
| Sensory Attribute | Plain | Moringa Supplemented | Traditional |
| Appearance | 5.9 ± 2.5 b | 5.5 ± 2.0 b | 4.5 ± 2.5 a |
| Colour | 5.8 ± 2.3 b | 5.6 ± 2.0 a,b | 4.8 ± 2.3 a |
| Aroma | 5.3 ± 2.5 b | 4.7 ± 2.1 a,b | 4.2 ± 2.3 a |
| Taste | 5.3 ± 2.1 a | 4.9 ± 2.1 a | 5.44 ± 2.4 a |
| Mouthfeel | 6.0 ± 2.1 a | 5.8 ± 1.9 a | 6.0 ± 2.2 a |
| Overall acceptability | 5.8 ± 2.0 b | 4.9 ± 2.0 a | 5.4 ± 2.1 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jideani, V.A.; Ratau, M.A.; Okudoh, V.I. Leuconostoc mesenteroides and Pediococcus pentosaceus Non-Alcoholic Pearl Millet Beverage Enriched with Moringa oleifera Leaf Powder: Nutritional and Sensory Characteristics. Processes 2021, 9, 2125. https://doi.org/10.3390/pr9122125
Jideani VA, Ratau MA, Okudoh VI. Leuconostoc mesenteroides and Pediococcus pentosaceus Non-Alcoholic Pearl Millet Beverage Enriched with Moringa oleifera Leaf Powder: Nutritional and Sensory Characteristics. Processes. 2021; 9(12):2125. https://doi.org/10.3390/pr9122125
Chicago/Turabian StyleJideani, Victoria A., Mmaphuti A. Ratau, and Vincent I. Okudoh. 2021. "Leuconostoc mesenteroides and Pediococcus pentosaceus Non-Alcoholic Pearl Millet Beverage Enriched with Moringa oleifera Leaf Powder: Nutritional and Sensory Characteristics" Processes 9, no. 12: 2125. https://doi.org/10.3390/pr9122125
APA StyleJideani, V. A., Ratau, M. A., & Okudoh, V. I. (2021). Leuconostoc mesenteroides and Pediococcus pentosaceus Non-Alcoholic Pearl Millet Beverage Enriched with Moringa oleifera Leaf Powder: Nutritional and Sensory Characteristics. Processes, 9(12), 2125. https://doi.org/10.3390/pr9122125







