Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characteristics
2.3. Experimental Procedure
3. Results and Discussion
3.1. Feedstock Composition
3.2. Screening Sodium Additives
3.2.1. Comparison of Leaching Efficiencies for Different Sodium Additives
3.2.2. Comparison of Melting Points for Different Sodium Additives
3.3. Roasting Parameter Analysis
3.3.1. Roasting Temperature
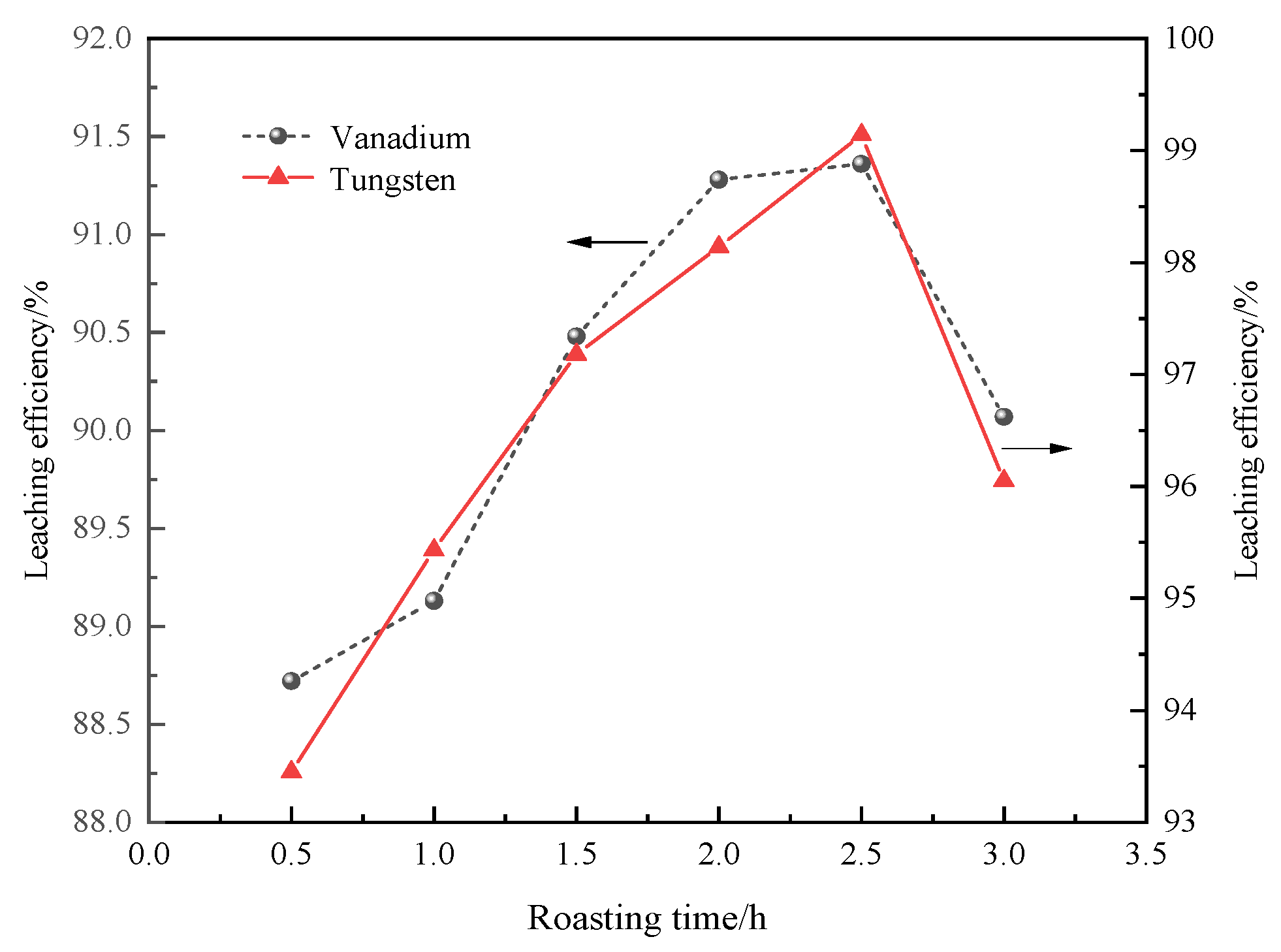
3.3.2. Roasting Time
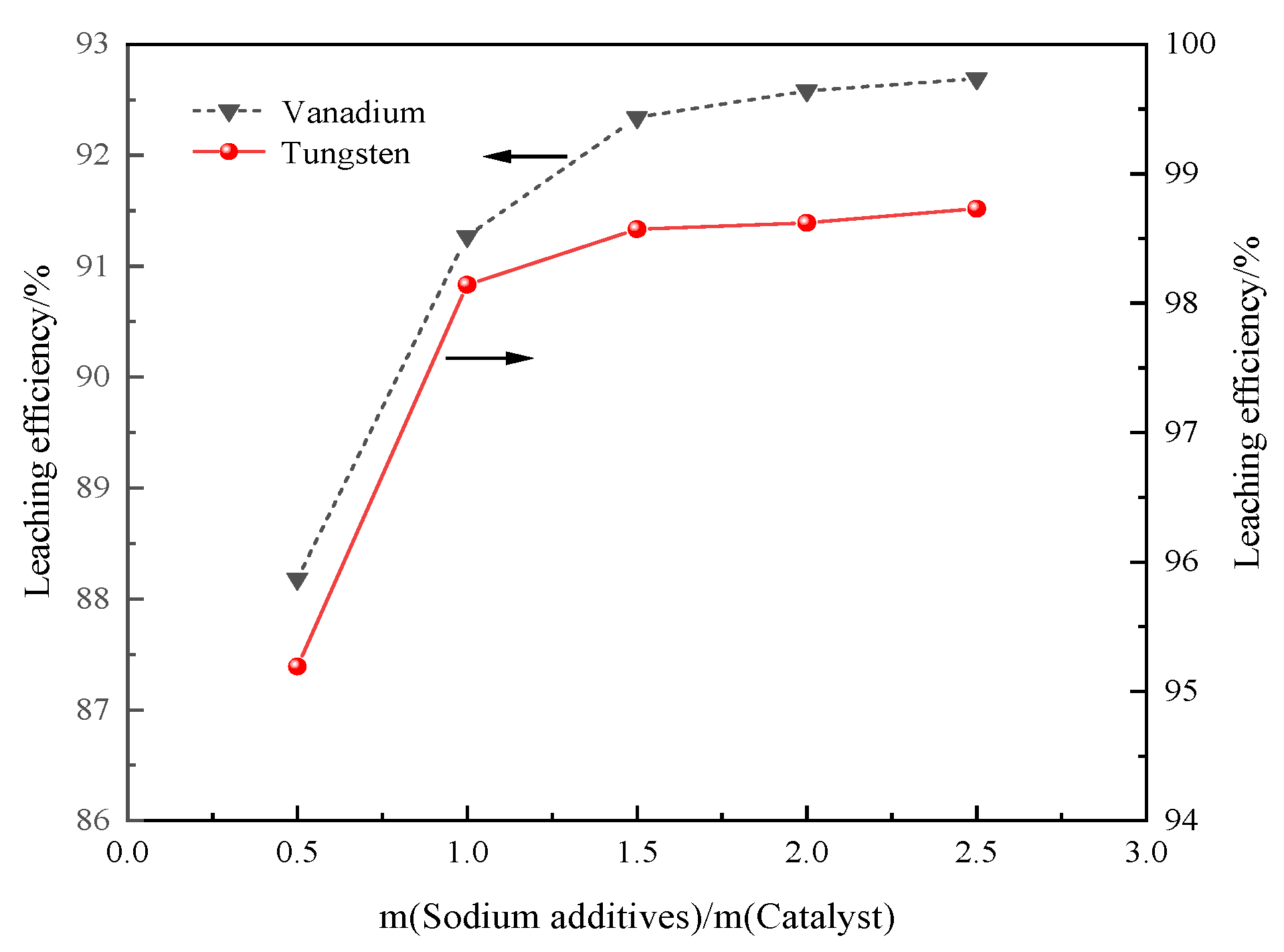
3.3.3. Mass Ratio of Sodium Additive and Catalyst
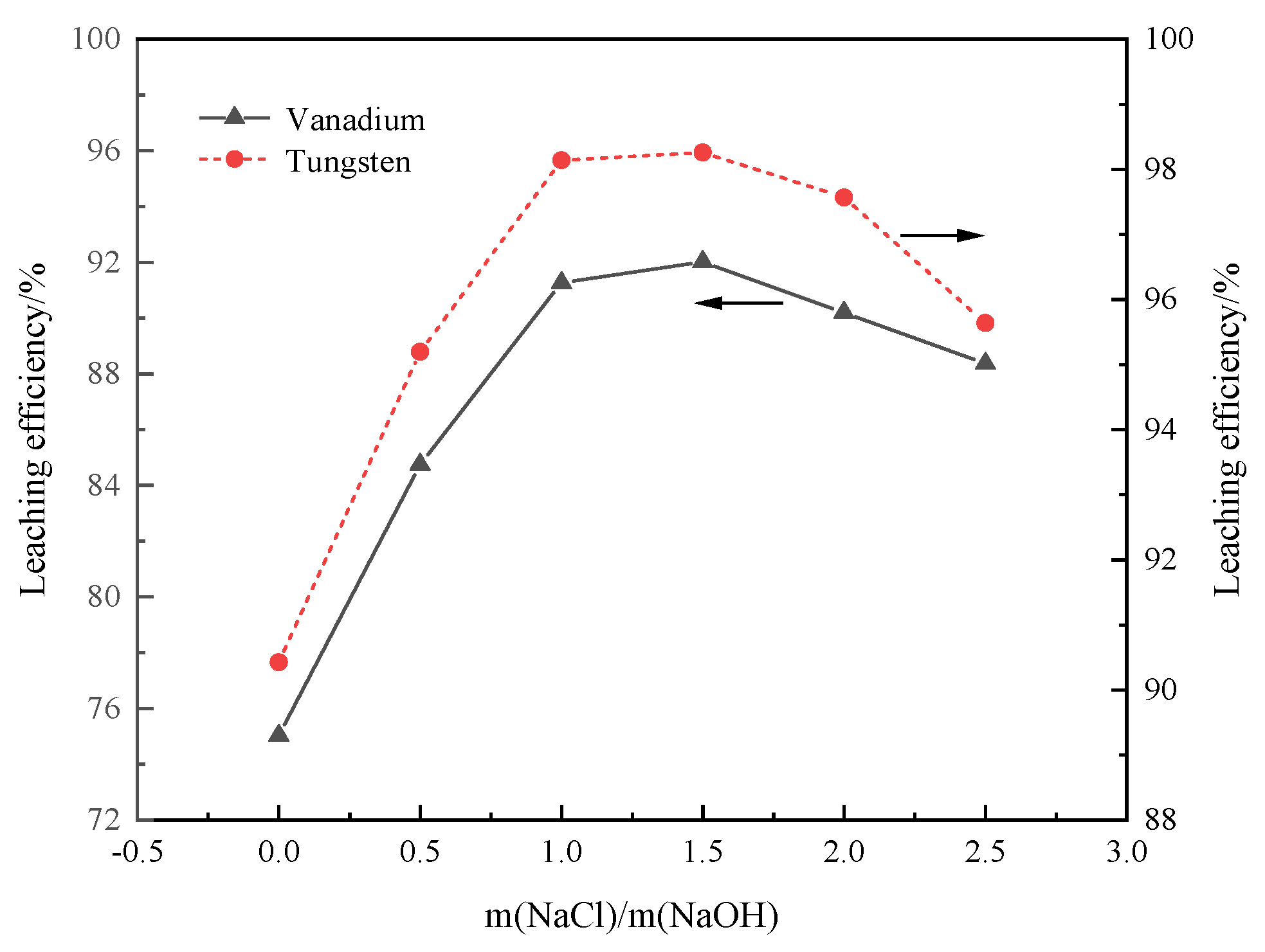
3.3.4. Mass Ratio of NaCl and NaOH
3.4. Orthogonal Experiment and Weight Matrix Analysis of Roasting Parameter
3.4.1. Orthogonal Experiment
3.4.2. Weight Matrix Analysis
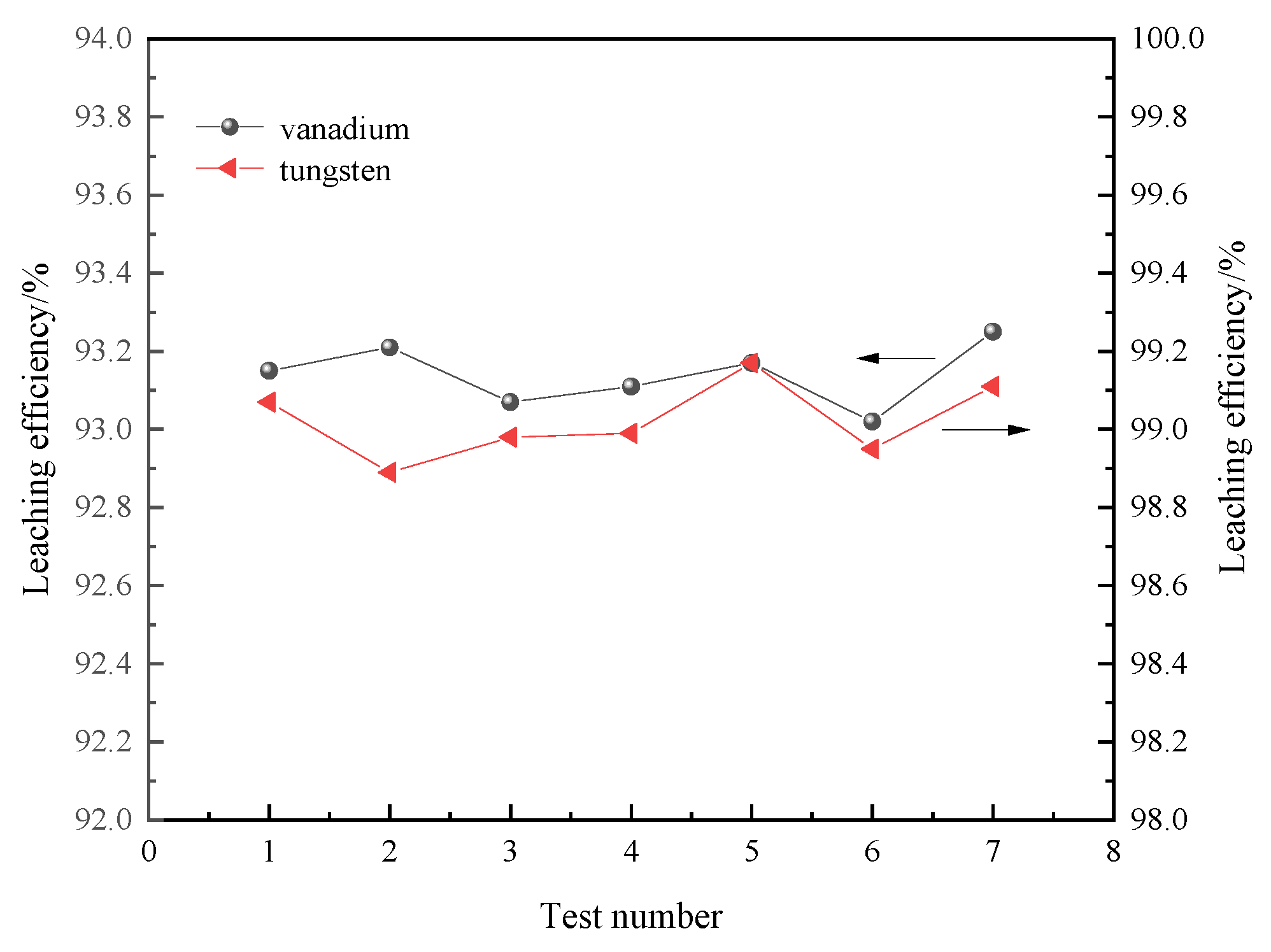
3.5. Repeated Test
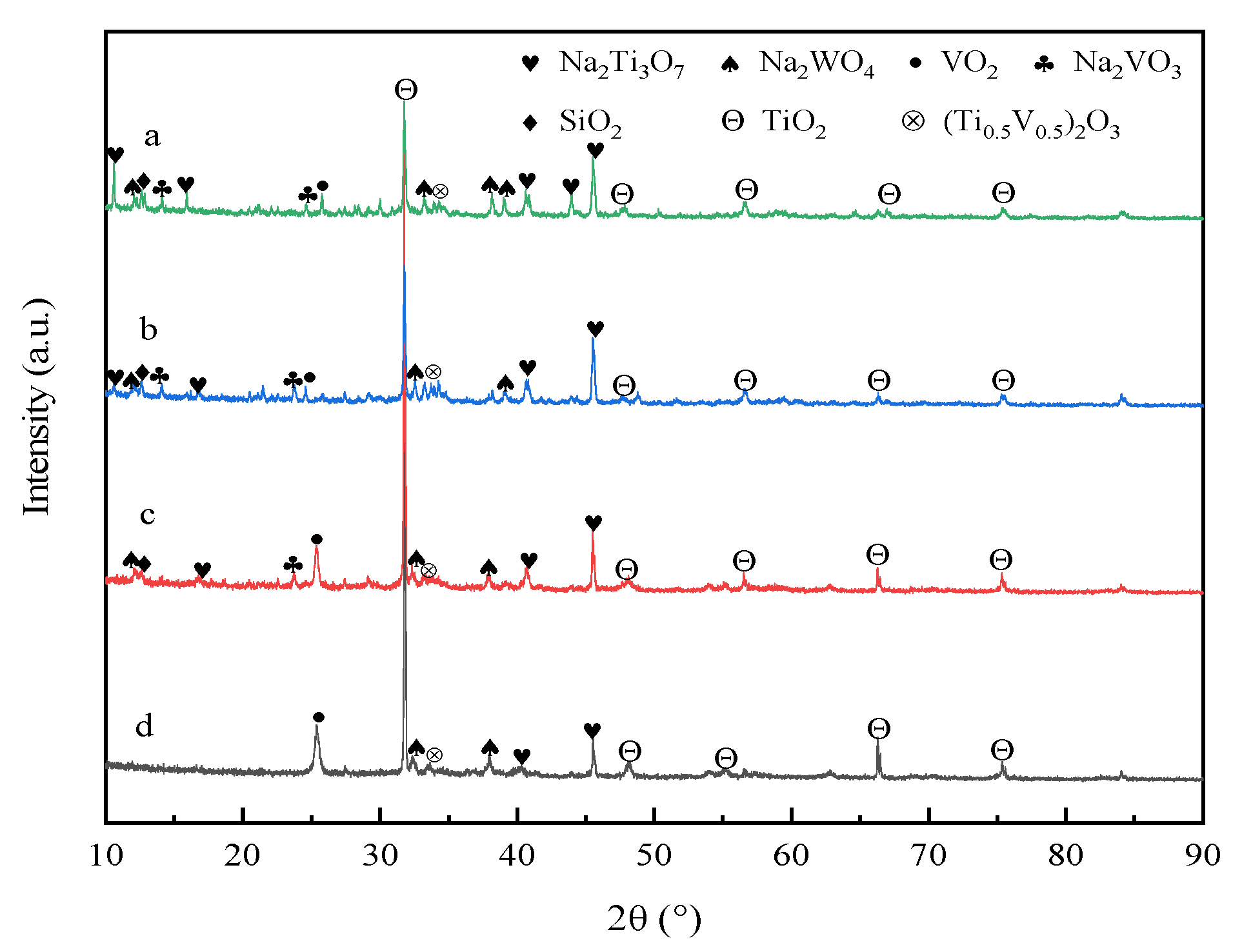
3.6. XRD Analysis of Roasted Clinker
3.7. Comparison of Vanadium and Tungsten Leaching Efficiencies for Different Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.M.; Sun, J.D.; Li, Q.S. Status and prospect of research on China’s energy structure optimization. Coal Eng. 2019, 51, 149–153. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2019&filename=MKSJ201902033&uniplaform=NZKPT&v=%25mmd2F9jPpLoXyCGxqu5sRrb8GaeO4eMIj0W5ub%25mmd2FnwDgahs6ZVXJwLX6fOoLZZw9oj5i (accessed on 3 October 2021).
- Zhang, T.Z. Discussion on the current situation and development trend of energy utilization in China. Territ. Nat. Resour. Study 2021, 5, 76–78. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2021&filename=GTZY202105020&uniplatform=NZKPT&v=gu9%25mmd2FHJ99I%25mmd2BIFv6IOvAE0z6MKFjO%25mmd2BrPNLJy%25mmd2FSeYJJoofDi0acvtjTqpmxVaNxn%25mmd2BNg (accessed on 3 October 2021).
- Nakajima, F.; Hamada, I. The state-of-the-art technology of NOx control. Catal. Today 1996, 29, 109–115. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A-Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B-Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Xie, X.H.; Hums, E.; Lu, J.D. Impact of the surface heterogeneity of commercial V2O5-WO3/TiO2 catalysts on the NH3-SCR-DeNOx reaction by kinetic modelling. Res. Chem. Intermediat. 2017, 43, 1409–1428. [Google Scholar] [CrossRef]
- Wu, W.F.; Wang, C.Y.; Bao, W.J.; Li, H.Q. Selective reduction leaching of vanadium and iron by oxalic acid from spent V2O5-WO3/TiO2 catalyst. Hydrometallurgy 2018, 179, 52–59. [Google Scholar] [CrossRef]
- Chen, C.M.; Jia, W.B.; Liu, S.T.; Cao, Y. Simultaneous NO removal and Hg0 oxidation over CuO doped V2O5-WO3/TiO2 catalysts in simulated coal-fired flue gas. Energy Fuels 2018, 32, 7025–7034. [Google Scholar] [CrossRef]
- Wang, X.M.; Du, X.S.; Zhang, L.; Chen, Y.R.; Yang, G.P.; Ran, J.Y. Promotion of NH4HSO4 decomposition in NO/NO2 contained atmosphere at low temperature over V2O5-WO3/TiO2 catalyst for NO reduction. Appl. Catal. A-Gen. 2018, 559, 112–121. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wu, Y.F.; Li, L.L.; Zuo, T. Sustainable approach for spent V2O5-WO3/TiO2 catalysts management: Selective recovery of heavy metal vanadium and production of value-added WO3-TiO2 photocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 12502–12510. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Wang, J.; Zhang, Y.; Zhang, Y.H.; Zhang, M.G.; Zhou, Y.F.; Phoutthavong, T.; Liang, P.; Zhang, H.W. Simultaneous removal of NO and Hg0 in flue gas over Co-Ce oxide modified rod-like MnO2 catalyst: Promoting effect of Co doping on activity and SO2 resistance. Fuel 2020, 276, 118018. [Google Scholar] [CrossRef]
- Lin, H.D.; Wang, Z.Y.; Song, B.H. Discussion about resource utilization for disabled SCR catalyst. Shandong Chem. Ind. 2013, 42, 8–10. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2013&filename=SDHG201304002&uniplatform=NZKPT&v=T%25mmd2BbgDMy%25mmd2FDMsL%25mmd2Fgvwstb9%25mmd2BDYTqBjkR%25mmd2Fb2Rds1cpZJoIYIp7OfD7xtcTS5rqkfduu (accessed on 3 October 2021).
- Qi, C.P.; Bao, W.J.; Wang, L.G.; Li, H.Q.; Wu, W.F. Study of the V2O5-WO3/TiO2 catalyst synthesized from waste catalyst on selective catalytic reduction of NOx by NH3. Catalysts 2017, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.H.; Moon, G.; Lee, J.Y.; Jyothi, R.K. Extraction of tungsten and vanadium from spent selective catalytic reduction catalyst for stationary application by pressure leaching process. J. Clean. Prod. 2018, 197, 163–169. [Google Scholar] [CrossRef]
- Cao, Y.J.; Yuan, J.F.; Du, H.; David, D.; Meng, L. A clean and efficient approach for recovery of vanadium and tungsten from spent SCR catalyst. Miner. Eng. 2021, 165, 106857. [Google Scholar] [CrossRef]
- Yao, J.X.; Cao, Y.B.; Wang, J.C.; Zhang, C.M.; Wang, W.J.; Bao, W.R.; Chang, L.P. Successive calcination-oxalate acid leaching treatment of spent SCR catalyst: A highly efficient and selective method for recycling tungsten element. Hydrometallurgy 2021, 201, 105576. [Google Scholar] [CrossRef]
- Gomez-Bueno, C.O.; Spink, D.R.; Rempel, G.L. Extraction of vanadium from Athabasca tar sands fly ash. Metall. Mater. Trans. B 1981, 12, 341–352. [Google Scholar] [CrossRef]
- Hairunnisha, S.; Sendil, G.K.; Rethinaraj, J.P.; Srinivasan, G.N.; Adaikkalam, P.; Kulandaisamy, S. Studies on the preparation of pure ammonium para tungstate from tungsten alloy scrap. Hydrometallurgy 2000, 85, 67–71. [Google Scholar] [CrossRef]
- Su, Q.F.; Miao, J.F.; Li, H.R.; Chen, Y.T.; Chen, J.S.; Wang, J.X. Optimizing vanadium and tungsten leaching with lowered silicon from spent SCR catalyst by pre-mixing treatment. Hydrometallurgy 2018, 181, 230–239. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhao, B.B.; Li, L.J.; Dong, Z.H.; Bai, R.G.; Wang, R.G. Study on selective separation of vanadium, titanium and tungsten from spent SCR denitration catalyst. Iron Steel Vanadium Titan. 2021, 42, 24–31. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2021&filename=GTFT202101004&uniplatforNZKPT&v=6b5uk1PPEnGbCaJk8vOr0giF55IRKLuVW21eC%25mmd2BDXHM3C9p5VuBqHXTLSBLdaAhw2 (accessed on 3 October 2021).
- Wu, W.F.; Li, H.Q.; Meng, Z.H.; Wang, C.Y.; Wang, X.R.; Zhao, C. Recovery of TiO2 from spent SCR denitration catalyst by alkali hydrothermal method. Chin. J. Pro. Eng. 2019, 19, 73–80. [Google Scholar]
- Wu, W.C.; Tsai, T.Y.; Shen, Y.W. Tungsten recovery from spent SCR catalyst using alkaline leaching and ion Exchange. Minerals 2016, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Huo, Y.T.; Chang, Z.D.; Li, W.J.; Liu, S.X.; Dong, B. Reuse and Valorization of Vanadium and Tungsten from waste V2O5-WO3/TiO2 catalyst. Waste Biomass Valori. 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, W.G.; Hwang, I.S.; Li, J.Y.; Han, C. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching. J. Ind. Eng. Chem. 2015, 28, 73–77. [Google Scholar] [CrossRef]
- Li, L.C.; Wang, L.; Zhao, X.J.; Qian, Q.; Song, S.S.; Li, X.B. Comparison of effect of different acid treatments on vanadium extraction of waste deNOx catalyst. Chin. J. Nonferrous Met. 2016, 26, 2230–2237. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2017&filename=ZYXZ201610024&uniplatform=NZKPT&=C%25mmd2Fw%25md2BSqYU3uPoKFK9YVKqsnJ2WPyTq8Z%25mmd2BNlnrdjycdTdu2fWHdHGiVyVkXERbBYBA (accessed on 3 October 2021).
- Zhang, B.B.; Yu, D.D.; Wang, F.; Li, J.F. Technology of vanadium recovery from deactivated denitration catalyst. Henan Sci. 2016, 34, 866–870. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2016&filename=HNKX201606008&uniplform=NZKPT&v=9PpMMjOEE8AtnHr%25mmd2F3VL5A47mj%25mmd2Fsy0j35kBJ57gnfSD4fnEaiRteWIspMlE5OQnH (accessed on 3 October 2021).
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. [Google Scholar] [CrossRef]
- Jia, X.M.; Chen, T.B.; Huang, Y.; Liu, H.W.; Liu, Z.C.; Li, P.Y.; Xiang, Q.Y. Recovery of titanium from spent SCR catalyst by sodium roasting. Iron Steel Vanadium Titan. 2020, 41, 1–5. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2021&filename=GTFT202006001&uniplatform=NZKPT&v=NysXK98p02GMZwqjFBCxL8NEI6qrs%25mmd2F7eeGDYqBsw6DD6451EBxdB5ptfbisyPjg (accessed on 3 October 2021).
- Zhang, Q.; Wu, Y.; Zuo, T. Green recovery of titanium and effective regeneration of TiO2 photocatalysts from spent selective catalytic reduction catalysts. ACS Sustain. Chem. Eng. 2018, 6, 3091–3101. [Google Scholar] [CrossRef]
- Choi, I.H.; Kim, H.R.; Moon, G.; Jyothi, R.K.; Lee, J.Y. Spent V2O5-WO3/TiO2 catalyst processing for valuable metals by soda roasting-water leaching. Hydrometallurgy 2018, 175, 292–299. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wu, Y.F.; Zuo, T.Y. Titanium extraction from spent selective catalytic reduction catalysts in a NaOH molten-salt system: Thermodynamic, experimental, and kinetic studies. Metall. Mater. Trans. B 2019, 50, 471–479. [Google Scholar] [CrossRef]
- Zhou, K.; Lu, B.; Wang, S.; Zhang, Y.P.; Teng, Y.T.; Li, J. Research on recovery process of Ti, V and W in waste SCR denitration catalyst. Electr. Power Technol. Environ. Prot. 2019, 35, 8–13. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2019&filename=DLHB201904003&uniplaform=NZKPT&v=hDR1hzvBV8Z8XIzWK52p%25mmd2FF%25mmd2B821J7h4uJDDm7pLyIMVumocJkwcS2w6SoKr2Qw1Os (accessed on 3 October 2021).
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.S.; Cho, B.K.; Choung, J.W.; Cha, M.S.; Yeo, G.K. Mn-Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B-Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Teng, Y.T.; Lu, B.; Zhuang, K.; Wang, S.; Liu, Y.; Wang, J.J. Regeneration treatment technology of deactivated vanadium tungsten and titanium denitration catalysts. J. Chin. Ceram. Soc. 2019, 4, 440–449. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2019&filename=GXYB201904004&uniplatform=NZKPT&v=5TPPS%25mmd2BRsKqGdo9d6nN4vsXd52TdOMjFlyvwZRzyarLkoGq7RTkSvsQzGH%25mmd2B8j9qv7 (accessed on 3 October 2021).
- Xu, G.Z. Studies on the role of sodium chloride in extracting vanadium pentoxide from the stone-like coal. Min. Metall. Eng. 1988, 8, 44–47. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD8589&filename=KYGC198804010&uniplatform=NZKPT&v=DX9m9z5pxxpU9fDDgECNXTBbEfNp3SgwKSM0G2DukYDWbgSZhHCLVOAIrxPvQiNX (accessed on 3 October 2021).
- Shi, L.; Wang, J.; Xie, J.H. Technology on vanadium extraction from bone coal by adding sodium chloride. Min. Metall. Eng. 2008, 28, 58–61. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2008&filename=KYGC200801017&uniplatform=NZKPT&v=MBqbS4KD4BsAwm74XvRPBT6VxBY75f2nKf4h0w8ejVKBllsRniK0ac0ayHpk0ctK (accessed on 3 October 2021).
- Wang, M.Y.; Wang, X.W.; Shen, J.F.; Wu, R.N. Extraction of vanadium from stone coal by modified salt-roasting process. J. Cent. South Univ. Technol. 2011, 18, 1940–1944. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Zhang, H.; Cui, L.; Yu, J.; Xu, G. Extraction of vanadium from stone coal by roasting in a fluidized bed reactor. Fuel 2015, 142, 180–188. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetites and other sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Wei, X.L.; Xue, B.J.; Zhao, Q. Optimization design of the stability for the plunger assembly of oil pumps based on multi-target orthogonal test design. J. Hebei Univ. Eng. (Nat. Sci. Ed.) 2010, 27, 95–99. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2010&filename=HJXU201003025&uniplatform=NZKPT&v=jqY8ef3dJlQ%25mmd2Bvn4qD0jt2zXEnL%25mmd2B3ulC7474UNO9Wjlc71aHnJfUBdJyAExVgqw9A (accessed on 3 October 2021).
- Yao, J.L.; Zhang, X.; Zhou, Z.; Liu, B.; Pang, Z.B. Optimization of process parameters of TiO2 photocatalytic oxidation coupled ceramic ultrafiltration membrane by matrix analysis. Autom. Instrum. 2018, 1, 107–110. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2018&filename=ZDYY201801030&uniplatform=NZKPT&v=E8ZnB%25mmd2FliCxtoW1KY9h7eEtlD%25mmd2Fu9LWd16zxEepguHVxB4eaZokJmCiFNFV0%25mmd2BreRhl (accessed on 3 October 2021).
- Cao, C.F.; Pang, Z.S.; Yuan, Z.Z.; Wang, R.X.; Nie, H.P.; Li, L.C. Study on the decomposition of spent SCR catalyst by Na2CO3-NaCl mixed roasting method. Nonferrous Met. Sci. Eng. 2021, 12, 63–69. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2021&filename=JXYS202103008&uniplatform=NZKPT&v=ZbacDTHDzC6x%25mmd2BwATs0f93MfusdaJFO718SoeB8NwuiQqemXS%25mmd2FaWOnSAS8%25mmd2Fn39bUy (accessed on 3 October 2021).
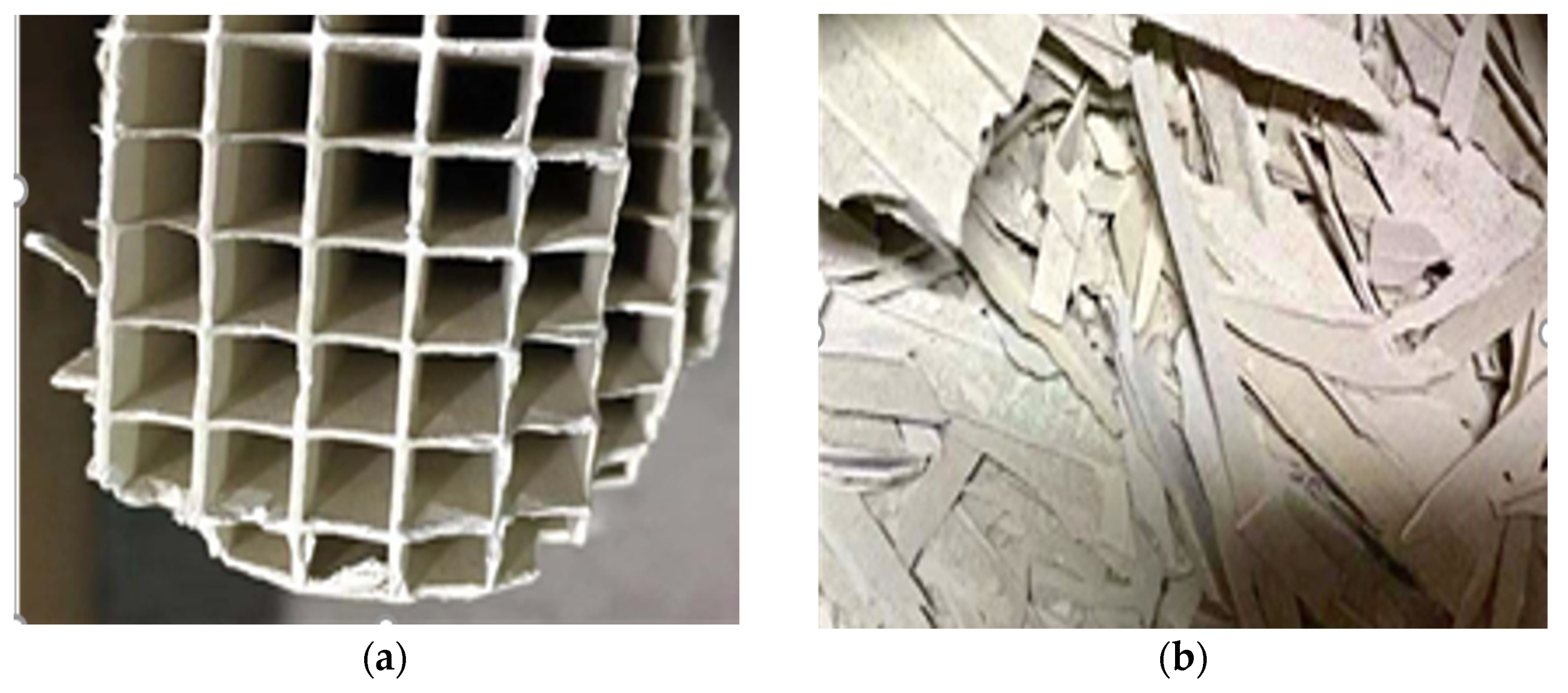
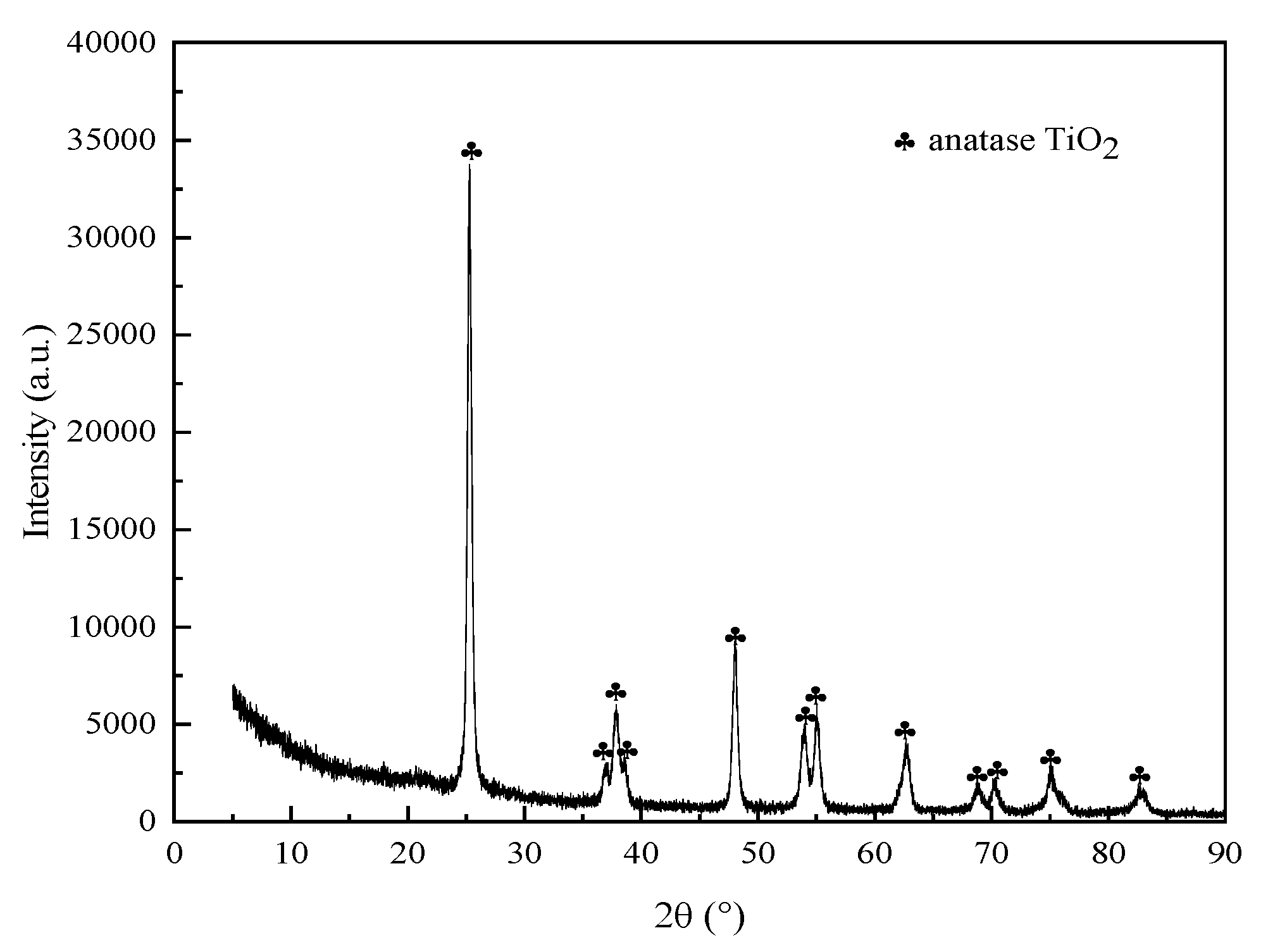
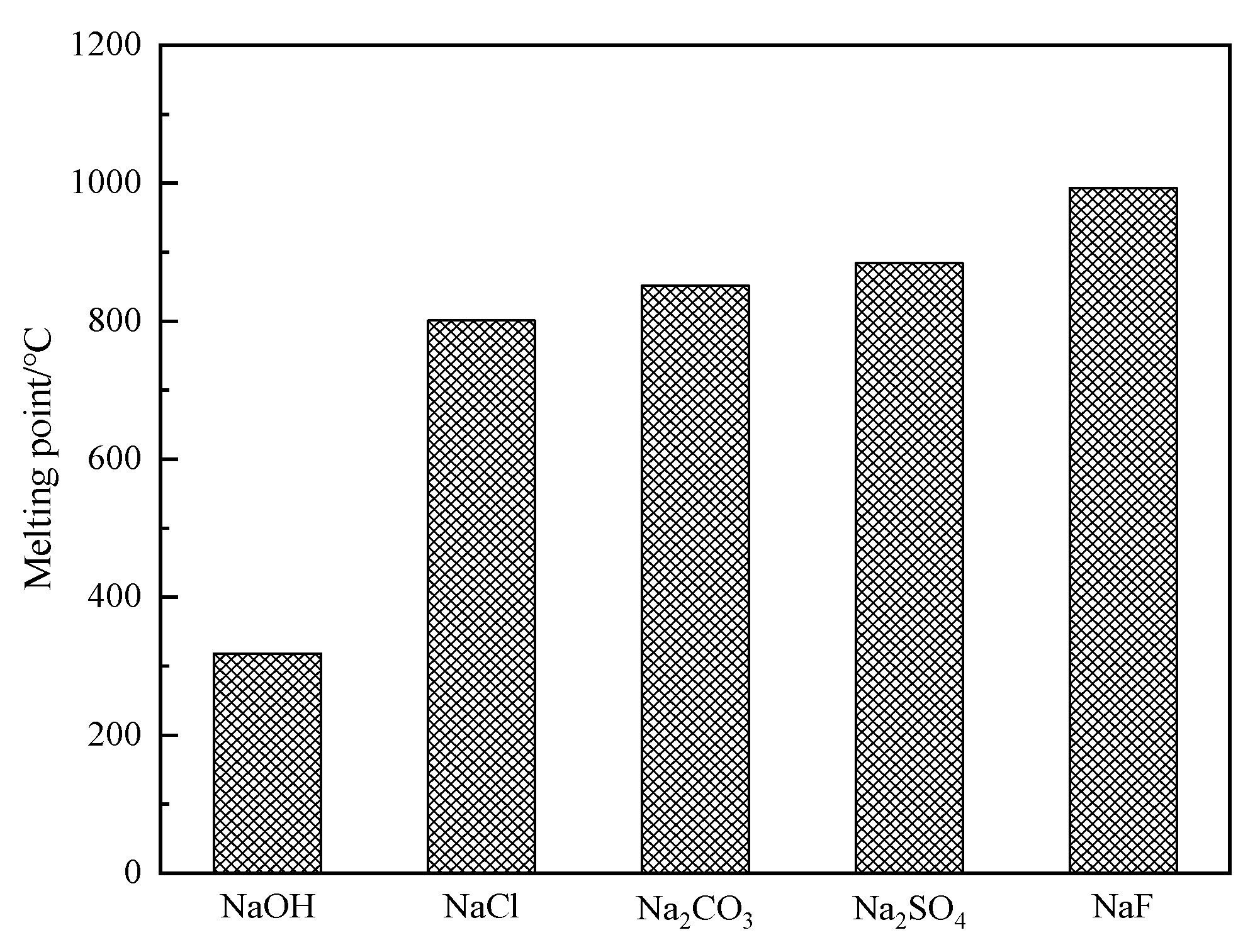
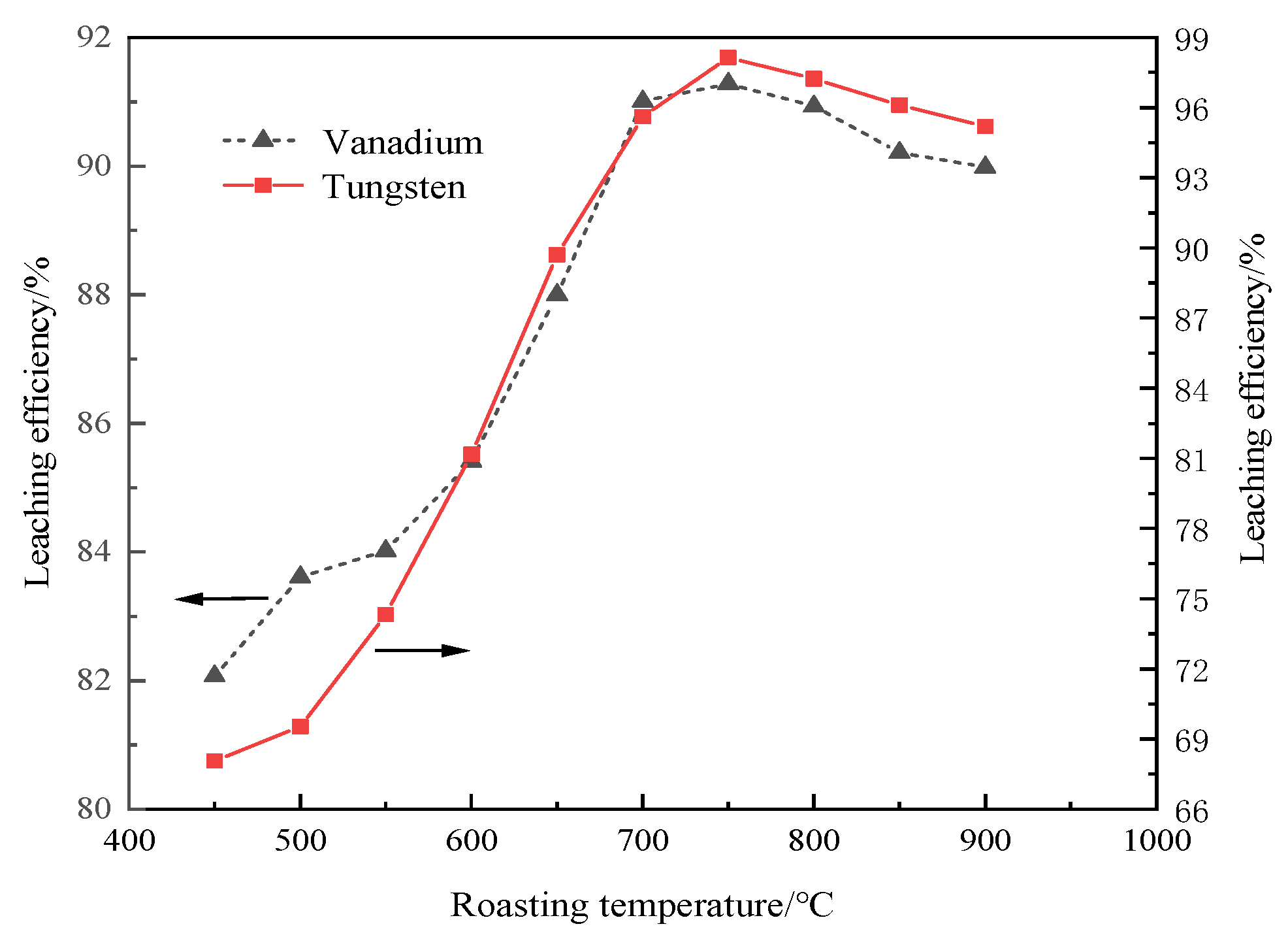
| Composition | TiO2 | SiO2 | WO3 | CaO | Al2O3 | V2O5 | SO3 | Others |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 81.46 | 7.37 | 4.75 | 2.11 | 1.68 | 0.82 | 0.67 | 1.15 |
| Sodium Additive | Leaching Efficiency of V/% | Leaching Efficiency of W/% |
|---|---|---|
| NaOH | 75.04 | 94.43 |
| NaCl | 76.51 | 19.01 |
| Na2SO4 | 76.30 | 17.24 |
| Na2CO3 | 73.25 | 90 |
| NaOH & Na2CO3 | 93.25 | 84.75 |
| NaOH & NaCl | 91.39 | 98.26 |
| NaOH & Na2SO4 | 88.27 | 95.88 |
| Standard Order | Roasting Temperature/°C (A) | Roasting Time/h (B) | m(Sodium Additives) /m(Catalyst) (C) | m(NaCl) /m(NaOH) (D) | Leaching Efficiency of Vanadium/% |
|---|---|---|---|---|---|
| 1 | 700 | 1.5 | 1.5 | 1.0 | 89.78 |
| 2 | 700 | 2.0 | 2.0 | 1.5 | 91.06 |
| 3 | 700 | 2.5 | 2.5 | 2.0 | 91.16 |
| 4 | 750 | 1.5 | 2.0 | 2.0 | 92.88 |
| 5 | 750 | 2.0 | 2.5 | 1.0 | 92.65 |
| 6 | 750 | 2.5 | 1.5 | 1.5 | 92.92 |
| 7 | 800 | 1.5 | 2.5 | 1.5 | 92.32 |
| 8 | 800 | 2.0 | 1.5 | 2.0 | 92.24 |
| 9 | 800 | 2.5 | 2.0 | 1.0 | 91.88 |
| k1 | 90.67 | 91.66 | 91.65 | 91.44 | |
| k2 | 92.82 | 91.98 | 91.94 | 92.10 | |
| k3 | 92.15 | 91.99 | 92.04 | 92.09 | |
| R | 2.15 | 0.33 | 0.40 | 0.66 |
| Standard Order | Roasting Temperature/°C (A) | Roasting Time/h (B) | m(Sodium Additives) /m(Catalyst) (C) | m(NaCl) /m(NaOH) (D) | Leaching Efficiency of Tungsten/% |
|---|---|---|---|---|---|
| 1 | 700 | 1.5 | 1.5 | 1.0 | 93.81 |
| 2 | 700 | 2.0 | 2.0 | 1.5 | 95.18 |
| 3 | 700 | 2.5 | 2.5 | 2.0 | 95.22 |
| 4 | 750 | 1.5 | 2.0 | 2.0 | 97.59 |
| 5 | 750 | 2.0 | 2.5 | 1.0 | 97.01 |
| 6 | 750 | 2.5 | 1.5 | 1.5 | 98.21 |
| 7 | 800 | 1.5 | 2.5 | 1.5 | 97.25 |
| 8 | 800 | 2.0 | 1.5 | 2.0 | 97.15 |
| 9 | 800 | 2.5 | 2.0 | 1.0 | 97.07 |
| k1 | 94.74 | 96.22 | 96.39 | 95.96 | |
| k2 | 97.60 | 96.45 | 96.61 | 96.88 | |
| k3 | 97.16 | 96.83 | 96.50 | 96.66 | |
| R | 2.87 | 0.62 | 0.22 | 0.92 |
| Test Index Layer | Test Index | |||
|---|---|---|---|---|
| Factor layer | Factor H1 | Factor H2 | … | Factor Hh |
| Level layer | H11H12H13H1m | H21H22H2m | … | Hh1Hh2Hhm |
| No. | Process | Roasting Conditions | Leaching Conditions | Leaching Efficiency of V/% | Leaching Efficiency of W/% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Na2CO3 roasting and water leaching | 900 °C, 2.0 h, and 30 wt.% Na2CO3 | 3:1 L/S mass ratio, 40 °C and 3.0 h | 49.05 | 99.02 | [32] |
| 2 | Na2CO3-NaCl roasting and water leaching | 750 °C, 2.0 h, 0.5:1.1 NaCl/Na2CO3 mole ratio and 1.2 Na2CO3/ catalyst mole ratio | 8:1 L/S mass ratio, 40 °C and 1.0 h | none | 99.10 | [42] |
| 3 | NaOH-NaCl roasting and water leaching | 750 °C, 2.5 h, 1.5 NaCl/Na2CO3 mass ratio and 2.5 additives/catalyst mass ratio | 6:1 L/S mass ratio, 90 °C and 1.0 h | 93.25 | 99.17 | this work |
| 4 | NaOH directly leaching | none | 3 M, 2.5 mL/g L/S ratio, 250 °C, and 2.0 h | 92 | 87 | [14] |
| 5 | NaOH directly leaching | none | 3% pulp density 0.3 L/S mass ratio, 90 °C, and 30 min | 87 | 91 | [22] |
| 6 | (NH4)2CO3 leaching | none | 3.0 M (NH4)2CO3, 1.5 M H2O2, 25:1 L/S ratio 70 °C, and 30 min | 98 | 99 | [15] |
| 7 | H2SO4 leaching | none | 45 wt.% with Na2SO3, 12 mL/g L/S ratio, 100 °C, and 180 min | 85 | none | [26] |
| 8 | H2C2O2 leaching | none | 20 mL/g L/S ratio 1.0 M, 90 °C, and 180 min | 84.22 | none | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yang, Q. Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting. Processes 2021, 9, 1923. https://doi.org/10.3390/pr9111923
Wang B, Yang Q. Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting. Processes. 2021; 9(11):1923. https://doi.org/10.3390/pr9111923
Chicago/Turabian StyleWang, Bo, and Qiaowen Yang. 2021. "Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting" Processes 9, no. 11: 1923. https://doi.org/10.3390/pr9111923
APA StyleWang, B., & Yang, Q. (2021). Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting. Processes, 9(11), 1923. https://doi.org/10.3390/pr9111923





