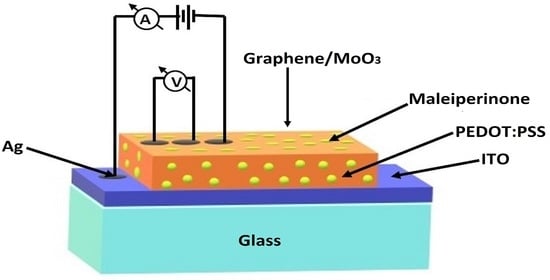
Deposit and Characterization of Semiconductor Films Based on Maleiperinone and Polymeric Matrix of (Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate)
Abstract
:1. Introduction
2. Materials and Methods
3. Theoretical Calculations
4. Results and Discussion
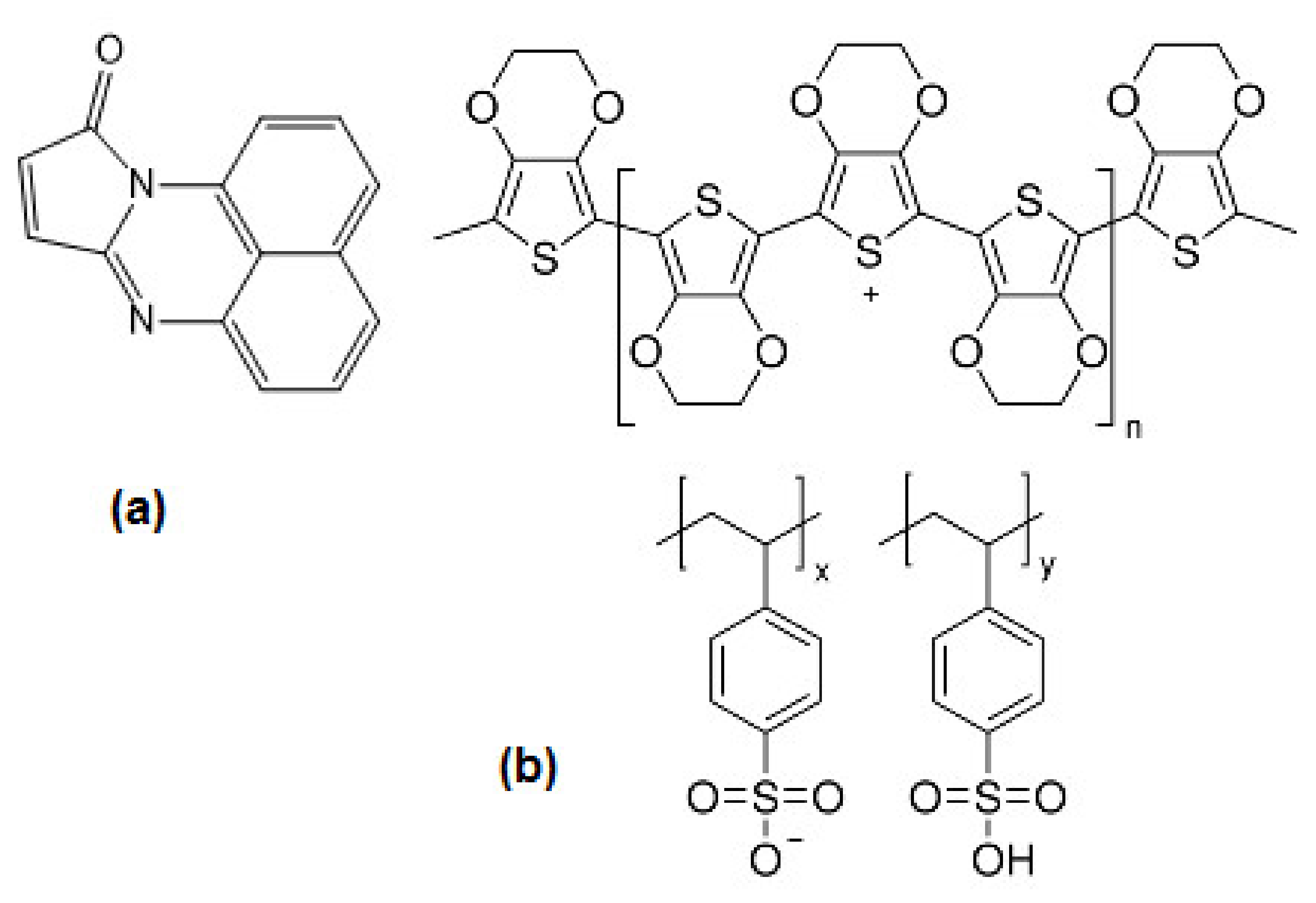
4.1. Deposit and Characterization of Pristine Maleiperinone Film
4.2. Theoretical Study
4.3. Deposit and Characterization of Hybrid Semiconductor Films
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular Semiconductors in Organic Photovoltaic Cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.E.; Ginley, D.S.; Jabbour, G.E. Organic-Based Photovoltaics: Toward low-cost power generation. MRS Bull. 2005, 30, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Yoo, S.; Domercq, B.; Kippelen, B. Efficient thin-film organic solar cells based on pentacene/C60 heterojunctions. Appl. Phys. Lett. 2004, 85, 5427–5429. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhu, H.; Meng, Q.; Gong, X.; Hu, W. Organic photoresponse materials and devices. Chem. Soc. Rev. 2012, 41, 1754–1808. [Google Scholar] [CrossRef]
- Dong, H.; Wang, C.; Hu, W. High performance organic semiconductors for field-effect transistors. Chem. Commun. 2010, 46, 5211–5222. [Google Scholar] [CrossRef] [PubMed]
- Scharber, M.C.; Sariciftci, N.S. Low band gap conjugated semiconducting polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Barbot, A.; Di Bin, C.; Lucas, B.; Ratier, B.; Aldissi, M. N-type doping and thermoelectric properties of co-sublimed cesium-carbonate-doped fullerene. J. Mater. Sci. 2013, 48, 2785–2789. [Google Scholar] [CrossRef]
- Husain, A.A.F.; Hasan, W.Z.W.; Shafie, S.; Hamidon, M.N.; Pandey, S.S. A review of transparent solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2018, 94, 779–791. [Google Scholar] [CrossRef]
- Cranston, R.R.; Lessard, B.H. Metal phthalocyanines: Thin-film formation, microstructure, and physical properties. RSC Adv. 2021, 11, 21716–21737. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: An overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef] [Green Version]
- Schubert, D.W.; Dunkel, T. Spin coating from a molecular point of view: Its concentration regimes, influence of molar mass and distribution. Mat. Res. Innovat. 2003, 7, 314–321. [Google Scholar] [CrossRef]
- Alfredo, N.V.; Likhatchev, D.; Ramirez, S.B.; Vazquez, J.R.; Valverde, G.C.; Alexandrova, L. Highly effective low temperature route to pyrroloperimidines synthesis and their copolymerization with styrene and methyl methacrylate. Polymer 2008, 49, 3654–3662. [Google Scholar] [CrossRef]
- Koca, R.; Ngren, E.H.; Kbrz, B.E.; Ylmaz, F. The synthesis of new pyrrolo[1,2-a]perimidin-10-one dyes via two convenient routes and its characterizations. Dyes Pigm. 2012, 95, 421–426. [Google Scholar] [CrossRef]
- Fernández-Gijón, C.A.; del Río-Portilla, F.; Ríos-Jara, D.; Fomine, S.; Santana, G.; Alexandrova, L. Itaco-perinone as a molecule with potential use in white light emitting materials. The effect of methyl- and methylene groups on the formation of perimidine and perinone structures. Tetrahedron 2015, 71, 7063–7069. [Google Scholar] [CrossRef]
- Nuñez Bahena, E.; Fernández Gijón, C.A.; Fomine, S.; Alexandrova, L.; Le Lagadec, R. Synthesis, characterization, and spectroscopic properties of allylic ruthenium(II) complexes of a highly conjugated perinone. Eur. J. Inorg. Chem. 2019, 2019, 3494–3502. [Google Scholar] [CrossRef]
- Erten, S.; Icli, S. Bilayer heterojunction solar cell based on naphthalene bis-benzimidazole. Inorg. Chim. Acta 2008, 361, 595–600. [Google Scholar] [CrossRef]
- Faraonov, M.A.; Romanenko, N.R.; Mikhailenko, M.V.; Kuzmin, A.V.; Khasanov, S.S.; Konarev, D.V. Structure and properties of radical anion and dianion salts of organic dye trans-perinone and its mixed salt with gallium(iii) phthalocyanine. New J. Chem. 2021, 45, 13599–13607. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Gao, J.; Gu, L.-J.; Wan, J.-H.; Wei, W.; Liu, F. Structural modification of thieno[3,4-c]pyrrole-4,6-dione: Structure–property relationships and application in solution-processed small-molecule organic solar cells. J. Mater. Chem. A Mater. Energy Sustain. 2013, 1, 5875. [Google Scholar] [CrossRef]
- Yeo, J.-S.; Yun, J.-M.; Kim, D.-Y.; Park, S.; Kim, S.-S.; Yoon, M.-H.; Kim, T.-W.; Na, S.-I. Significant vertical phase separation in solvent-vapor-annealed poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) composite films leading to better conductivity and work function for high-performance indium tin oxide-free optoelectronics. ACS Appl. Mater. Interfaces 2012, 4, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate) (PEDOT:PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Neophytou, M.; Griffiths, J.; Fraser, J.; Kirkus, M.; Chen, H.; Nielsen, C.B.; McCulloch, I. High mobility, hole transport materials for highly efficient PEDOT:PSS replacement in inverted perovskite solar cells. J. Mater. Chem. C Mater. Opt. Electron. Devices 2017, 5, 4940–4945. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bi, X.; Zhao, X.; Zhao, Z.; Zhu, J.; Dai, S.; Lu, Y.; Yang, S. Isopropanol-treated PEDOT:PSS as electron transport layer in polymer solar cells. Org. Electron. 2014, 15, 3445–3451. [Google Scholar] [CrossRef]
- Yeon, C.; Kim, G.; Lim, J.W.; Yun, S.J. Highly conductive PEDOT:PSS treated by sodium dodecyl sulfate for stretchable fabric heaters. RSC Adv. 2017, 7, 5888–5897. [Google Scholar] [CrossRef] [Green Version]
- Kepić, D.; Markovic, Z.; Jovanović, S.P.; Perusko, D.; Budimir, M.; Holclajtner-Antunović, I.D.; Pavlović, V.; Marković, B.T. Preparation of PEDOT:PSS thin films doped with graphene and graphene quantum dots. Synth. Met. 2014, 198, 150–154. [Google Scholar] [CrossRef]
- Chen, Q.; Zabihi, F.; Eslamian, M. Improved functionality of PEDOT:PSS thin films via graphene doping, fabricated by ultrasonic substrate vibration-assisted spray coating. Synth. Met. 2016, 222, 309–317. [Google Scholar] [CrossRef]
- Hilal, M.; Han, J.I. Improving the conductivity of PEDOT:PSS to nearly 1 million S/m with graphene on an ITO-glass substrate. Synth. Met. 2018, 245, 276–285. [Google Scholar] [CrossRef]
- Lattante, S. Electron and Hole Transport Layers: Their Use in Inverted Bulk Heterojunction Polymer Solar Cells. Electronics 2014, 3, 132–164. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, X.; Zhang, H.; Chen, J.; Wang, S.; Yang, H. Construction of 2D/0D/2D face-to-face contact g-C3N4@Au@Bi4Ti3O12 heterojunction photocatalysts for degradation of rhodamine B. J. Electron. Mater. 2020, 49, 5248–5259. [Google Scholar] [CrossRef]
- Leung, S.-F.; Zhang, Q.; Xiu, F.; Yu, D.; Ho, J.C.; Li, D.; Fan, Z. Light management with nanostructures for optoelectronic devices. J. Phys. Chem. Lett. 2014, 5, 1479–1495. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B Condens. Matter 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Fox, Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venables, J.A. Atomic processes in crystal growth. Surf. Sci. 1994, 299–300, 798–817. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. The Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Nashville, TN, USA, 2005. [Google Scholar]
- Novotny, M.; Bulir, J.; Bensalah-Ledoux, A.; Guy, S.; Fitl, P.; Vrnata, M.; Lancok, J.; Moine, B. Optical properties of zinc phthalocyanine thin films prepared by pulsed laser deposition. Appl. Phys. A Mater. Sci. Process. 2014, 117, 377–381. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Rivera, M.; Alonso-Huitrón, J.C.; Rodriguez, A.; Álvarez-Bada, J.R. Electrical and optical properties of copper-complexes thin films grown by the vacuum thermal evaporation technique. Mater. Chem. Phys. 2013, 138, 392–398. [Google Scholar] [CrossRef]
- Breckenridge, R.G.; Russell, B.R.; Hahn, E.E. Proceedings of the Photoconductivity Conference; Wiley: New York, NY, USA, 1956. [Google Scholar]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Hassanien, A.M.; Abu-Samaha, F.S.H.; Elesh, E. Effect of illumination on the structural and optical properties of Cu (II) tetraphenyl porphyrin (CuTPP) thin films. Opt. Commun. 2014, 325, 116–121. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Al-Ghamdi, A.A.; Asiri, A.M. Optical properties of thermally evaporated tin-phthalocyanine dichloride thin films, SnPcCl2. Phys. B Condens. Matter. 2004, 344, 398–406. [Google Scholar] [CrossRef]
- Tsiper, E.V.; Soos, Z.G.; Gao, W.; Kahn, A. Electronic polarization at surfaces and thin films of organic molecular crystals: PTCDA. Chem. Phys. Lett. 2002, 360, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Zubair, M.; Mustafa, M.; Ali, A.; Doh, Y.H.; Choi, K.H. Improvement of solution based conjugate polymer organic light emitting diode by ZnO–graphene quantum dots. J. Mater. Sci. Mater. Electron. 2015, 26, 3344–3351. [Google Scholar] [CrossRef]
- Pasha, A.; Khasim, S.; Khan, F.A.; Dhananjaya, N. Fabrication of gas sensor device using poly (3,4-ethylenedioxythiophene)-poly (styrenesulfonate)-doped reduced graphene oxide organic thin films for detection of ammonia gas at room temperature. Iran. Polym. J. 2019, 28, 183–192. [Google Scholar] [CrossRef]
- Murphy, A.R.; Fréchet, J.M.J. Organic semiconducting oligomers for use in thin film transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef]
- Soliman, I.M.; El-Nahass, M.M.; Khalifa, B.A. Characterization and photovoltaic performance of organic device based on AlPcCl/p-Si heterojunction. Synth. Met. 2015, 209, 55–59. [Google Scholar] [CrossRef]
- Zeyada, H.M.; El-Nahass, M.M.; El-Menyawy, E.M.; El-Sawah, A.S. Electrical and photovoltaic characteristics of indium phthalocyanine chloride/p-Si solar cell. Synth. Met. 2015, 207, 46–53. [Google Scholar] [CrossRef]
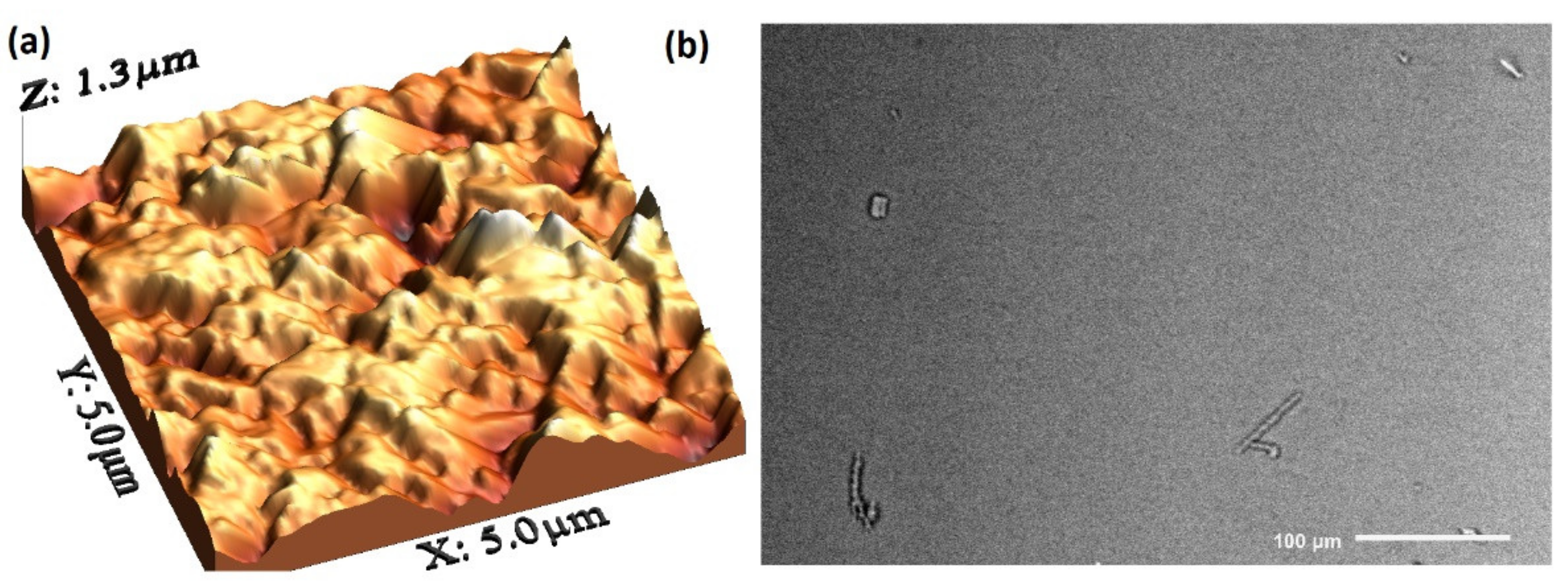
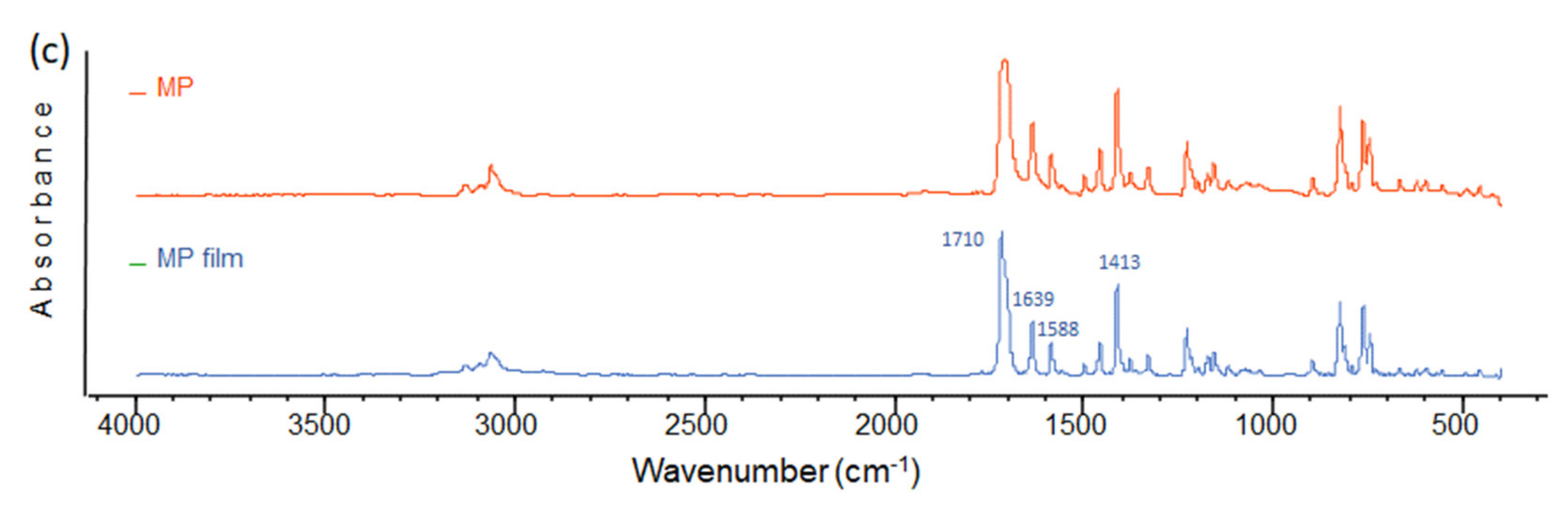
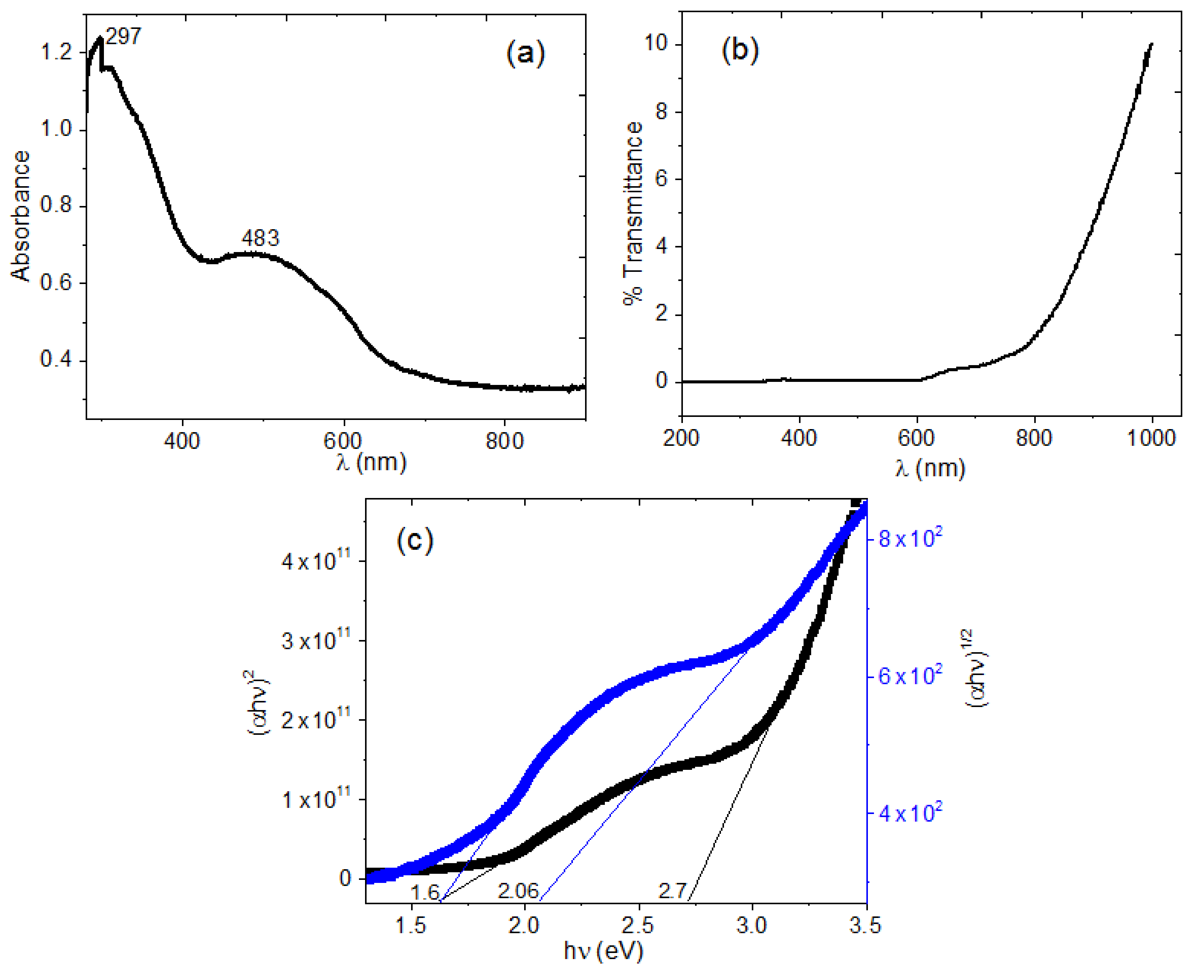
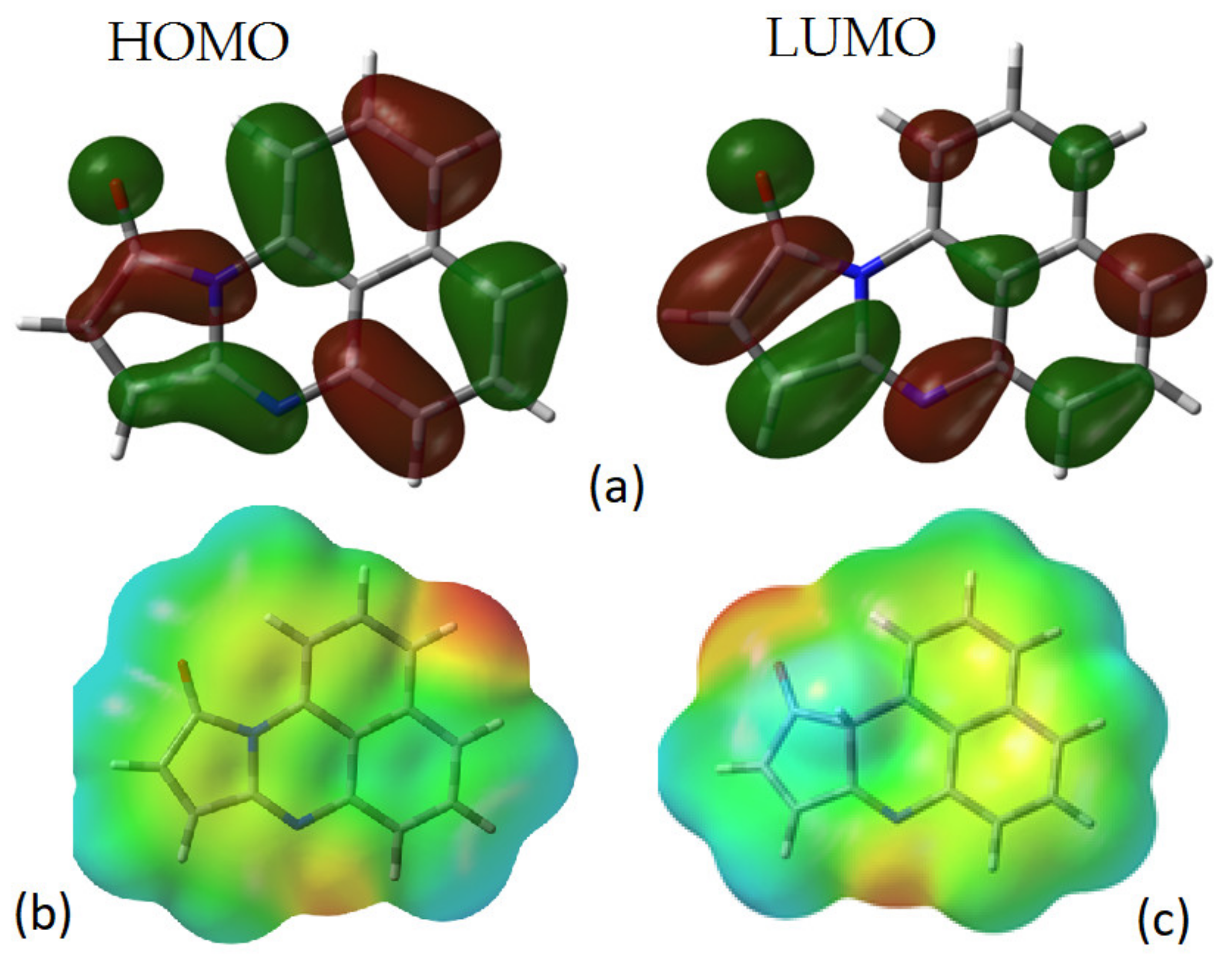
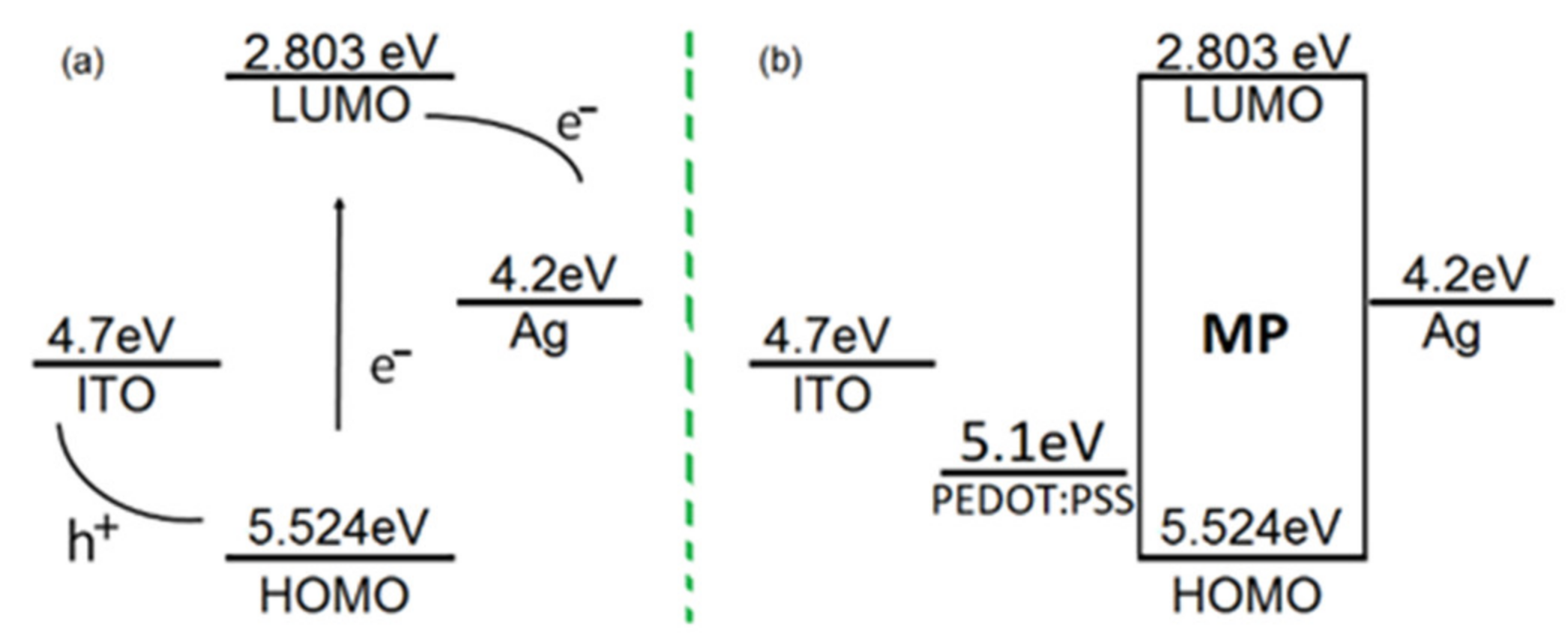
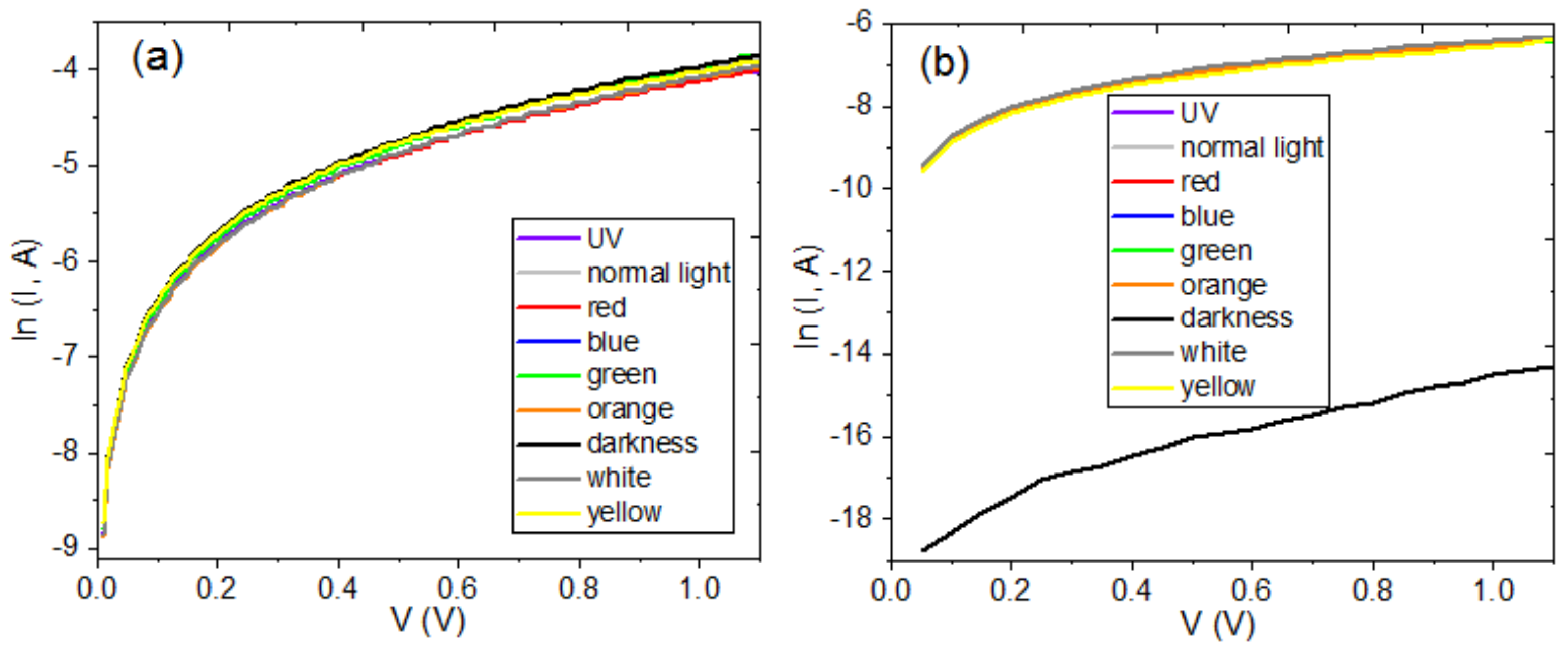
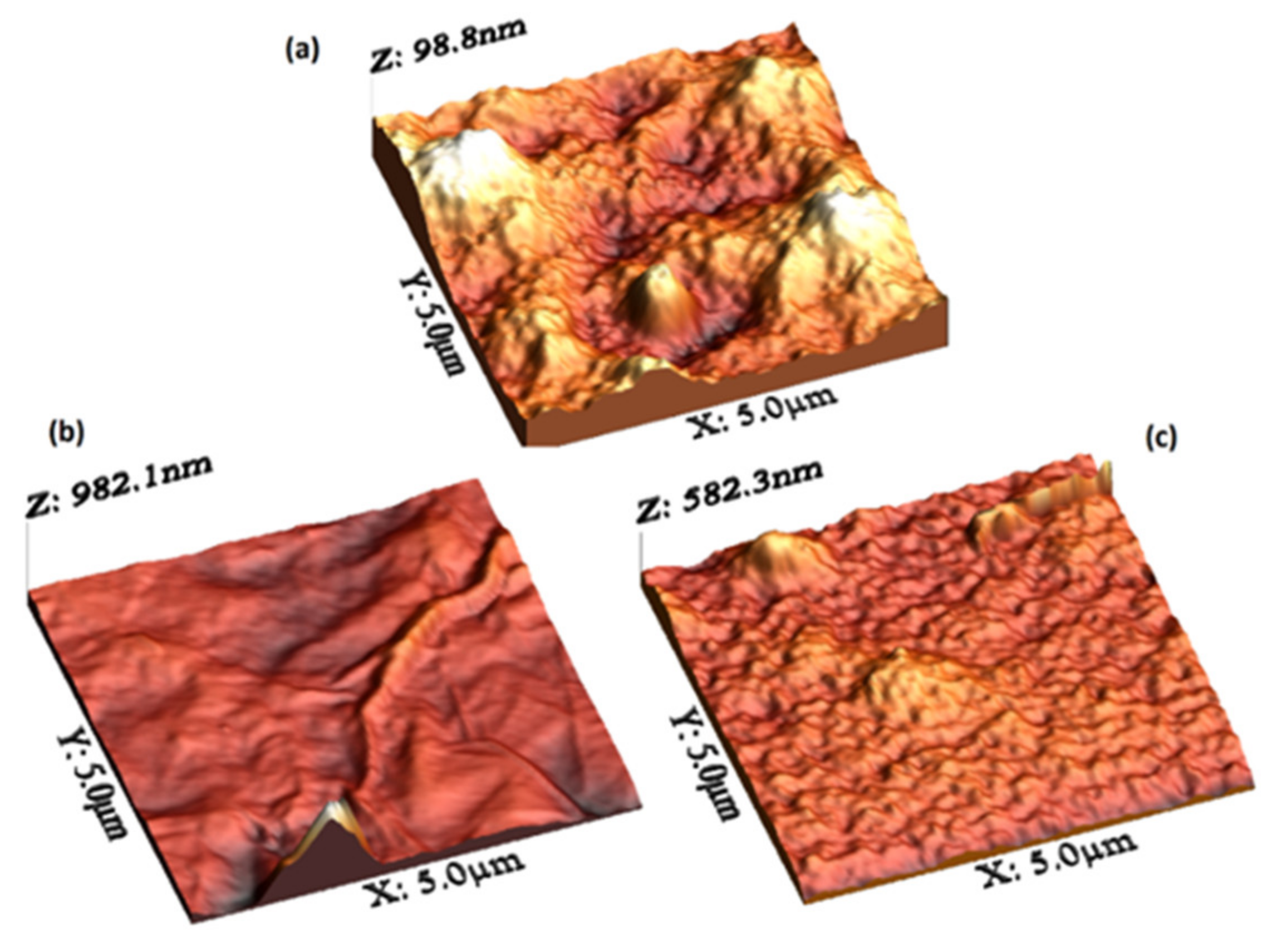

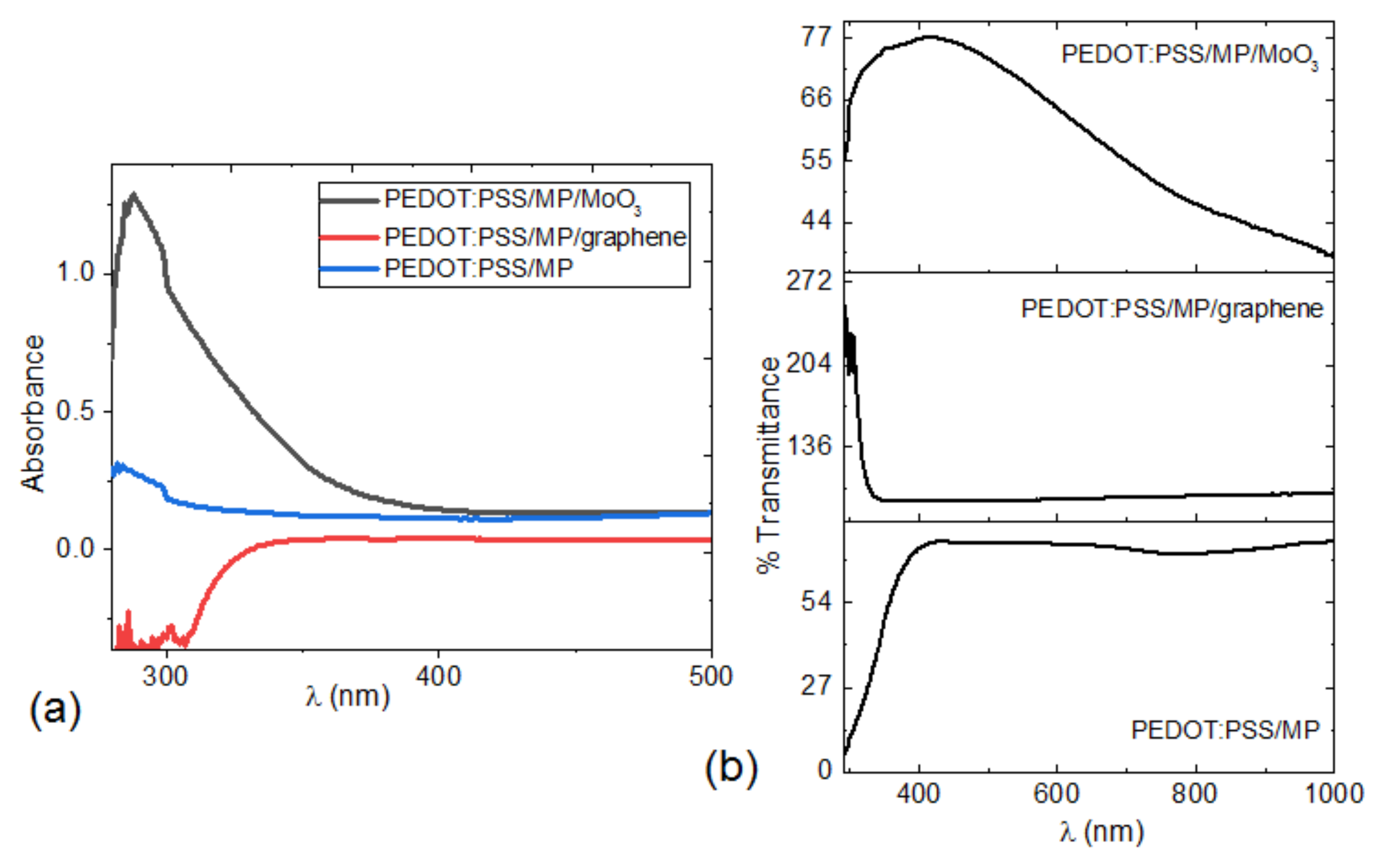

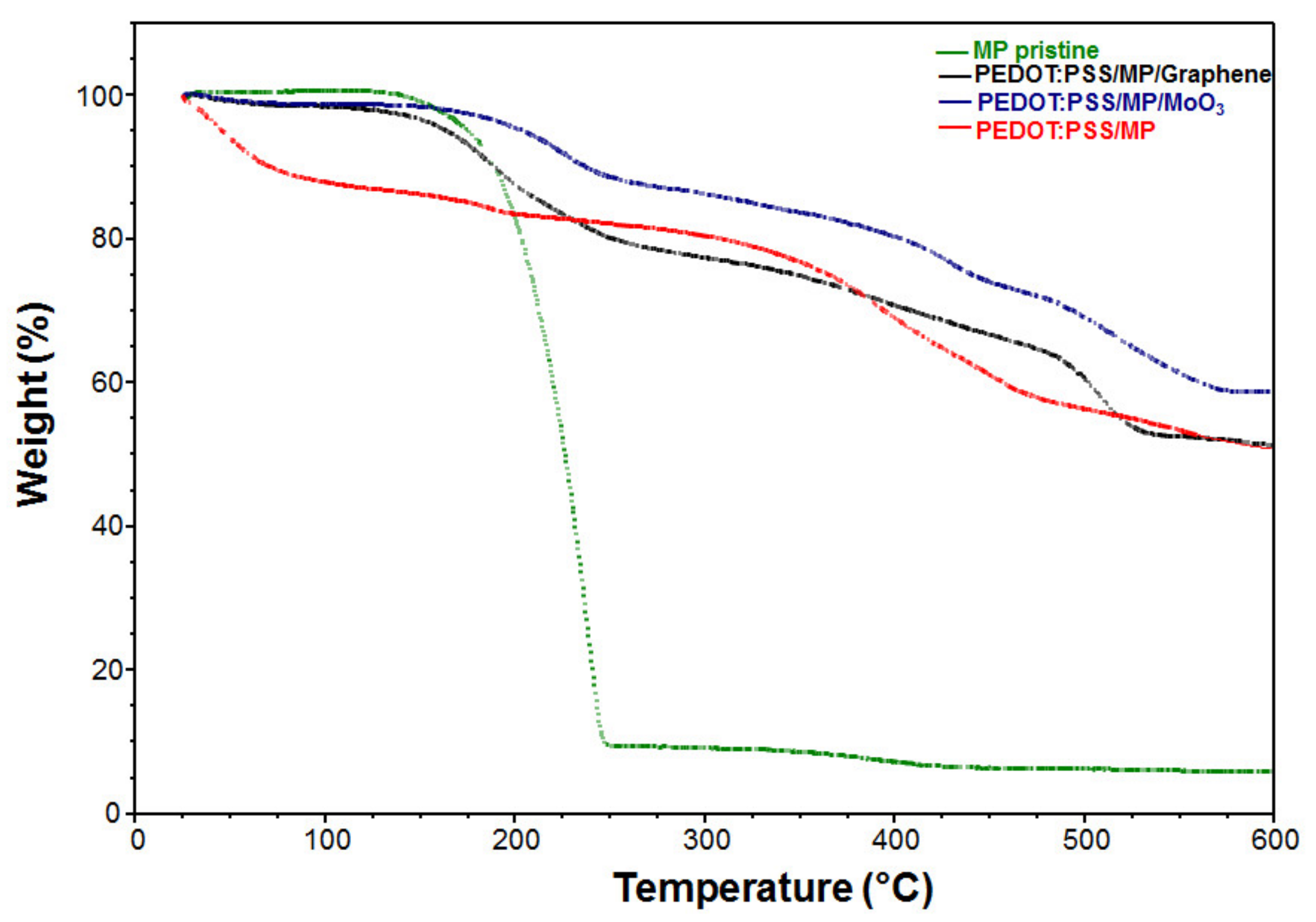
| Film | Thickness (μm) | RMS (nm) | Onset Band Gap(eV) | Energy Band Gap. Direct Transitions (eV) | Energy Band Gap. Indirect Transitions (eV) |
|---|---|---|---|---|---|
| Pristine MP | 1.4 | 16.95 | 1.6 | 2.71 | 2.06 |
| PEDOT:PSS/MP | 0.988 | 15.92 | - | 3.92 | 3.60 |
| PEDOT:PSS/MP/graphene | 1.06 | 58.75 | - | - | - |
| PEDOT:PSS/MP/MoO3 | 1.0 | 34.97 | - | 3.72 | 2.83 |
| Sample | 0.5 V | 1.0 V |
|---|---|---|
| ITO/MP/Ag | 3.6 × 104 Ω | 1.8 × 104 Ω |
| ITO/PEDOT:PSS/MP/Ag | 2.2 × 108 Ω | 1.1 × 108 Ω |
| ITO/PEDOT:PSS/MP/graphene/Ag | 50 Ω | 43 Ω |
| ITO/PEDOT:PSS/MP/MoO3/Ag | 59 Ω | 54 Ω |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Vergara, M.E.; Ramirez, S.B.; Alanis, R.L.; Ramírez, G.M.d.O.; Baeza Alvarado, M.D.; Fomina, L.; Rios, C.; Salcedo, R. Deposit and Characterization of Semiconductor Films Based on Maleiperinone and Polymeric Matrix of (Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate). Processes 2021, 9, 1776. https://doi.org/10.3390/pr9101776
Sánchez Vergara ME, Ramirez SB, Alanis RL, Ramírez GMdO, Baeza Alvarado MD, Fomina L, Rios C, Salcedo R. Deposit and Characterization of Semiconductor Films Based on Maleiperinone and Polymeric Matrix of (Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate). Processes. 2021; 9(10):1776. https://doi.org/10.3390/pr9101776
Chicago/Turabian StyleSánchez Vergara, María Elena, Sergio Barrientos Ramirez, Rafael Loaiza Alanis, Georgina Montes de Oca Ramírez, María Dolores Baeza Alvarado, Lioudmila Fomina, Citlalli Rios, and Roberto Salcedo. 2021. "Deposit and Characterization of Semiconductor Films Based on Maleiperinone and Polymeric Matrix of (Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate)" Processes 9, no. 10: 1776. https://doi.org/10.3390/pr9101776
APA StyleSánchez Vergara, M. E., Ramirez, S. B., Alanis, R. L., Ramírez, G. M. d. O., Baeza Alvarado, M. D., Fomina, L., Rios, C., & Salcedo, R. (2021). Deposit and Characterization of Semiconductor Films Based on Maleiperinone and Polymeric Matrix of (Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate). Processes, 9(10), 1776. https://doi.org/10.3390/pr9101776