Best Conditions for the Production of Natural Isopentyl Acetate (Banana Aroma) from Cheese Industry Waste: An Experimental Precursor Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain
2.2. Whey Preparation
2.3. Isopentyl Acetate Production
2.4. Monitoring Process
2.5. 1H-NMR
2.6. Optical Rotation
2.7. Mathematical Modeling
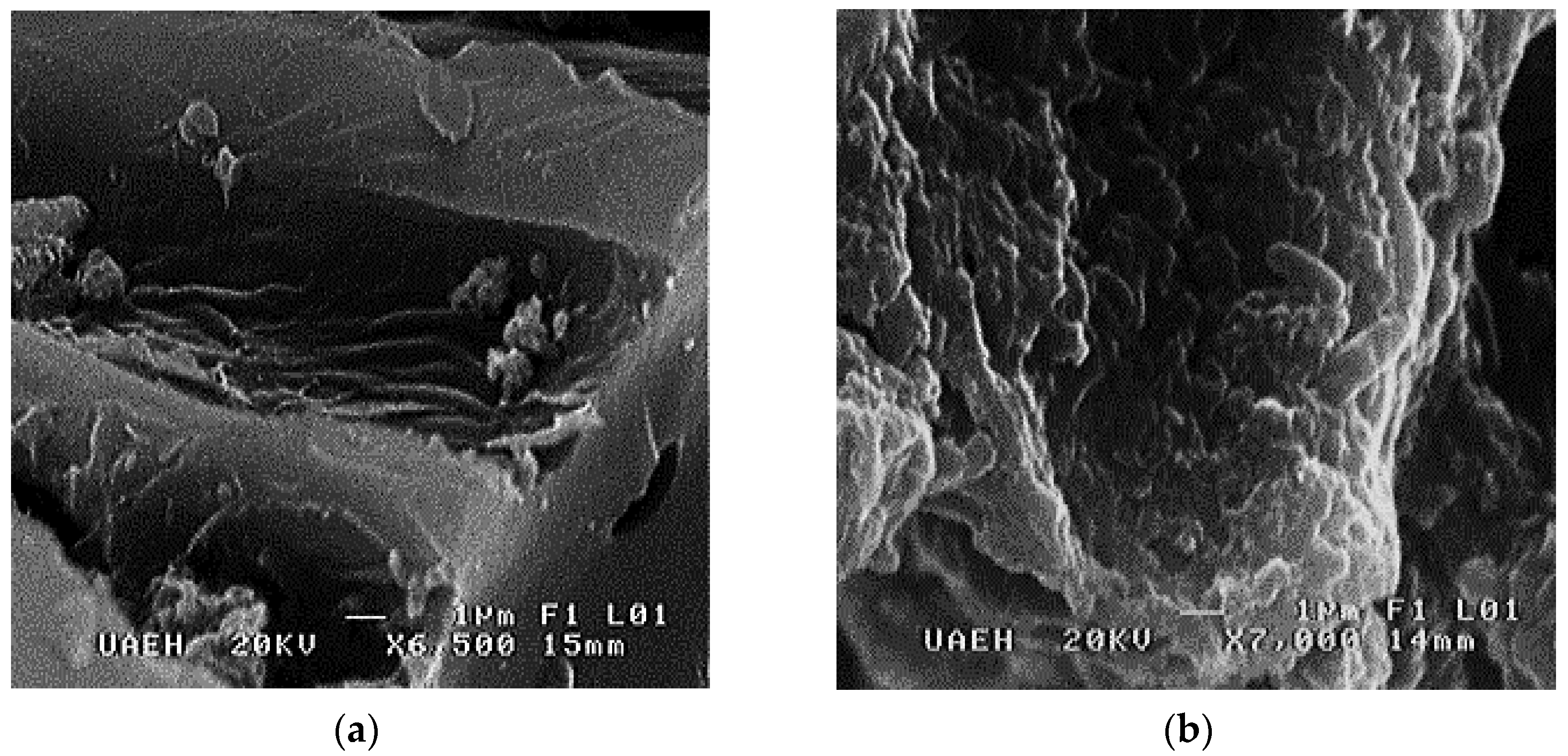
2.8. Scanning Electron Microscopy (SEM)
2.9. Statistical Analysis
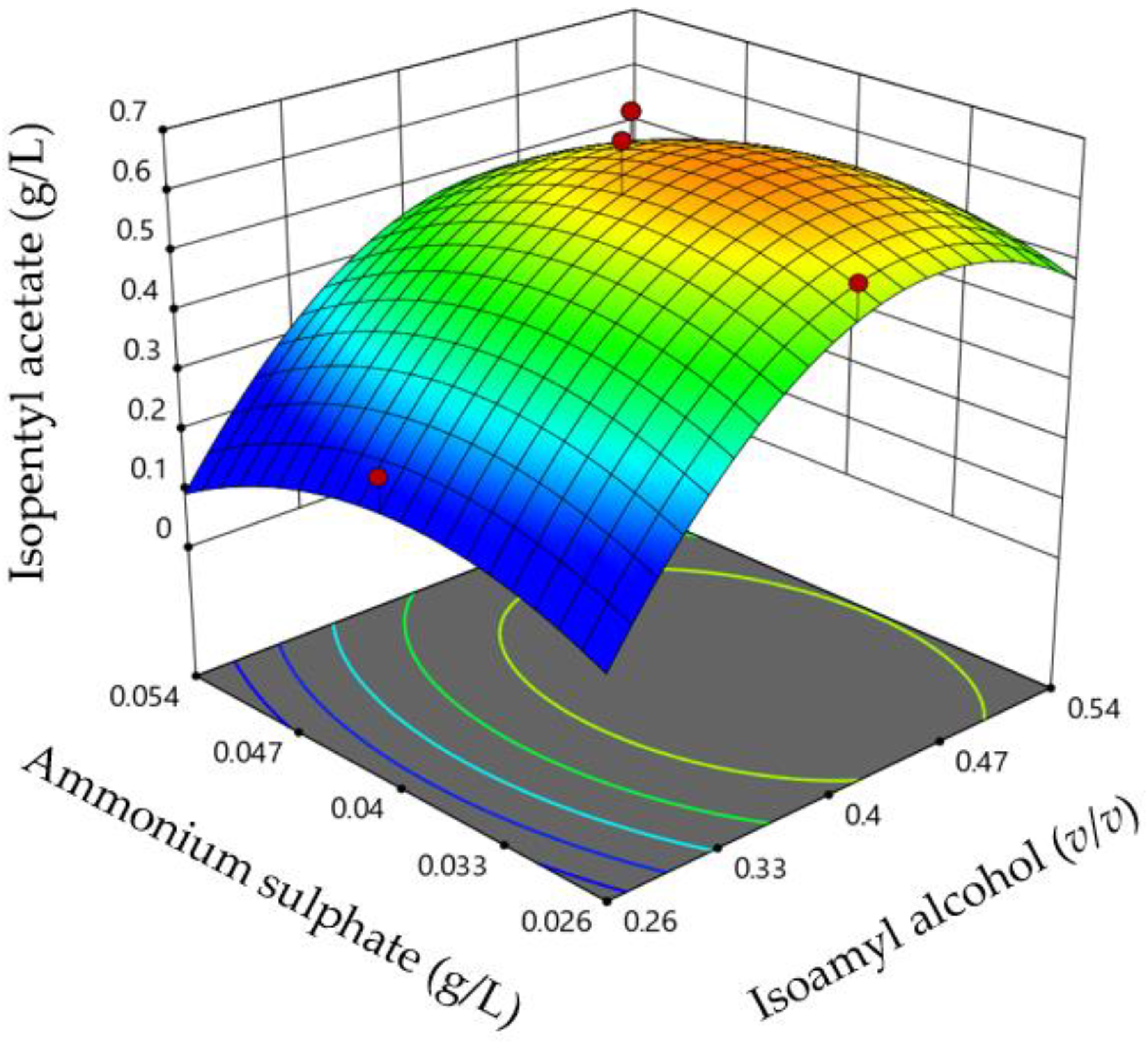
| Run | Isoamyl Alcohol (X1) | Ammonium Sulphate (X2) | Pmaxa | (g/L) | rpb | (g/L h) | ||
|---|---|---|---|---|---|---|---|---|
| Actual (v/v) | Code | Actual (g/L) | Code | Observed | Predicted | Actual (v/v) | ||
| 1 | 0.4 | 0 | 0.04 | 0 | 7.73 | 7.64 | 0.59 | 0.60 |
| 2 | 0.4 | 0 | 0.054 | 1414 | 4.68 | 5.96 | 0.42 | 0.46 |
| 3 | 0.3 | −1 | 0.05 | 1 | 8.02 | 7.14 | 0.30 | 0.31 |
| 4 | 0.3 | −1 | 0.03 | −1 | 9.95 | 9.52 | 0.26 | 0.34 |
| 5 | 0.4 | 0 | 0.04 | 0 | 8.12 | 7.64 | 0.56 | 0.60 |
| 6 | 0.5 | 1 | 0.03 | −1 | 8.57 | 8.14 | 0.57 | 0.58 |
| 7 | 0.4 | 0 | 0.026 | 1414 | 8.37 | 9.32 | 0.59 | 0.53 |
| 8 | 0.4 | 0 | 0.04 | 0 | 7.65 | 7.64 | 0.69 | 0.60 |
| 9 | 0.4 | 0 | 0.04 | 0 | 7.96 | 7.64 | 0.57 | 0.60 |
| 10 | 0.5 | 1 | 0.05 | 1 | 6.22 | 5.76 | 0.58 | 0.51 |
| 11 | 0.26 | −1.414 | 0.04 | 0 | 7.47 | 8.62 | 0.28 | 0.22 |
| 12 | 0.4 | 0 | 0.04 | 0 | 8.76 | 7.64 | 0.60 | 0.60 |
| 13 | 0.54 | 1.414 | 0.04 | 0 | 5.79 | 6.66 | 0.48 | 0.53 |
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asunis, F.; Giorgia, G.; Paolo, D.; Marco, I.; Piet, N.; Aldo, M.; Alessandra, P.; Raffaella, P.; Andreina, R.; Daniela, S. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111–240. [Google Scholar] [CrossRef]
- Dessı, P.; Asunis, F.; Harish, R.; Francesco, G.; Cocco, G.; Aldo, M.; Piet, N.; Lens, L. Fermentative hydrogen production from cheese whey with in-line, concentration gradient-driven butyric acid extraction. Int. J. Hydrog. Energy 2020, 45, 24453–24466. [Google Scholar] [CrossRef]
- Guo, D.; Sijia, K.; Hong, P.; Xiaoyun, L.; Xun, L. De novo biosynthesis of 2-phenylethanol in engineered Pichia pastoris. Enzym. Microb. Technol. 2020, 133, 10941–10959. [Google Scholar]
- Paulino, B.N.; Sales, A.; Felipe, L.; Pastore, G.M.; Molina, G.; Bicas, J.L. Recent advances in the microbial and enzymatic production of aroma compounds. Curr. Opin. Food Sci. 2021, 37, 98–106. [Google Scholar] [CrossRef]
- Conde, B.L.; Castro, R.J.; Villagómez, I.J.R.; Páez, L.J.B.; Gómez, A.C. Evaluation of waste of the cheese industry for the production of aroma of roses (phenylethyl alcohol). Waste Biomass Valorization 2016, 8, 1343–1350. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by enterobacter aerogenes 2822. Int. J. Hydrog. Energy 2021, 46, 1777–1800. [Google Scholar] [CrossRef]
- Soto, I.N.; Araiz, C.O.; Martínez, L.E.; Barragán, P.A.; Ochoa, S.H. Solid/gas biocatalysis for aroma production: An alternative process of white biotechnology. Biochem. Eng. J. 2020, 164, 107767. [Google Scholar] [CrossRef]
- Azudin, N.Y.; Sangaran, S.; Shukor, S.R.A. Non-enzymatic synthesis route for production of isoamyl acetate in a solvent-free system using miniaturized intensified reactor. J. Environ. Chem. Eng. 2019, 8, 103186. [Google Scholar] [CrossRef]
- Sánchez, C.A.K.; Athés, V.; Moussa, M.; López, M.J.; Páez, L.J.B.; Soto, C.O.N.; Trelea, I.C. Modeling of isoamyl acetate production by fermentation with Pichia fermentans in an aerated system coupled to in situ extraction. Process. Biochem. 2018, 65, 11–20. [Google Scholar] [CrossRef]
- Zare, M.; Golmakani, M.-T.; Niakousari, M. Lipase synthesis of isoamyl acetate using different acyl donors: Comparison of novel esterification techniques. LWT 2019, 101, 214–219. [Google Scholar] [CrossRef]
- Aïder, M.; Ahasanul, K.; Natela, G. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 10881–10888. [Google Scholar]
- Faccia, M.; Trani, A.; Natrella, G.; Gambacorta, G. Short communication: Chemical-sensory and volatile compound characterization of ricotta forte, a traditional fermented whey cheese. J. Dairy Sci. 2018, 101, 5751–5757. [Google Scholar] [CrossRef]
- Páez, L.J.B.; Arias, G.A.; Rutiaga, Q.O.; Barrio, E.Q.; Soto, C.N. Yeasts isolated from the alcoholic fermentation of Agave durangensis during mezcal production. Food Biotecnol. 2010, 4, 27–34. [Google Scholar]
- Conde, B.L.; López, M.A.; Gómez, A.C.A.; Pineda, M.C.F.; Conde, M.C. Economic projection of 2-phenylethanol pro-duction from whey. Food Bioprod. Process. 2019, 115, 10–19. [Google Scholar] [CrossRef]
- Hortsch, R.; Löser, C.; Bley, T. A two-stage CSTR cascade for studying the effect of inhibitory and toxic substances in bioprocesses. Eng. Life Sci. 2008, 8, 650–657. [Google Scholar] [CrossRef]
- Deseure, J.; Obeid, J.; Willison, C.J.; Magnin, J.P. Reliable determination of the growth and hydrogen production param-eters of the photosynthetic bacterium Rhodobacter capsulatus in fed batch culture using a combination of the Gompertz function and the Luedeking-Piret model. Heliyon 2021, 7, e07394. [Google Scholar] [CrossRef]
- Santhosh, A.J.; Tura, A.D.; Jiregna, I.T.; Gemechu, W.F.; Ashok, N.; Ponnusamy, M. Optimization of CNC turning parameters using face centred CCD approach in RSM and ANN-genetic algorithm for AISI 4340 alloy steel. Results Eng. 2021, 11, 100251. [Google Scholar] [CrossRef]
- Salcedo, P.S.I.; Calla, C.A.; Aliaga, A.M.T.; Melgar, C. CF Volatile ester production using citrus waste through fermenta-tion technology. Rev. Cienc. 2016, 4, 83–91. [Google Scholar]
- Arrizon, J.J.; Ñeco, C.V.; Cervantes, V.F.; Delgado, L.A.; Ballesteros, A.O.; Plou, F.J. Synthesis of b(1 → 3) and b(1 → 6) galactooligosaccharides from lactose and whey using a recombinant b-galactosidase from Pantoea anthophila. Electron. J. Biotechnol. 2021, 49, 14–21. [Google Scholar]
- Jawad, R.; Drake, A.F.; Elleman, C.; Martin, G.P.; Warren, F.J.; Perston, B.B.; Ellis, P.R.; Hassoun, M.A.; Royall, P.G. Stability of sugar solutions: A novel study of the epimerization kinetics of lactose in water. Mol. Pharm. 2014, 11, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.J.M.A.; Wolff, K.; Nokhodchi, A.; Martin, G.P.; Royall, P.G. Variability in the α and β anomer content of commercially available lactose. Int. J. Pharm. 2019, 555, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Texeira, R.M. Endless versatility in the biotechnological applications of Kluyveromyces marxianus LAC genes. Biotechnol. Adv. 2009, 24, 212–225. [Google Scholar] [CrossRef]
- Calleros, C.L.; Ivanhoe, L.C.; Vernon, C.E.J. Texture and microstructure of low-fat and low-cholesterol panela type cheeses: Different methodologies. Ing. Agríc. Biosist. 2009, 1, 39–48. [Google Scholar]
- Lee, J.-W.; Trinh, C.T. Towards renewable flavors, fragrances, and beyond. Curr. Opin. Biotechnol. 2020, 61, 168–180. [Google Scholar] [CrossRef]
- Yong, J.C.; Lu, Y.; Quan, S.L. Evaluation of five commercial non-Saccharomyces yeasts in fermentation of soy (tofu) whey into an alcoholic beverage. Food Microbiol. 2018, 76, 533–542. [Google Scholar]
- Santos, D.P.; Angela, M.; Meireles, A.; Julian, M. Production of isoamyl acetate by enzymatic reactions in batch and packed bed reactors with supercritical CO2. J. Supercrit. Fluids 2017, 127, 71–80. [Google Scholar] [CrossRef]
- Barney, B.M. Metabolic engineering: The sweet smell of biosynthesis. Nat. Chem. Biol. 2014, 10, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Yilmaztekin, M.; Erten, H.; Cabaroglu, T. Enhanced production of isoamyl acetate from beet molasses with addition of fusel oil by Williopsis saturnus var. saturnus. Food Chem. 2009, 112, 290–294. [Google Scholar] [CrossRef]
| Factor | Independent Variables | Level | ||||||
|---|---|---|---|---|---|---|---|---|
| −1.414 (−α) | −1 | 0 | 1 | 1.414 (+α) | ||||
| X1 | Isoamyl alcohol (v/v) | 0.26 | 0.3 | 0.4 | 0.5 | 0.54 | ||
| X2 | Ammonium sulphate (g/L) | 0.026 | 0.03 | 0.04 | 0.05 | 0.054 | ||
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| MODEL | 0.2044 | 5 | 0.0409 | 8.6 | 0.0067 |
| X1 | 0.0977 | 1 | 0.0977 | 20.55 | 0.0027 |
| X2 | 0.0049 | 1 | 0.0049 | 1.03 | 0.3442 |
| X1X2 | 0.0002 | 1 | 0.0002 | 0.0398 | 0.8476 |
| X12 | 0.091 | 1 | 0.091 | 19.13 | 0.0033 |
| X22 | 0.02 | 1 | 0.02 | 4.21 | 0.0793 |
| Residual | 0.0333 | 7 | 0.0048 | ||
| Adjustment | 0.0233 | 3 | 0.0078 | 3.09 | 0.1519 |
| Pure Error | 0.01 | 4 | 0.0025 | ||
| Cor Total | 0.2377 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Aldapa, C.A.; Castro-Rosas, J.; López-Molina, A.; Conde-Mejía, C.; Pineda-Muñoz, C.F.; Jiménez-González, A.; Medina-Moreno, S.A.; Falcón-León, M.P.; Conde-Báez, L. Best Conditions for the Production of Natural Isopentyl Acetate (Banana Aroma) from Cheese Industry Waste: An Experimental Precursor Approach. Processes 2021, 9, 1880. https://doi.org/10.3390/pr9111880
Gómez-Aldapa CA, Castro-Rosas J, López-Molina A, Conde-Mejía C, Pineda-Muñoz CF, Jiménez-González A, Medina-Moreno SA, Falcón-León MP, Conde-Báez L. Best Conditions for the Production of Natural Isopentyl Acetate (Banana Aroma) from Cheese Industry Waste: An Experimental Precursor Approach. Processes. 2021; 9(11):1880. https://doi.org/10.3390/pr9111880
Chicago/Turabian StyleGómez-Aldapa, Carlos Alberto, Javier Castro-Rosas, Antioco López-Molina, Carolina Conde-Mejía, Cuauhtémoc Francisco Pineda-Muñoz, Angélica Jiménez-González, Sergio Alejandro Medina-Moreno, Martha Patricia Falcón-León, and Laura Conde-Báez. 2021. "Best Conditions for the Production of Natural Isopentyl Acetate (Banana Aroma) from Cheese Industry Waste: An Experimental Precursor Approach" Processes 9, no. 11: 1880. https://doi.org/10.3390/pr9111880
APA StyleGómez-Aldapa, C. A., Castro-Rosas, J., López-Molina, A., Conde-Mejía, C., Pineda-Muñoz, C. F., Jiménez-González, A., Medina-Moreno, S. A., Falcón-León, M. P., & Conde-Báez, L. (2021). Best Conditions for the Production of Natural Isopentyl Acetate (Banana Aroma) from Cheese Industry Waste: An Experimental Precursor Approach. Processes, 9(11), 1880. https://doi.org/10.3390/pr9111880







