An Investigation into the Metabolic Differences between Conventional and High Seeding Density Fed-Batch Cell Cultures by Applying a Segmented Modeling Approach
Abstract
1. Introduction
- Analysis and comparison of metabolic phases in conventional and high seeding density processes using segmented modeling.
- Application of a segmented model for feeding optimization with the HSD process using measured experiments.
- Metabolic flux analyses and extension of segmented modeling by integration of intracellular fluxes.
2. Materials and Methods
2.1. Cell Lines, Seed Train and Cultivation Processes
2.2. In-Process Analytics
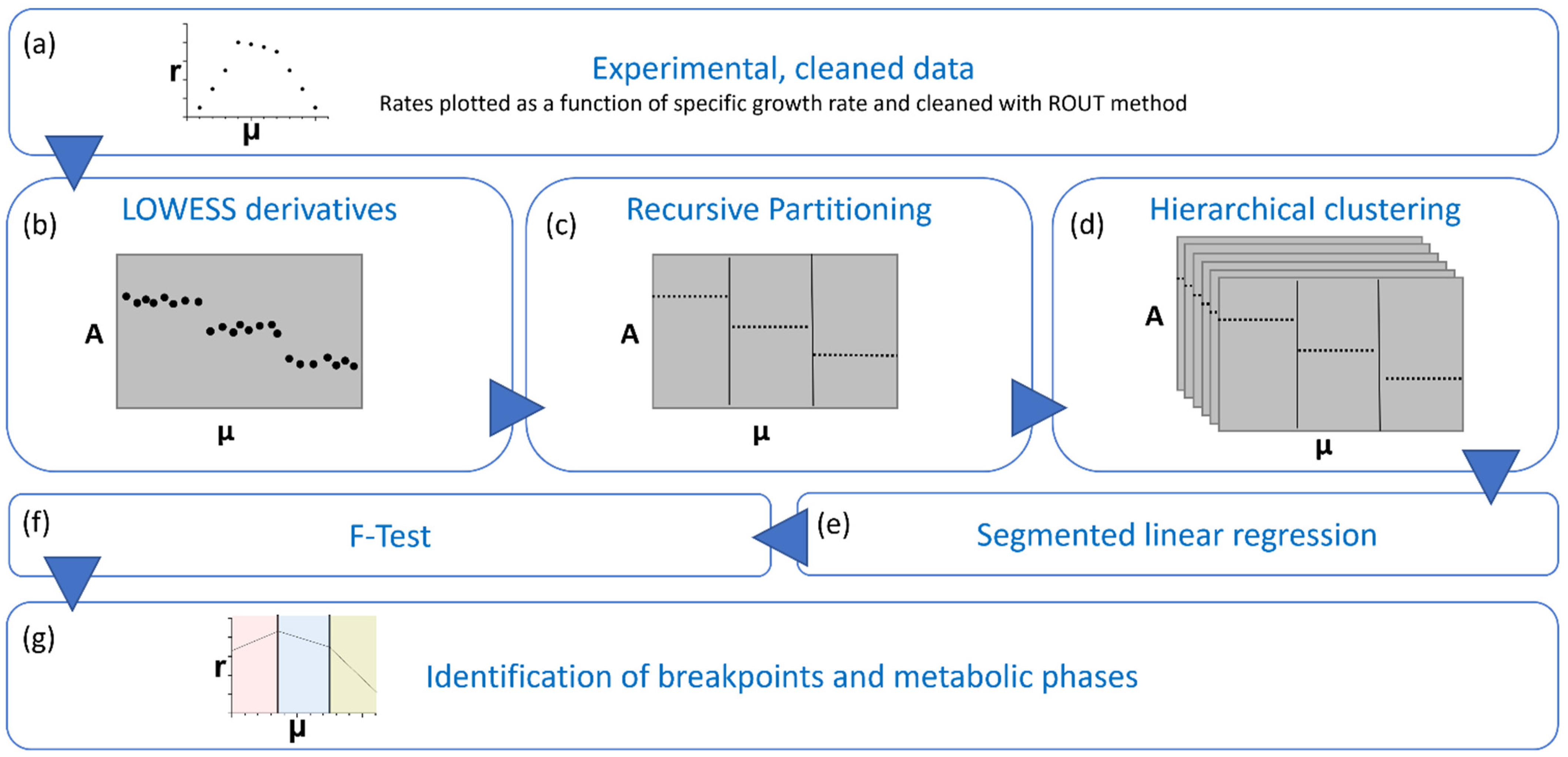
3. Data Analysis and Mathematical Model
3.1. Metabolic Steady State
3.2. Phase Identification
3.3. Segmented Modeling
3.4. Metabolic Flux Analysis
4. Results and Discussion
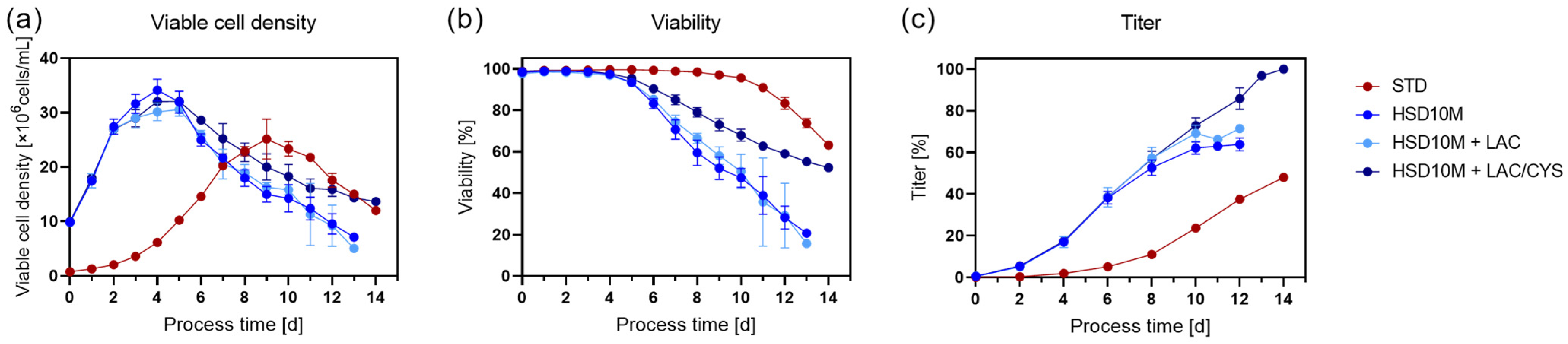
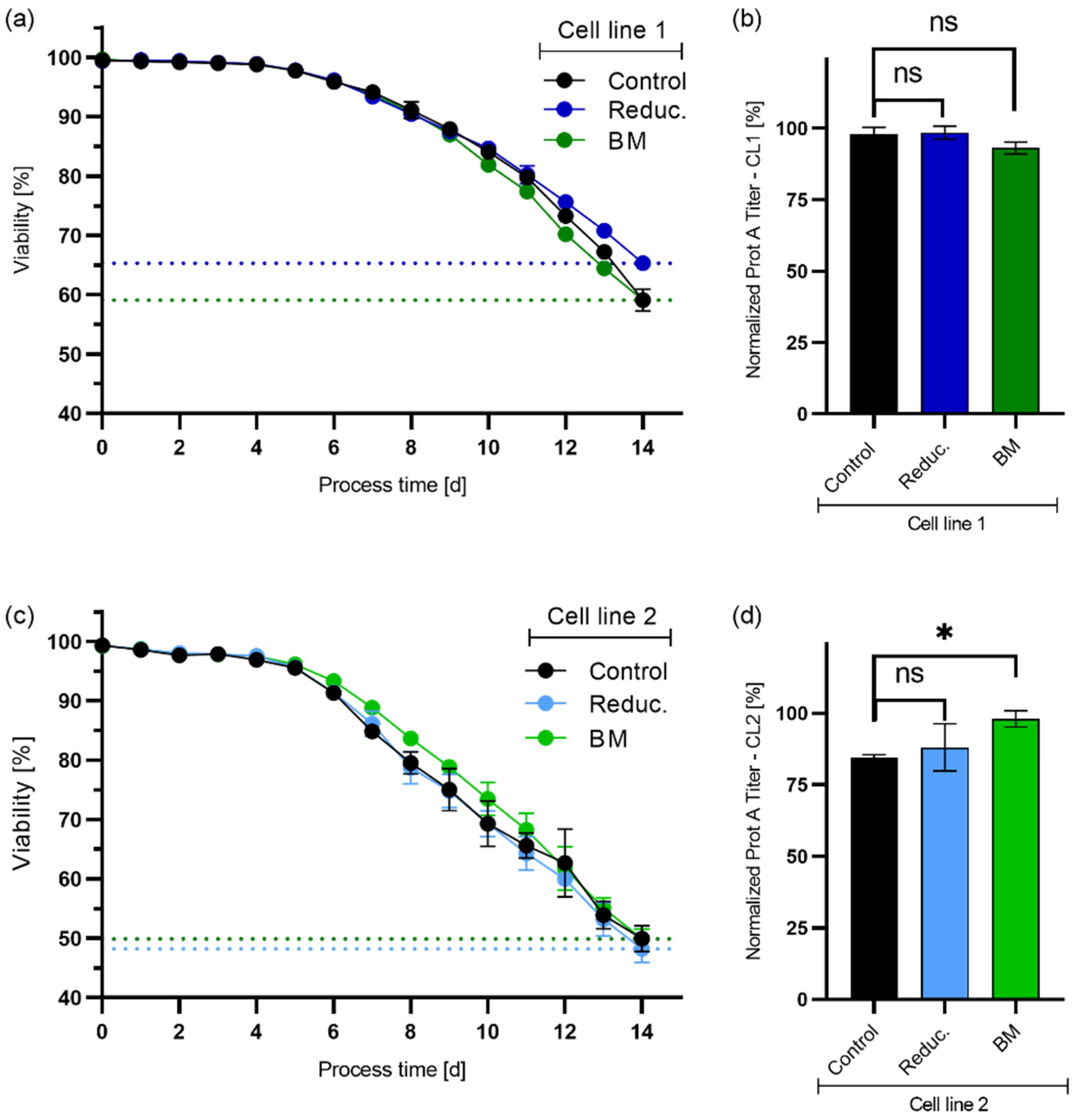
4.1. Process Performance: Standard vs. High Seeding Density Fed-Batch
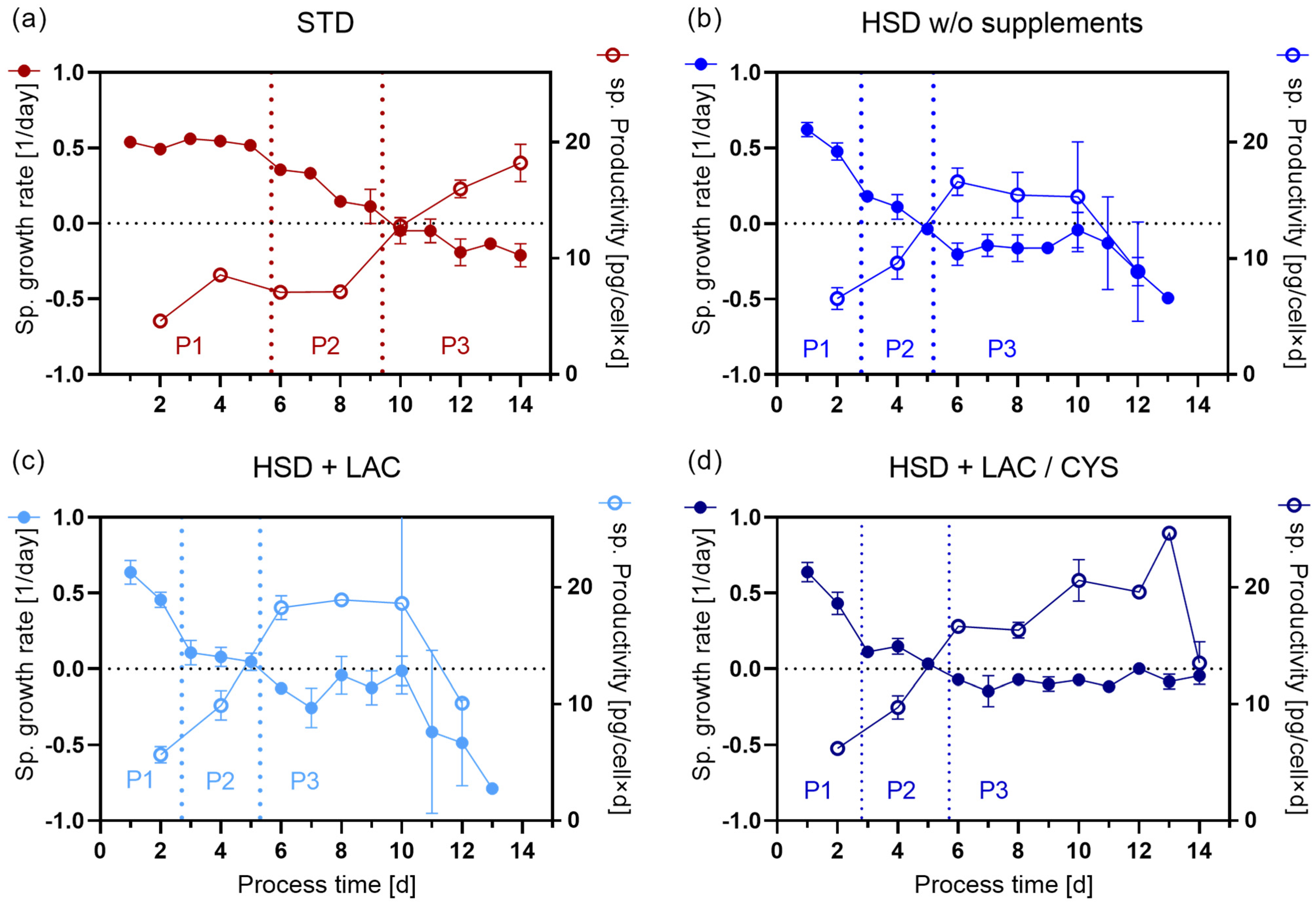
4.2. Identification of Metabolic Phases Using Extracellular Fluxes
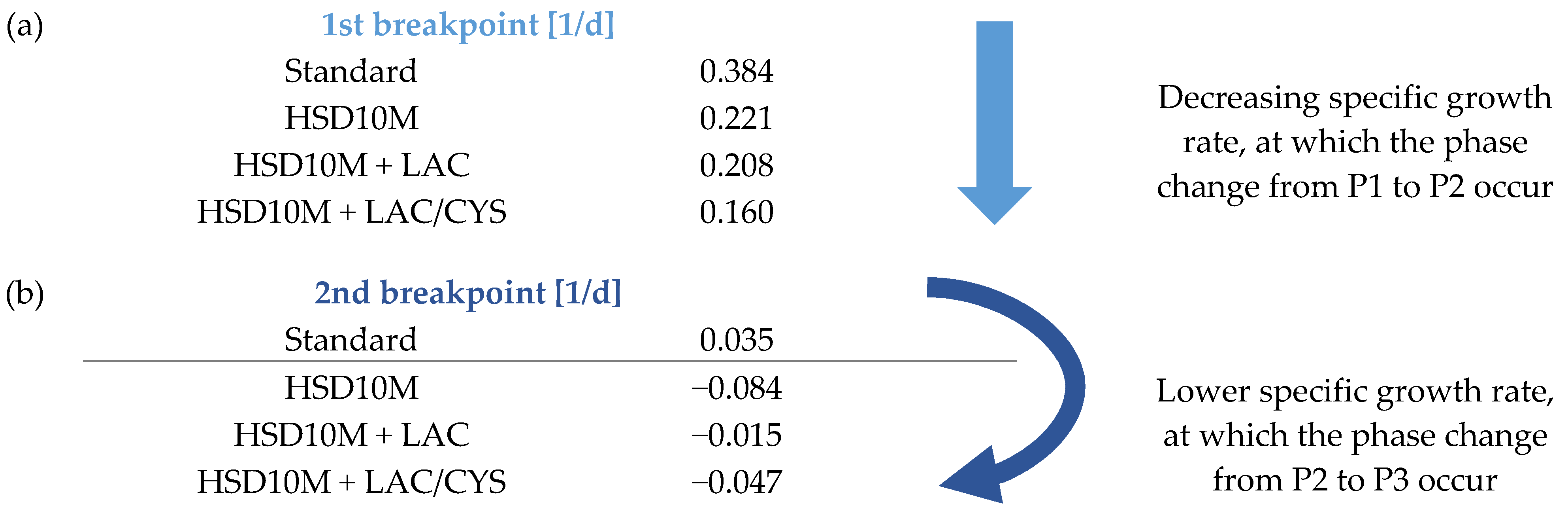
4.2.1. Comparison of Identified Breakpoints
4.2.2. Comparison of the Growth Behavior and Length of Identified Phases
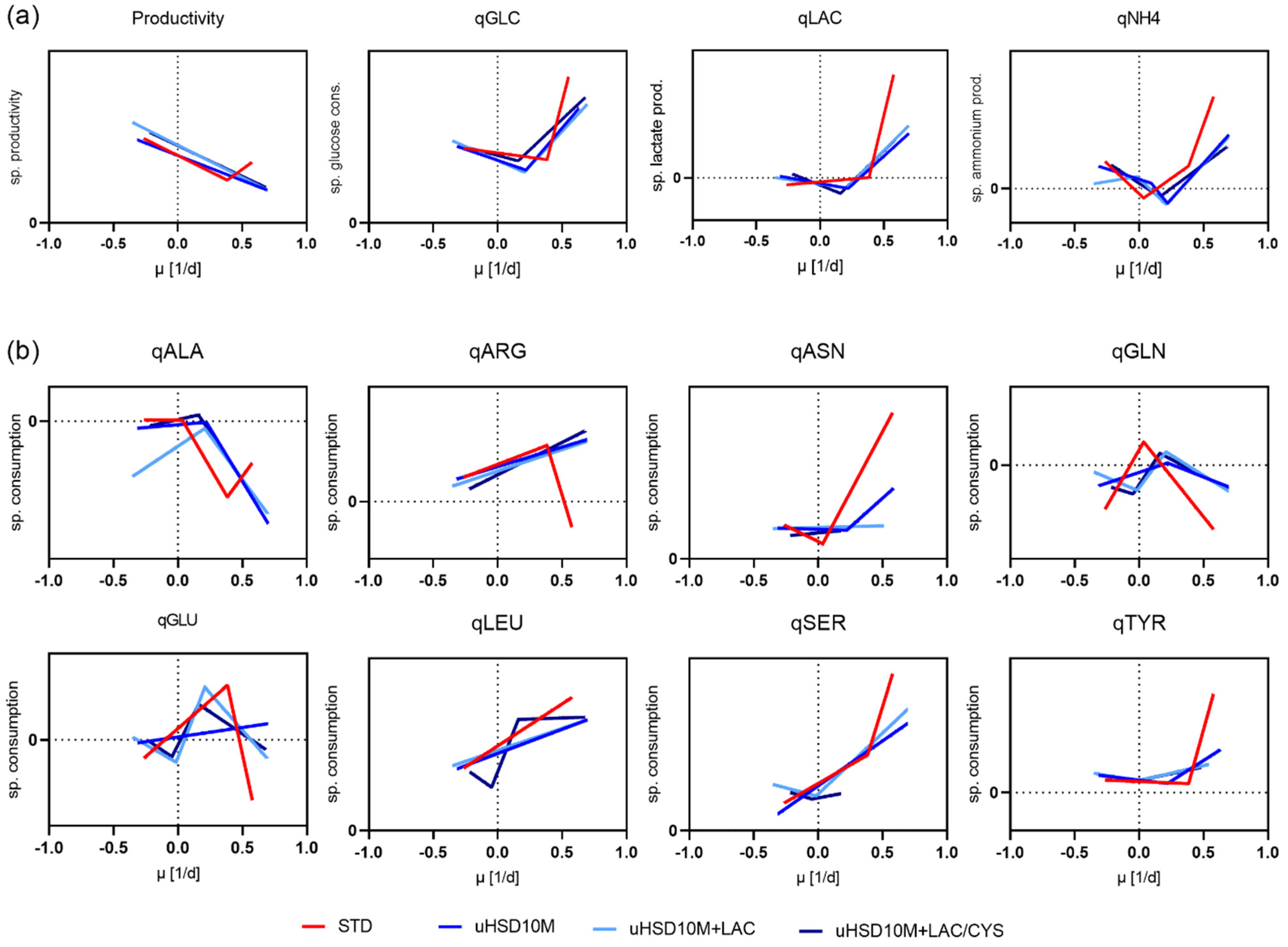
4.2.3. Comparison of the Rates in the Identified Phases
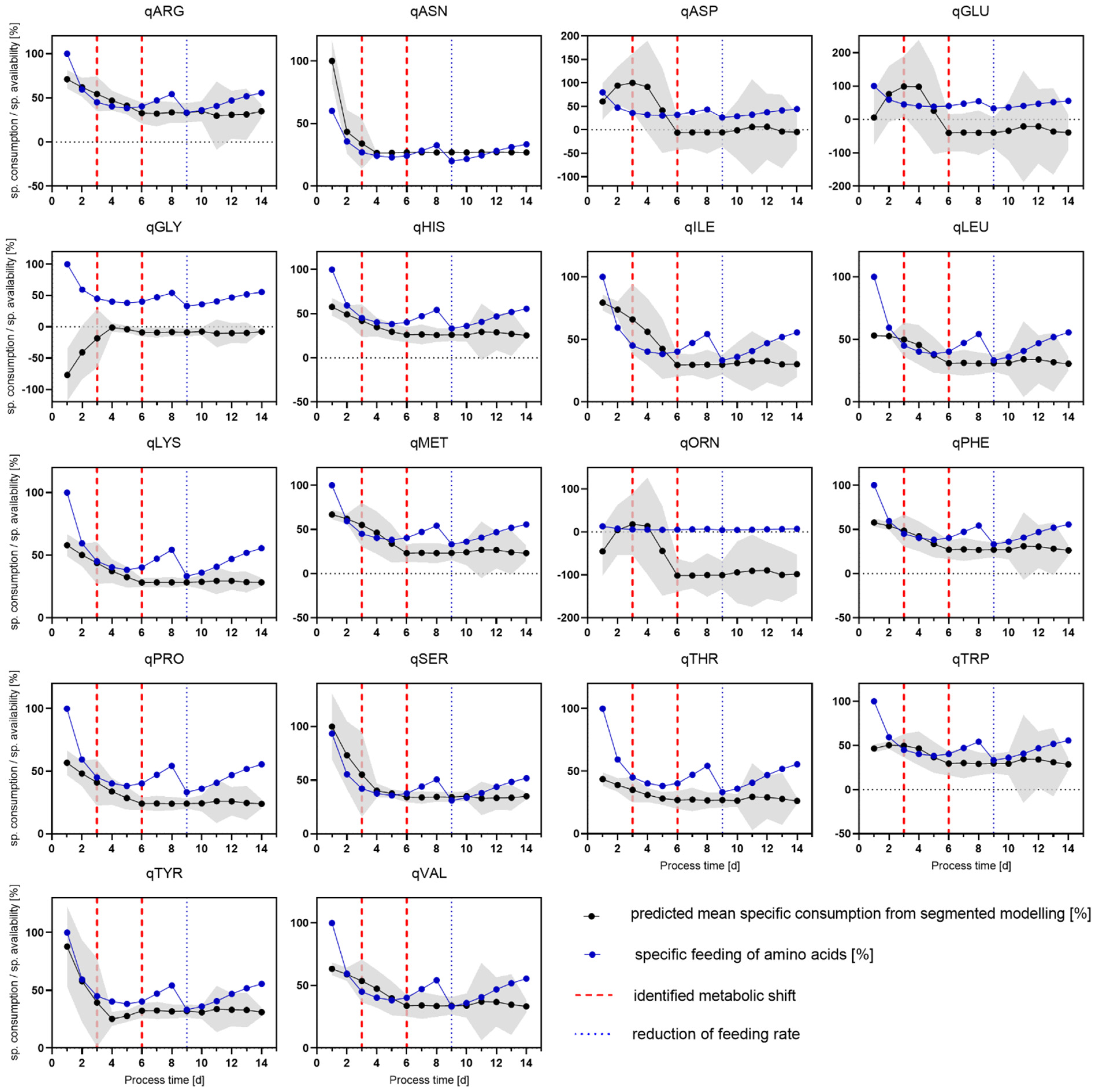
4.3. Feed Optimization Using Segmented Modeling
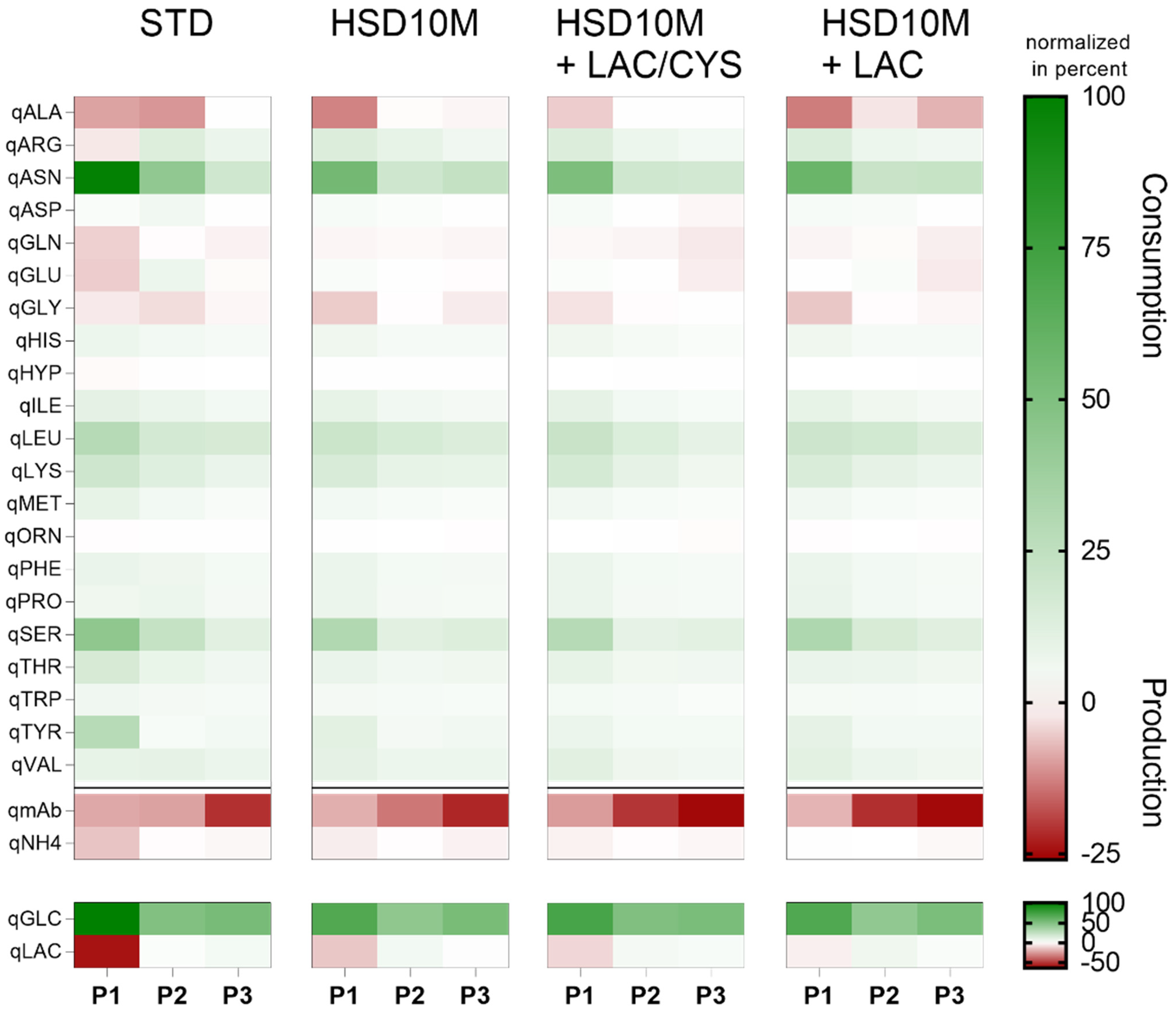
4.3.1. Reduction in Overfeeding
4.3.2. Addition of Depleted Amino Acids
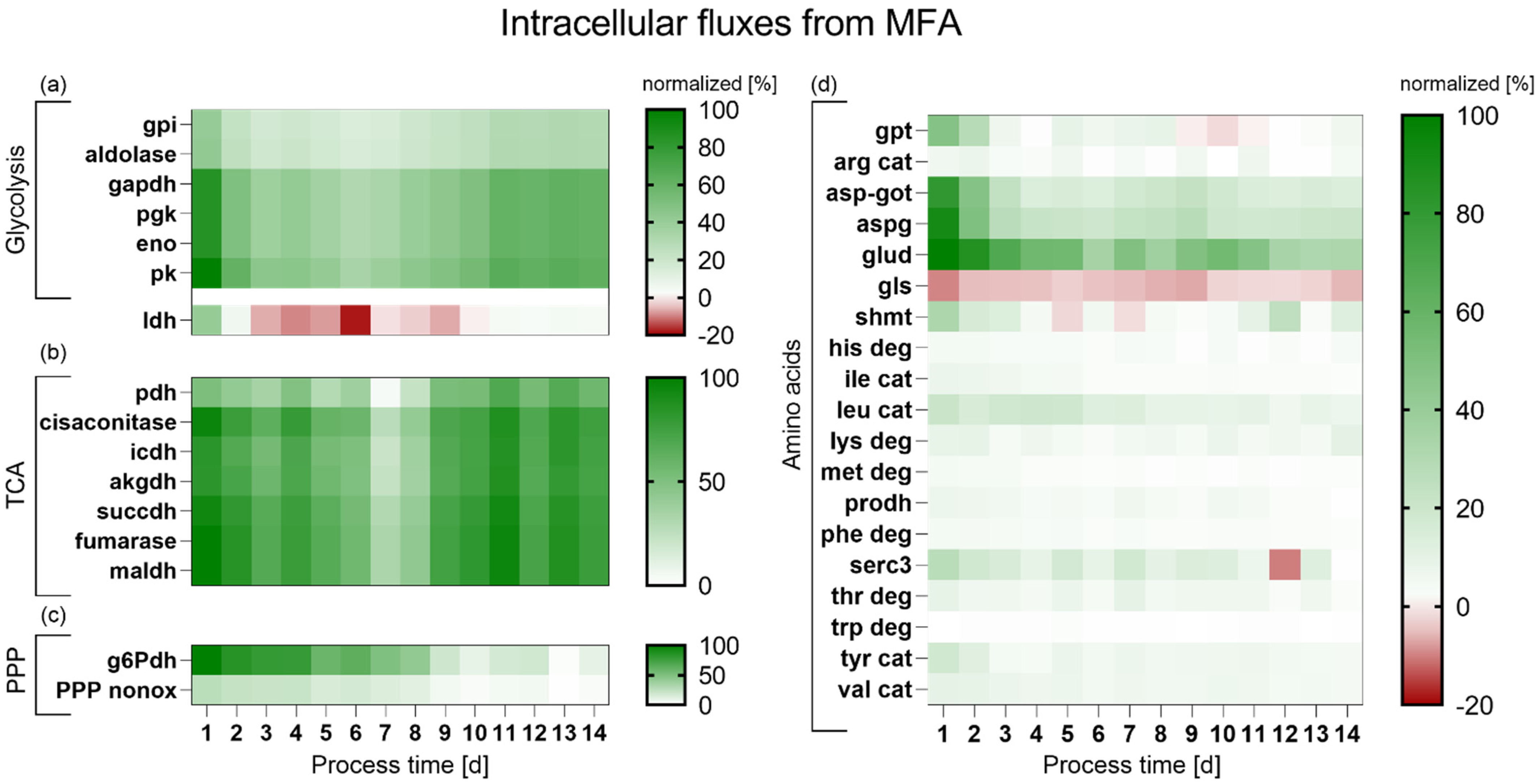
4.4. Metabolic Flux Analysis of the HSD Process Supplemented with LAC/CYS
4.5. Extended Segmented Modeling by Integration of Intracellular Fluxes
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tihanyi, B.; Nyitray, L. Recent Advances in CHO Cell Line Development for Recombinant Protein Production. Drug. Discov. Today Technol. 2020, 38, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ritacco, F.V.; Wu, Y.; Khetan, A. Cell Culture Media for Recombinant Protein Expression in Chinese Hamster Ovary (CHO) Cells: History, Key Components, and Optimization Strategies. Biotechnol. Progr. 2018, 34, 1407–1426. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Lin, Y.; Mi, C.-L.; Pang, J.-Y.; Wang, T.-Y. Progress in Fed-Batch Culture for Recombinant Protein Production in CHO Cells. Appl. Microbiol. Biotechnol. 2023, 107, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Marquart, K.F.; Pieper, L.A.; Fieder, J.; Gamer, M.; Gorr, I.; Schulz, P.; Bradl, H. MiRNA Engineering of CHO Cells Facilitates Production of Difficult-to-express Proteins and Increases Success in Cell Line Development. Biotechnol. Bioeng. 2017, 114, 1495–1510. [Google Scholar] [CrossRef]
- Brunner, M.; Kolb, K.; Keitel, A.; Stiefel, F.; Wucherpfennig, T.; Bechmann, J.; Unsoeld, A.; Schaub, J. Application of Metabolic Modeling for Targeted Optimization of High Seeding Density Processes. Biotechnol. Bioeng. 2021, 118, 1793–1804. [Google Scholar] [CrossRef]
- Ahn, W.S.; Antoniewicz, M.R. Towards Dynamic Metabolic Flux Analysis in CHO Cell Cultures. Biotechnol. J. 2012, 7, 61–74. [Google Scholar] [CrossRef]
- Yang, W.C.; Lu, J.; Kwiatkowski, C.; Yuan, H.; Kshirsagar, R.; Ryll, T.; Huang, Y. Perfusion Seed Cultures Improve Biopharmaceutical Fed-batch Production Capacity and Product Quality. Biotechnol. Progr. 2014, 30, 616–625. [Google Scholar] [CrossRef]
- Gong, X.; Li, D.; Li, X.; Fang, Q.; Han, X.; Wu, Y.; Yang, S.; Shen, B.Q. Fed-Batch Culture Optimization of a Growth-Associated Hybridoma Cell Line in Chemically Defined Protein-Free Media. Cytotechnology 2006, 52, 25–38. [Google Scholar] [CrossRef]
- Stadermann, A.; Gamer, M.; Fieder, J.; Lindner, B.; Fehrmann, S.; Schmidt, M.; Schulz, P.; Gorr, I.H. Structural Analysis of Random Transgene Integration in CHO Manufacturing Cell Lines by Targeted Sequencing. Biotechnol. Bioeng. 2022, 119, 868–880. [Google Scholar] [CrossRef]
- Brunner, M.; Bechmann, J.; Bollgoenn, E.; Unsoeld, A. Mammalian Cell Culture Processes. Application WO-2021165302-A1, 26 August 2021. [Google Scholar]
- Prade, E.; Zeck, A.; Stiefel, F.; Unsoeld, A.; Mentrup, D.; Gutierrez, E.A.; Gorr, I.H. Cysteine in Cell Culture Media Induces Acidic IgG1 Species by Disrupting the Disulfide Bond Network. Biotechnol. Bioeng. 2021, 118, 1091–1104. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Lei, H.; Hao, F.; Liu, X.; Wang, Y.; Tang, H. Simultaneous Quantification of Amino Metabolites in Multiple Metabolic Pathways Using Ultra-High Performance Liquid Chromatography with Tandem-Mass Spectrometry. Sci. Rep. 2017, 7, 1423. [Google Scholar] [CrossRef] [PubMed]
- Provost, A.; Bastin, G.; Agathos, S.N.; Schneider, Y.-J. Metabolic Design of Macroscopic Bioreaction Models: Application to Chinese Hamster Ovary Cells. Bioproc. Biosyst. Eng. 2006, 29, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Yahia, B.B.; Gourevitch, B.; Malphettes, L.; Heinzle, E. Segmented Linear Modeling of CHO Fed-batch Culture and Its Application to Large Scale Production. Biotechnol. Bioeng. 2017, 114, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Yang, T.H.; Heinzle, E. Towards a Metabolic and Isotopic Steady State in CHO Batch Cultures for Reliable Isotope-based Metabolic Profiling. Biotechnol. J. 2009, 4, 247–263. [Google Scholar] [CrossRef]
- Pirt, S.J. Maintenance Energy: A General Model for Energy-Limited and Energy-Sufficient Growth. Arch. Microbiol. 1982, 133, 300–302. [Google Scholar] [CrossRef]
- Luedeking, R.; Piret, E.L. A Kinetic Study of the Lactic Acid Fermentation. Batch Process at Controlled PH. J. Biochem. Microbiol. 1959, 1, 393–412. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting Outliers When Fitting Data with Nonlinear Regression—A New Method Based on Robust Nonlinear Regression and the False Discovery Rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
- Murtagh, F. A Survey of Recent Advances in Hierarchical Clustering Algorithms. Comput. J. 1983, 26, 354–359. [Google Scholar] [CrossRef]
- Malash, G.F.; El-Khaiary, M.I. Piecewise Linear Regression: A Statistical Method for the Analysis of Experimental Adsorption Data by the Intraparticle-Diffusion Models. Chem. Eng. J. 2010, 163, 256–263. [Google Scholar] [CrossRef]
- Nolan, R.P.; Lee, K. Dynamic Model for CHO Cell Engineering. J. Biotechnol. 2012, 158, 24–33. [Google Scholar] [CrossRef]
- Morales, Y.; Bosque, G.; Vehí, J.; Picó, J.; Llaneras, F. PFA Toolbox: A MATLAB Tool for Metabolic Flux Analysis. BMC Syst. Biol. 2016, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Doppler, P.; Klein, T.; Herwig, C.; Fricke, J. Elevated PCO2 Affects the Lactate Metabolic Shift in CHO Cell Culture Processes. Eng. Life Sci. 2018, 18, 204–214. [Google Scholar] [CrossRef]
- Wahrheit, J.; Nicolae, A.; Heinzle, E. Dynamics of Growth and Metabolism Controlled by Glutamine Availability in Chinese Hamster Ovary Cells. Appl. Microbiol. Biot. 2014, 98, 1771–1783. [Google Scholar] [CrossRef]
- Xing, Z.; Kenty, B.; Koyrakh, I.; Borys, M.; Pan, S.-H.; Li, Z.J. Optimizing Amino Acid Composition of CHO Cell Culture Media for a Fusion Protein Production. Process Biochem. 2011, 46, 1423–1429. [Google Scholar] [CrossRef]
- Padawer, I.; Ling, W.L.W.; Bai, Y. Case Study: An Accelerated 8-day Monoclonal Antibody Production Process Based on High Seeding Densities. Biotechnol. Progr. 2013, 29, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ryan, P.W.; Ray, B.H.; Leister, K.J.; Sirimuthu, N.M.S.; Ryder, A.G. Rapid Characterization and Quality Control of Complex Cell Culture Media Solutions Using Raman Spectroscopy and Chemometrics. Biotechnol. Bioeng. 2010, 107, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Raju, R.; Kshirsagar, R.; Ivanov, A.R.; Gilbert, A.; Zang, L.; Karger, B.L. Multi-Omics Study on the Impact of Cysteine Feed Level on Cell Viability and MAb Production in a CHO Bioprocess. Biotechnol. J. 2019, 14, 1800352. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, N.; Rose, S.T.; Morgan, J.A. Metabolic Flux Analysis of CHO Cell Metabolism in the Late Non-growth Phase. Biotechnol. Bioeng. 2011, 108, 82–92. [Google Scholar] [CrossRef]
- Stepper, L.; Filser, F.A.; Fischer, S.; Schaub, J.; Gorr, I.; Voges, R. Pre-Stage Perfusion and Ultra-High Seeding Cell Density in CHO Fed-Batch Culture: A Case Study for Process Intensification Guided by Systems Biotechnology. Bioproc. Biosyst. Eng. 2020, 43, 1431–1443. [Google Scholar] [CrossRef]
- Zagari, F.; Jordan, M.; Stettler, M.; Broly, H.; Wurm, F.M. Lactate Metabolism Shift in CHO Cell Culture: The Role of Mitochondrial Oxidative Activity. New Biotechnol. 2013, 30, 238–245. [Google Scholar] [CrossRef]
- Vodopivec, M.; Lah, L.; Narat, M.; Curk, T. Metabolomic Profiling of CHO Fed-batch Growth Phases at 10, 100, and 1000 L. Biotechnol. Bioeng. 2019, 116, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Traustason, B. Amino Acid Requirements of the Chinese Hamster Ovary Cell Metabolism during Recombinant Protein Production. BioRxiv 2019. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W.-M. Regulation of Mammalian Nucleotide Metabolism and Biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wong, C.L.; Vijayasankaran, N.; Hudson, T.; Amanullah, A. Feeding Lactate for CHO Cell Culture Processes: Impact on Culture Metabolism and Performance. Biotechnol. Bioeng. 2012, 109, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Coulet, M.; Kepp, O.; Kroemer, G.; Basmaciogullari, S. Metabolic Profiling of CHO Cells during the Production of Biotherapeutics. Cells 2022, 11, 1929. [Google Scholar] [CrossRef] [PubMed]
- Mulukutla, B.C.; Kale, J.; Kalomeris, T.; Jacobs, M.; Hiller, G.W. Identification and Control of Novel Growth Inhibitors in Fed-batch Cultures of Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2017, 114, 1779–1790. [Google Scholar] [CrossRef]
- Ahn, W.S.; Antoniewicz, M.R. Metabolic Flux Analysis of CHO Cells at Growth and Non-Growth Phases Using Isotopic Tracers and Mass Spectrometry. Metab. Eng. 2011, 13, 598–609. [Google Scholar] [CrossRef]
- Goudar, C.; Biener, R.; Boisart, C.; Heidemann, R.; Piret, J.; de Graaf, A.; Konstantinov, K. Metabolic Flux Analysis of CHO Cells in Perfusion Culture by Metabolite Balancing and 2D [13C, 1H] COSY NMR Spectroscopy. Metab. Eng. 2010, 12, 138–149. [Google Scholar] [CrossRef]
- Schaub, J.; Clemens, C.; Kaufmann, H.; Schulz, T.W. Genomics and Systems Biology of Mammalian Cell Culture. Adv. Biochem. Eng. Biotechnol. 2011, 127, 133–163. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumm, T.L.; Ehsani, A.; Schaub, J.; Stiefel, F. An Investigation into the Metabolic Differences between Conventional and High Seeding Density Fed-Batch Cell Cultures by Applying a Segmented Modeling Approach. Processes 2023, 11, 1094. https://doi.org/10.3390/pr11041094
Krumm TL, Ehsani A, Schaub J, Stiefel F. An Investigation into the Metabolic Differences between Conventional and High Seeding Density Fed-Batch Cell Cultures by Applying a Segmented Modeling Approach. Processes. 2023; 11(4):1094. https://doi.org/10.3390/pr11041094
Chicago/Turabian StyleKrumm, Teresa Laura, Alireza Ehsani, Jochen Schaub, and Fabian Stiefel. 2023. "An Investigation into the Metabolic Differences between Conventional and High Seeding Density Fed-Batch Cell Cultures by Applying a Segmented Modeling Approach" Processes 11, no. 4: 1094. https://doi.org/10.3390/pr11041094
APA StyleKrumm, T. L., Ehsani, A., Schaub, J., & Stiefel, F. (2023). An Investigation into the Metabolic Differences between Conventional and High Seeding Density Fed-Batch Cell Cultures by Applying a Segmented Modeling Approach. Processes, 11(4), 1094. https://doi.org/10.3390/pr11041094







