Optimising Tropical Fruit Juice Quality Using Thermosonication-Assisted Extraction via Blocked Face-Centered Composite Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Pulp Preparation
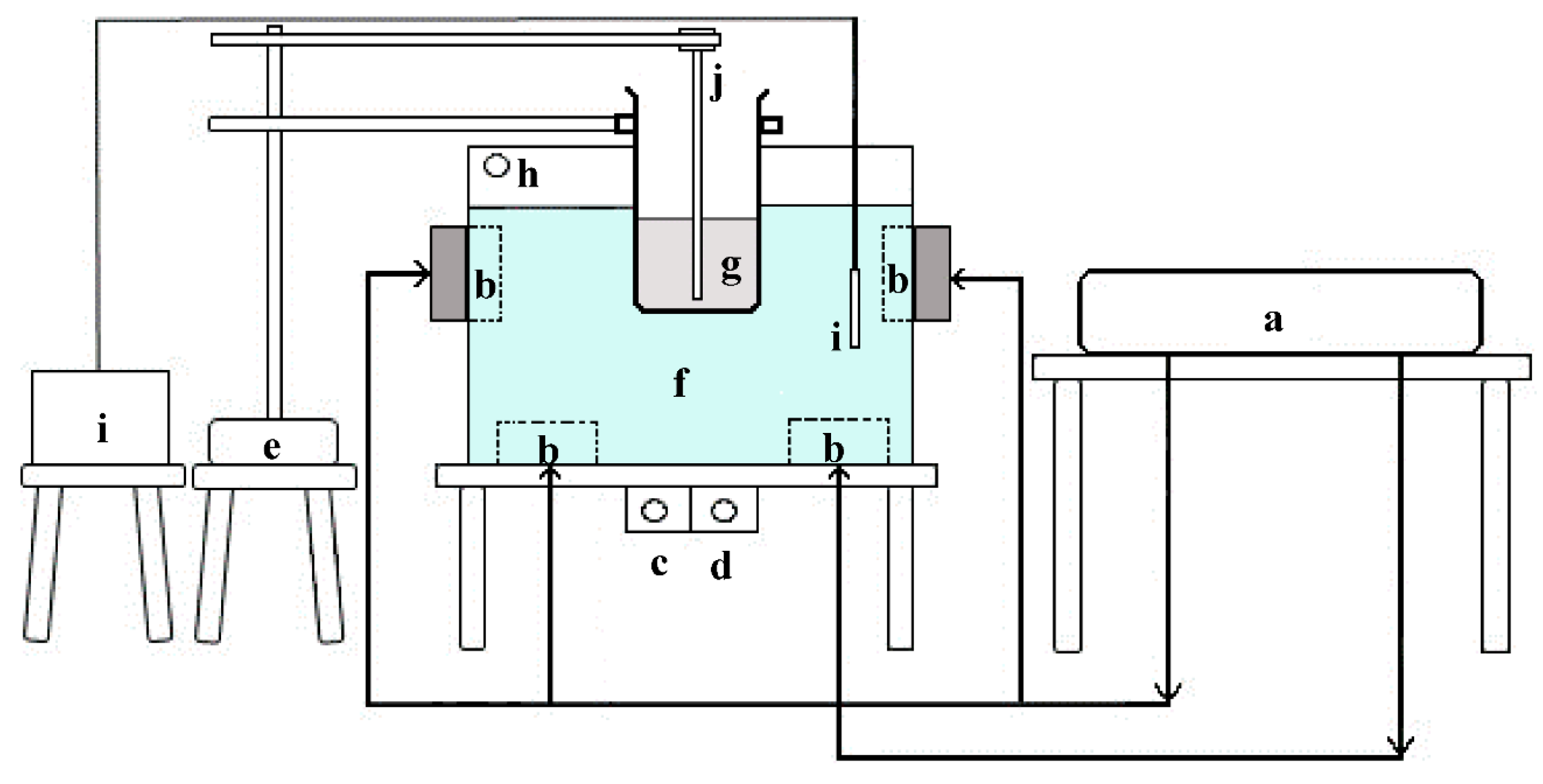
2.2. Thermosonic-Assisted Extraction
2.3. Blocked Face-Centred Central Composite Design
2.4. Response Analyses
3. Results and Discussion
3.1. Materials Characterisation
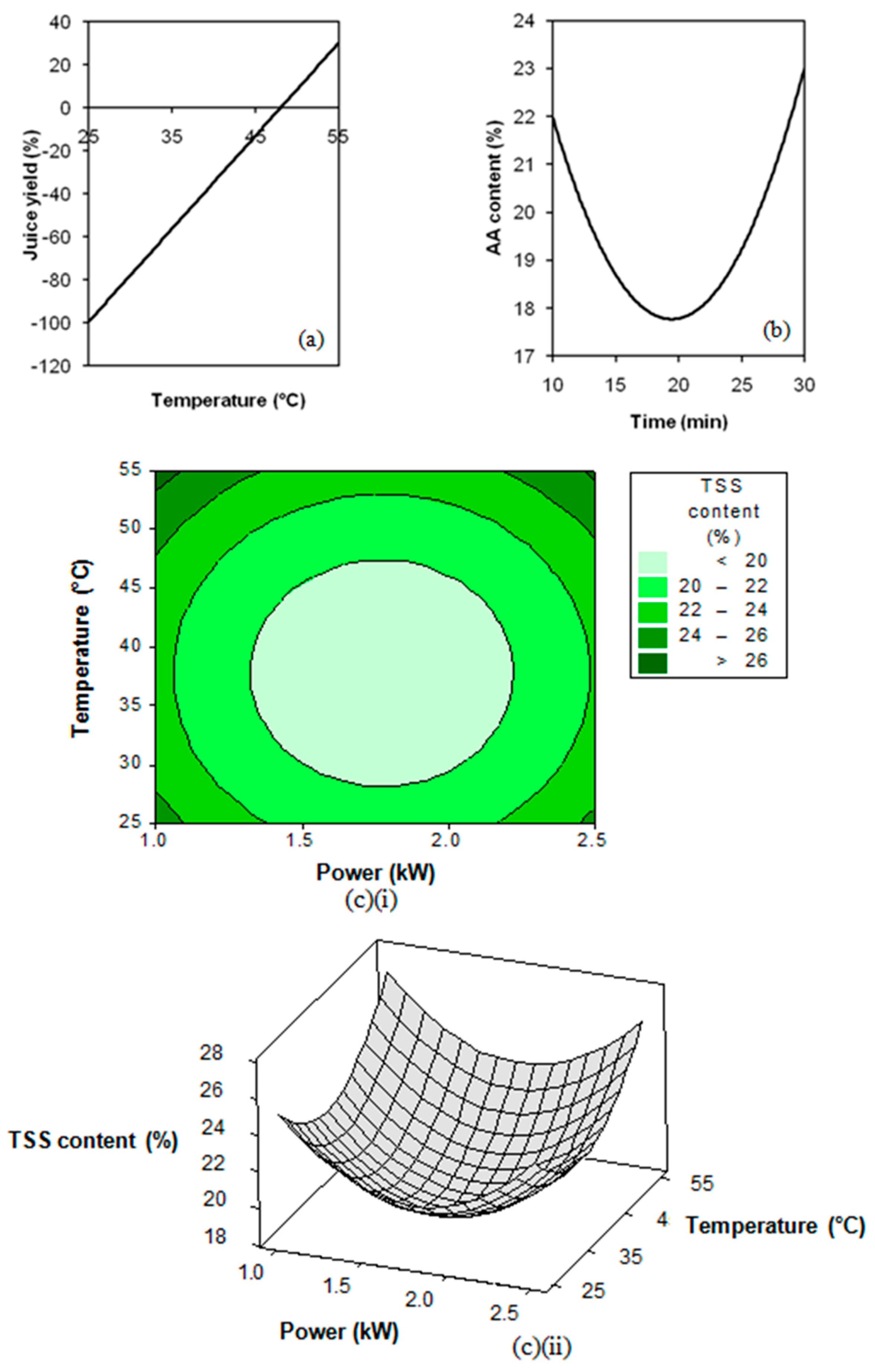
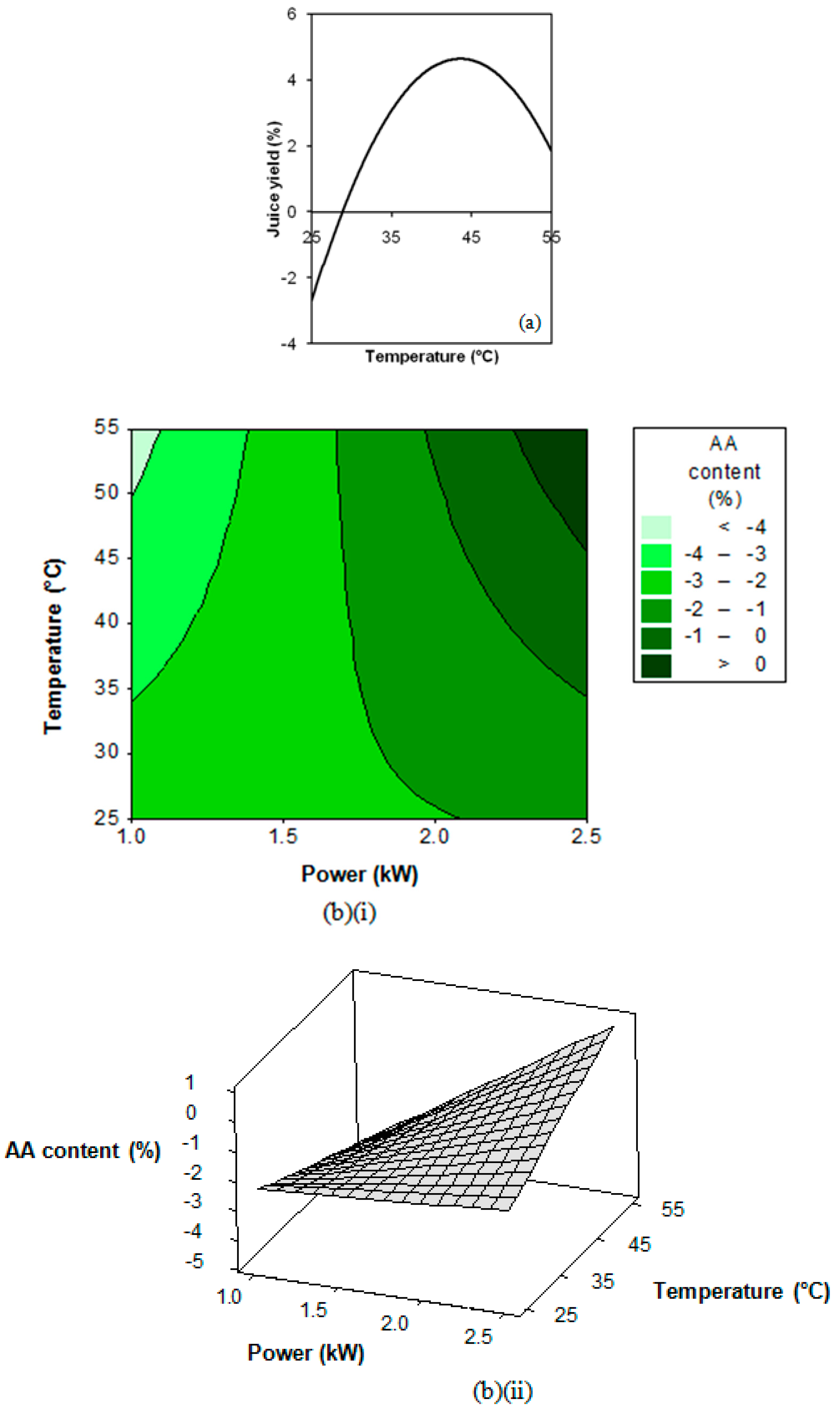
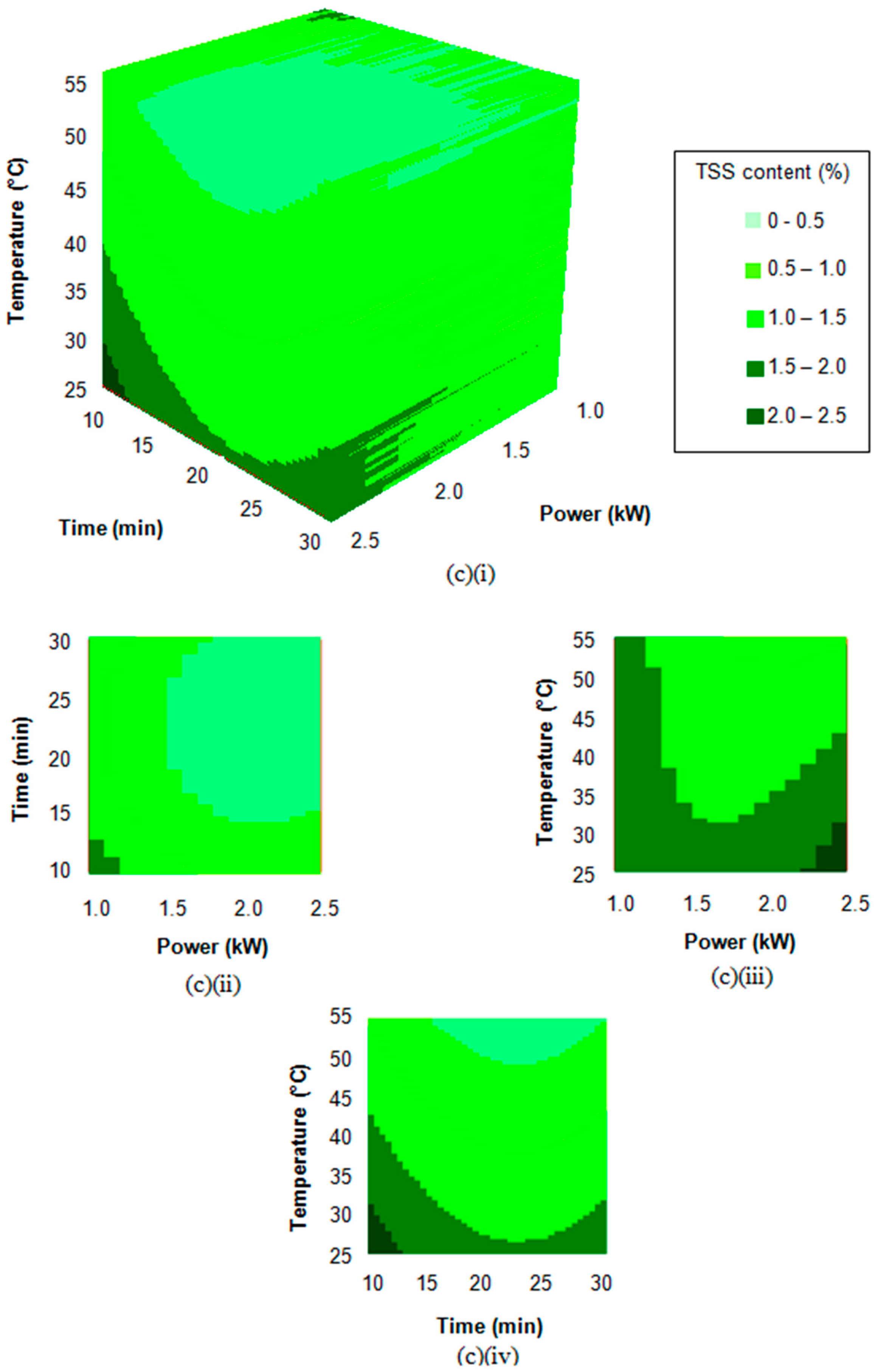
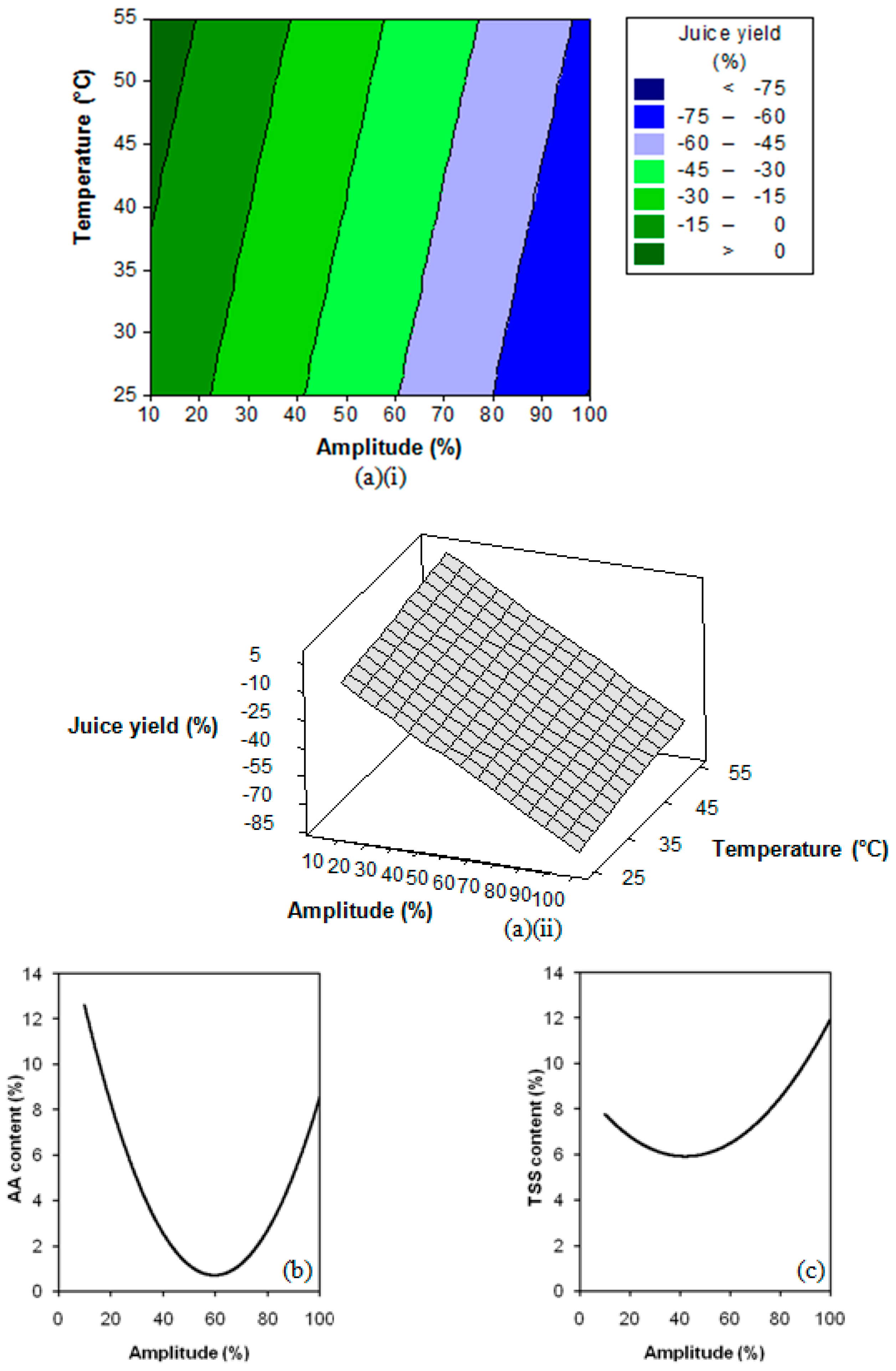
3.2. Response Surface Regression Analysis
3.3. Extraction Process Optimisation
3.4. Adequacy of Models and Verification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atawodi, S. Nigerian foodstuffs with prostate cancer chemopreventive polyphenols. Infect. Agents Cancer. 2011, 6 (Suppl. 2), S9. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Worrell, D.B.; Carrington, C.M.S.; Huber, D.J. Growth, maturation and ripening of soursop (Annona muricata L.) fruit. Sci. Hortic-Amsterdam. 1994, 57, 7–15. [Google Scholar] [CrossRef]
- Ko, Y.-M.; Wu, T.-Y.; Wu, Y.-C.; Chang, F.-R.; Guh, J.-Y.; Chuang, L.-Y. Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells. J. Ethnopharmacol. 2011, 137, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Chek Zaini, H.; Zaiton, H.; Zanariah, C.W.; Sakinah, N. High Fiber Cookies Made from Pink Guava (Psidium guajava) Decanter/Agro Waste. 2020. Available online: https://www.doc-developpement-durable.org/file/Arbres-Fruitiers/FICHES_ARBRES/goyavier_Psidium%20guajava/High%20fiber%20cookies%20made%20from%20pink%20guava.pdf (accessed on 18 November 2020).
- Nwokocha, L.M.; Williams, P.A. New starches: Physicochemical properties of sweetsop (Annona squamosa) and soursop (Anonna muricata) starches. Carbohyd Polym. 2009, 78, 462–468. [Google Scholar] [CrossRef]
- Economos, C.; Clay, W.D. Food, Nutrition and Agriculture: Nutritional and Health Benefits of Citrus Fruits. 1999. Available online: http://www.fao.org/docrep/x2650T/x2650t03.htm (accessed on 18 November 2020).
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour degradation and quality parameters of sonicated orange juice using response surface methodology. LWT Food Sci. Technol. 2008, 41, 1876–1883. [Google Scholar] [CrossRef]
- Adekunte, A.; Tiwari, B.K.; Scannell, A.; Cullen, P.J.; O’Donnell, C. Modelling of yeast inactivation in sonicated tomato juice. Int. J. Food Microbiol. 2010, 137, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.S.B.C.; Min-Tze, L.; Karim, A.A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Muthukumarappan, K.; Cullen, P.J. Effect of sonication on orange juice quality parameters during storage. Int. J. Food Sci. Tech. 2009, 44, 586–595. [Google Scholar] [CrossRef]
- Valero, M.; Recrosio, N.; Saura, D.; Muñoz, N.; Martí, N.; Lizama, V. Effects of ultrasonic treatments in orange juice processing. J. Food Eng. 2007, 80, 509–516. [Google Scholar] [CrossRef]
- Chemat, F.; Zill, E.H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Koshani, R.; Ziaee, E.; Niakousari, M.; Golmakani, M.T. Optimization of thermal and thermosonication treatments on pectin methyl esterase inactivation of sour orange juice (Citrus aurantium). J. Food Process. Preserv. 2014, 39, 567–573. [Google Scholar] [CrossRef]
- Tribess, T.B.; Tadini, C.C. Inactivation kinetics of pectin methylesterase in orange juice as a function of pH and temperature/time process conditions. J. Sci. Food Agric. 2006, 86, 1328–1335. [Google Scholar] [CrossRef]
- de Carvalho, J.M.; Maia, G.A.; da Fonseca, A.V.V.; de Sousa, P.H.M.; Rodrigues, S. Effect of processing on physicochemical composition, bioactive compounds and enzymatic activity of yellow mombin (Spondias mombin L.) tropical juice. J. Food Sci. Technol. 2013, 52, 1182–1187. [Google Scholar] [CrossRef][Green Version]
- Wu, J.; Gamage, T.V.; Vilkhu, K.S.; Simons, L.K.; Mawson, R. Effect of thermosonication on quality improvement of tomato juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 86–195. [Google Scholar] [CrossRef]
- Terefe, N.S.; Gamage, M.; Vilkhu, K.; Simons, L.; Mawson, R.; Versteeg, C. The kinetics of inactivation of pectin methylesterase and polygalacturonase in tomato juice by thermosonication. Food Chem. 2009, 117, 20–27. [Google Scholar] [CrossRef]
- Torres, E.F.; Bayarri, S.; Sampedro, F.; Martinez, A.; Carbonell, J.V. Improvement of the fresh taste intensity of processed clementine juice by separate pasteurization of its serum and pulp. Food Sci. Technol. Int. 2008, 14, 525–529. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Lee, K. Response surface optimization of ultrasonic-assisted extraction of carotenoids from oil palm (Elaeis guineensis Jacq.) fronds. Food Sci. Nutr. 2013, 1, 209–221. [Google Scholar] [CrossRef]
- Sin, H.N.; Yusof, S.; Abdul Hamid, N.S.; Rahman, R. Optimization of hot water extraction for sapodilla juice using response surface methodology. J. Food Eng. 2006, 74, 352–358. [Google Scholar] [CrossRef]
- Lee, W.C.; Yusof, S.; Hamid, N.S.A.; Baharin, B.S. Optimizing conditions for hot water extraction of banana juice using response surface methodology (RSM). J. Food Eng. 2006, 75, 473–479. [Google Scholar] [CrossRef]
- Cendres, A.; Chemat, F.; Maingonnat, J.F.; Renard, C.M.G.C. An innovative process for extraction of fruit juice using microwave heating. LWT Food Sci. Technol. 2011, 44, 1035–1041. [Google Scholar] [CrossRef]
- Chin, L.N.; Tan, C.M.; Pa, N.F.; Yusof, Y.A. Method and Apparatus for High Intensity Ultrasonic Treatment of Baking Materials. U.S. Patent 2013/01894.07 A1, 25 July 2013. [Google Scholar]
- Carley, K.M.; Kamneva, N.Y.; Reminga, J. Response Surface Methodology; Technical Report CMU-ISRI-04-136; CASOS: Pittsburgh, PA, USA, 2004. [Google Scholar]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wires Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Lenth, R.V. Response-surface methods in R using RSM. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef]
- Noordin, M.Y.; Venkatesh, V.C.; Sharif, S.; Elting, S.; Abdullah, A. Application of response surface methodology in describing the performance of coated carbide tools when turning AISI 1045 steel. J. Mater. Process Technol. 2004, 145, 46–58. [Google Scholar] [CrossRef]
- Cheng, L.H.; Soh, C.Y.; Liew, S.C.; Teh, F.F. Effects of sonication and carbonation on guava juice quality. Food Chem. 2007, 104, 1396–1401. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Trappey, A.F.; Johnson, C.E.; Wilson, P.W. Characterization of juice extraction methods utilizing fresh mayhaw (Crataegus opaca Hook.) fruit. Int. J. Fruit Sci. 2008, 8, 318–331. [Google Scholar] [CrossRef]

| Batch | Run | Block | Intensity a | Time (minutes) | Temperature (°C) |
|---|---|---|---|---|---|
| 1 b | 1 | 1 | −1 | −1 | −1 |
| 2 | 1 | 1 | 1 | −1 | |
| 3 | 1 | 1 | −1 | 1 | |
| 4 | 1 | −1 | 1 | 1 | |
| 5 | 1 | 0 | 0 | 0 | |
| 6 | 1 | 0 | 0 | 0 | |
| 7 | 2 | 1 | −1 | −1 | |
| 8 | 2 | −1 | 1 | −1 | |
| 9 | 2 | −1 | −1 | 1 | |
| 10 | 2 | 1 | 1 | 1 | |
| 11 | 2 | 0 | 0 | 0 | |
| 12 | 2 | 0 | 0 | 0 | |
| 13 | 3 | −1 | 0 | 0 | |
| 14 | 3 | 1 | 0 | 0 | |
| 15 | 3 | 0 | −1 | 0 | |
| 16 | 3 | 0 | 1 | 0 | |
| 17 | 3 | 0 | 0 | −1 | |
| 18 | 3 | 0 | 0 | 1 | |
| 19 | 3 | 0 | 0 | 0 | |
| 20 | 3 | 0 | 0 | 0 |
| Response | Fresh Fruit Pulp | Pulp Mixture Sample | ||||
|---|---|---|---|---|---|---|
| Guava | Pomelo | Soursop | Guava | Pomelo | Soursop | |
| Juice yield (%) | ND | ND | ND | 8.705 ±4.619 | 71.532 ±7.980 | 19.402 ±5.381 |
| AA content (mg AA/100mL) | 51.422 ±11.751 | 38.080 ±3.017 | 13.669 ±4.404 | 18.834 ±4.684 | 20.829 ±1.857 | 6.460 ±1.638 |
| TSS content (°Brix) | 8.024 ±1.594 | 10.648 ±0.466 | 15.026 ±0.868 | 3.231 ±0.601 | 5.156 ±0.211 | 6.109 ±0.501 |
| Response | Model |
|---|---|
| Guava | |
| Control | |
| Direct thermo-sonication | |
| Indirect thermo-sonication | |
| Pomelo | |
| Control | |
| Direct thermo-sonication | |
| Indirect thermo-sonication | |
| Soursop | |
| Control | |
| Direct thermo-sonication | |
| Indirect thermo-sonication |
| Factors | Guava | Pomelo | Soursop |
|---|---|---|---|
| Control | |||
| Motion frequency (rpm) | 20 | NS | 180 |
| Time (minutes) | 120 | 120 | 120 |
| Temperature (°C) | 55 | 55 | 55 |
| Direct thermosonication | |||
| Amplitude (%) | 100 | 33 | 10 |
| Time (minutes) | 10 | 10 | NS |
| Temperature (°C) | 39 | 54 | 55 |
| Indirect thermosonication | |||
| Power (kW) | 1 | 2.5 | 1 |
| Time (minutes) | 30 | 23 | 21 |
| Temperature (°C) | 55 | 54 | 55 |
| Parameters Changes (%) | Control | Direct Thermosonication | Indirect Thermosonication | |||
|---|---|---|---|---|---|---|
| Predicted | Experiment | Predicted | Experiment | Predicted | Experiment | |
| Guava | ||||||
| Juice yield | 6.7 | 7.6 | −85.0 | −79.3 | 30.1 | 28.1 |
| AA content | 22.9 | 25.1 | 32.4 | 32.5 | 23.0 | 27.1 |
| TSS content | 25.7 | 26.5 | 26.3 | 25.9 | 26.8 | 27.4 |
| Pomelo | ||||||
| Juice yield | 5.8 | 8.7 | 1.3 | 0.6 | 2.4 | 3.7 |
| AA content | −2.4 | −3.5 | −1.1 | −2.5 | 0.7 | 1.2 |
| TSS content | 1.5 | 1.7 | 2.6 | 2.1 | 2.2 | 3.4 |
| Soursop | ||||||
| Juice yield | 53.6 | 48.3 | 7.2 | 9.2 | 21.0 | 20. 8 |
| AA content | −9.5 | −8.8 | 12.6 | 14.7 | −6.7 | −5.1 |
| TSS content | 22.1 | 25.2 | 7.8 | 7.7 | 10.9 | 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, N.; Chin, N.L. Optimising Tropical Fruit Juice Quality Using Thermosonication-Assisted Extraction via Blocked Face-Centered Composite Design. Processes 2021, 9, 3. https://doi.org/10.3390/pr9010003
Abdullah N, Chin NL. Optimising Tropical Fruit Juice Quality Using Thermosonication-Assisted Extraction via Blocked Face-Centered Composite Design. Processes. 2021; 9(1):3. https://doi.org/10.3390/pr9010003
Chicago/Turabian StyleAbdullah, Norazlin, and Nyuk Ling Chin. 2021. "Optimising Tropical Fruit Juice Quality Using Thermosonication-Assisted Extraction via Blocked Face-Centered Composite Design" Processes 9, no. 1: 3. https://doi.org/10.3390/pr9010003
APA StyleAbdullah, N., & Chin, N. L. (2021). Optimising Tropical Fruit Juice Quality Using Thermosonication-Assisted Extraction via Blocked Face-Centered Composite Design. Processes, 9(1), 3. https://doi.org/10.3390/pr9010003





