Biodegradation Kinetic Studies of Phenol and p-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells
Abstract
:1. Introduction
2. Kinetic Model Development
2.1. Growth Kinetics of Free Cells Batch System
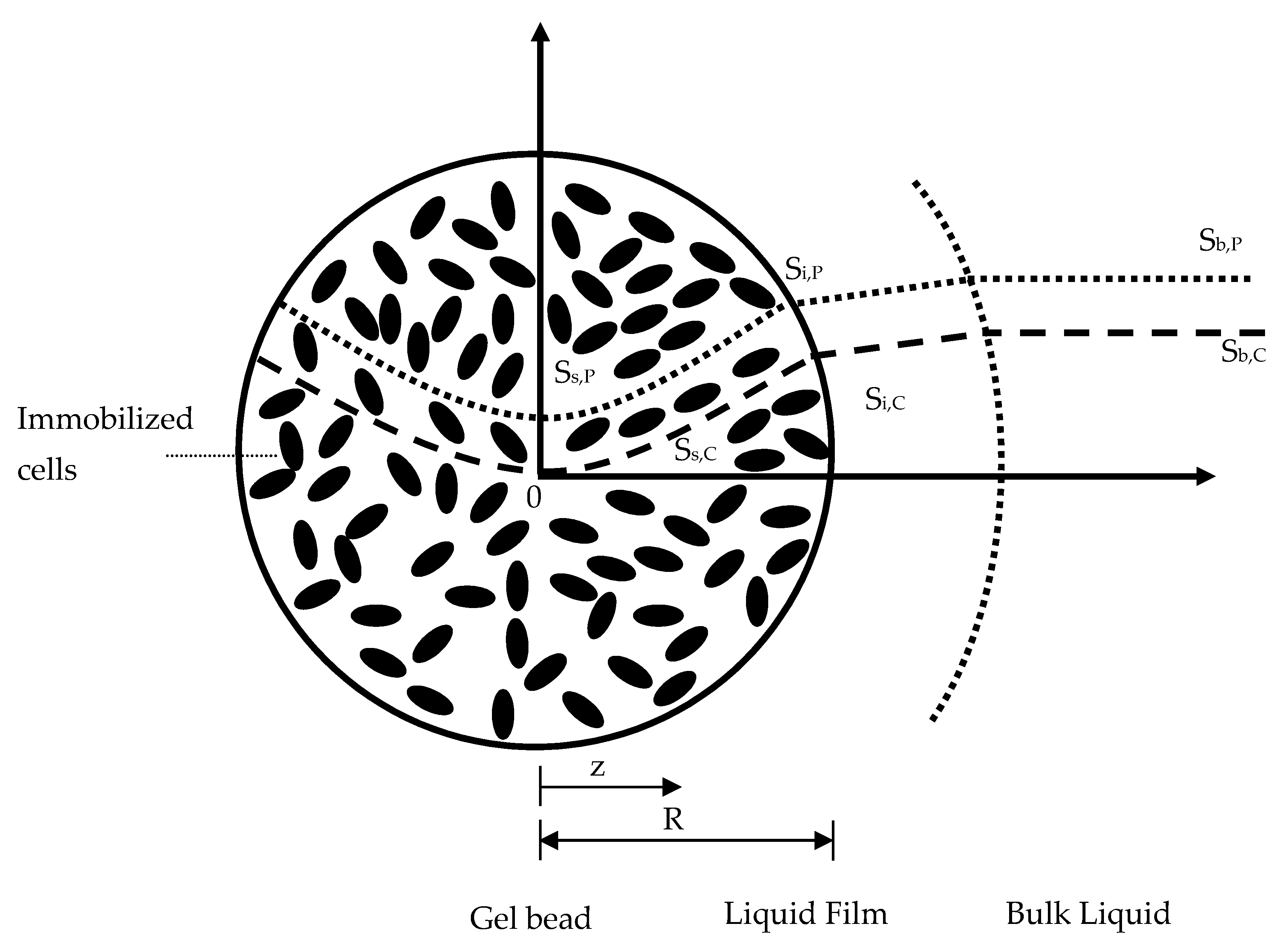
2.2. Conceptual Basis of Immobilized Cells in the Continuous Stirred-Tank Bioreactor
2.3. Kinetic Model of the Entrapped Cell in the Continuous Stirred-Tank Bioreactor
3. Materials and Methods
3.1. Chemicals
3.2. Cell Cultivation for Immobilization
3.3. Entrapment of Cells in Ca-Alginate
3.4. Batch Experiments
3.5. Analysis of P. putida Cells, Phenol and p-Cresol
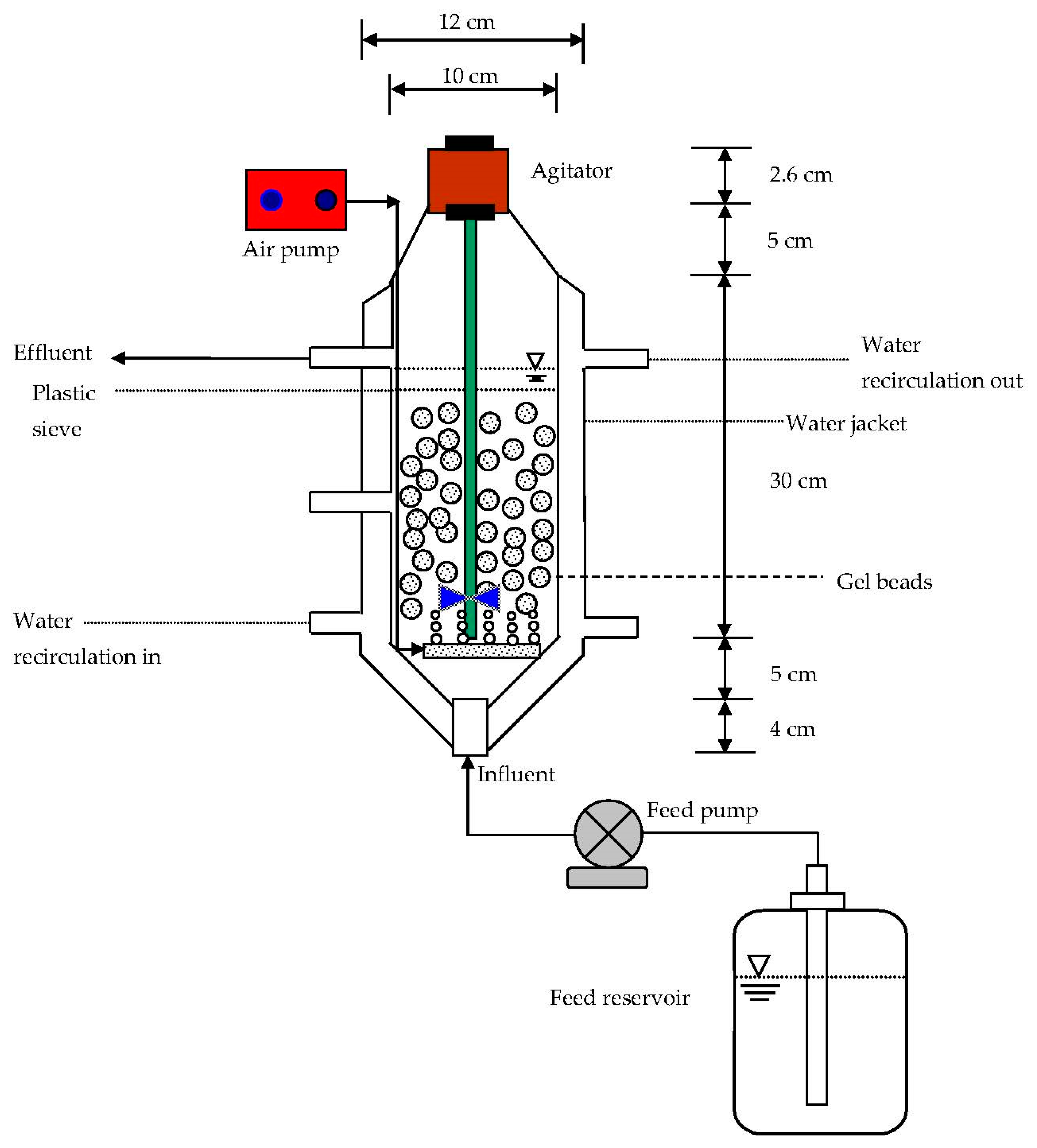
3.6. Continuous-Flow Bioreactor
4. Results and Discussion
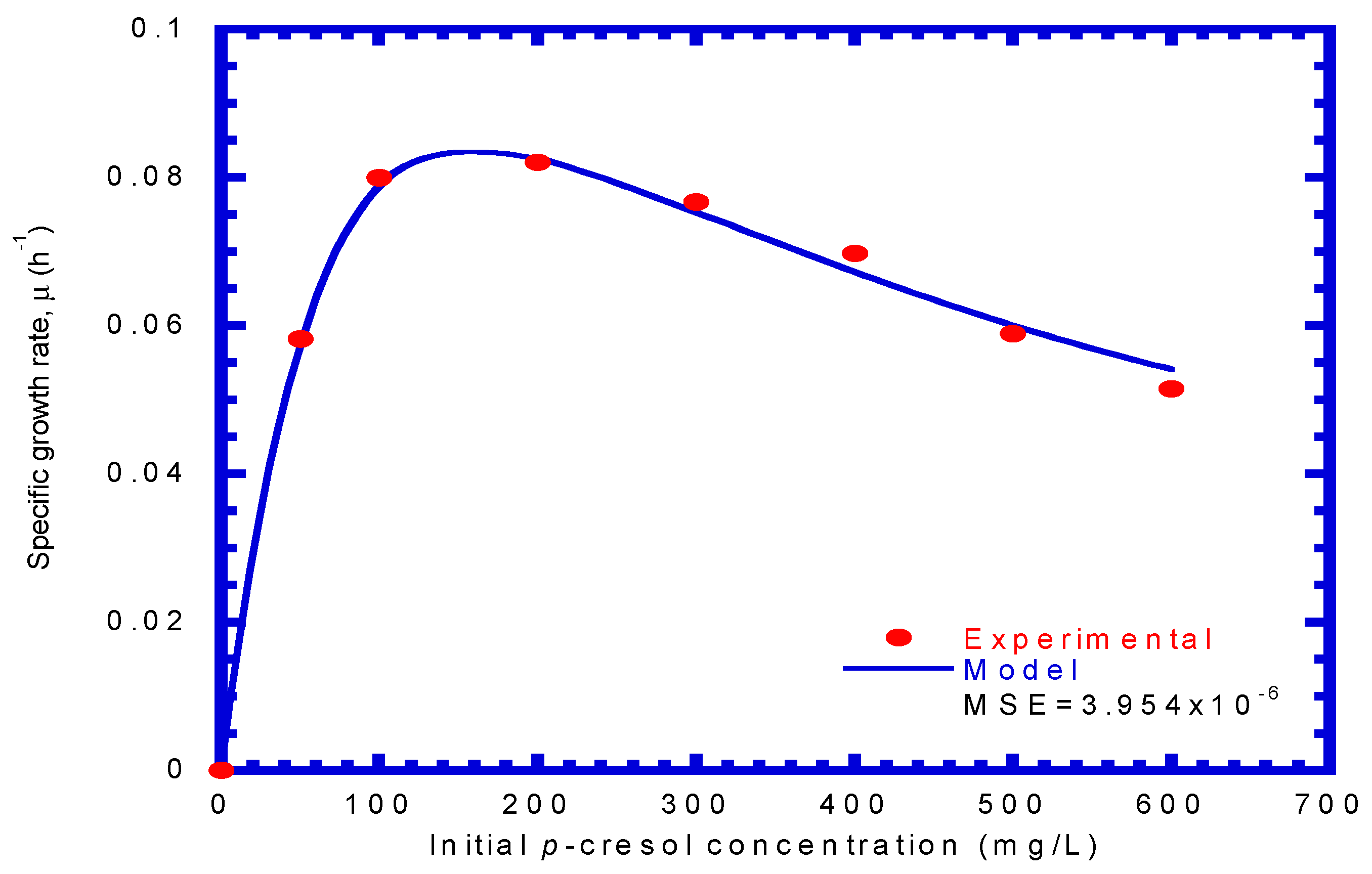
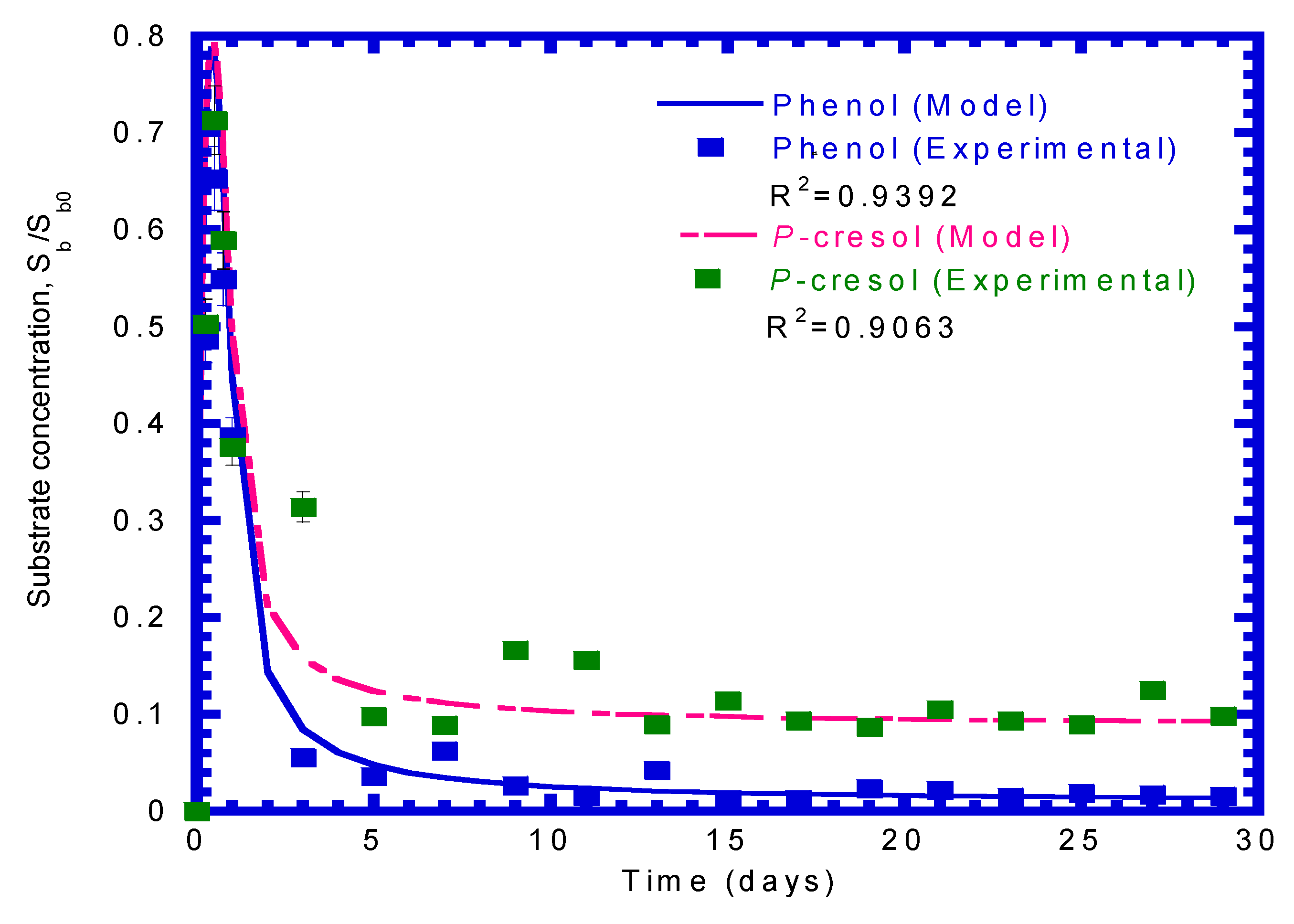
4.1. Biodegradation of Phenol or p-Cresol in Batch Experiments
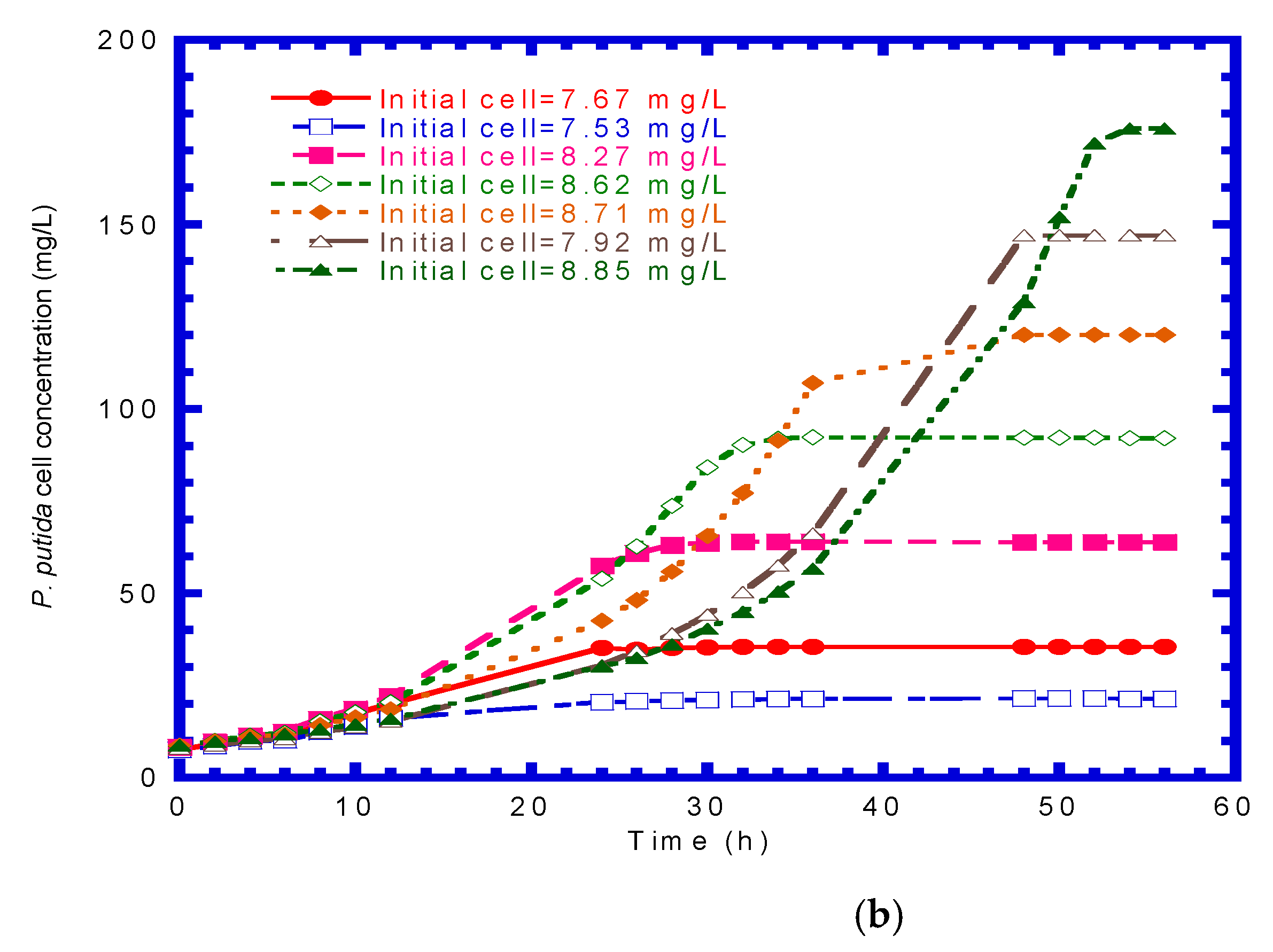
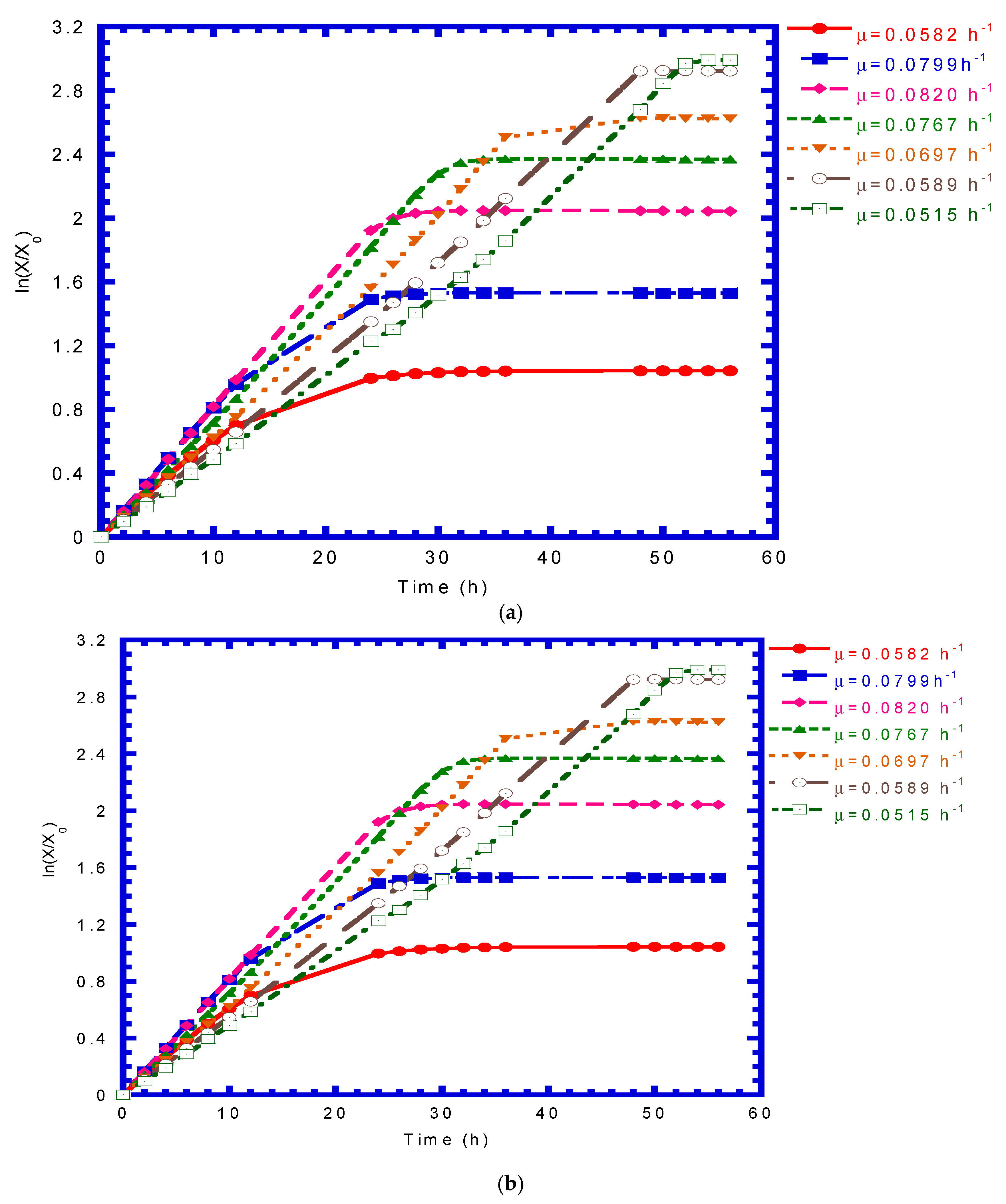
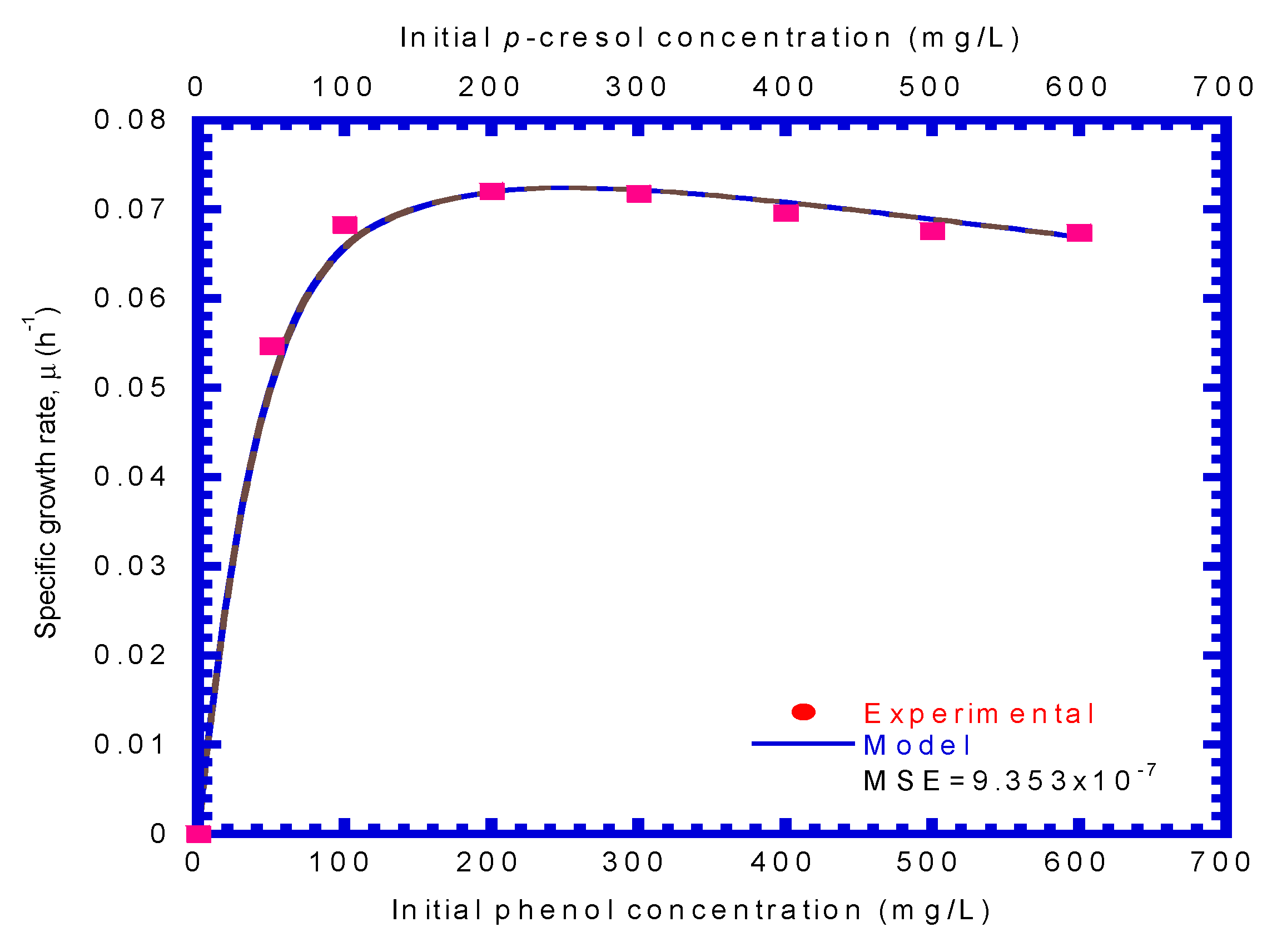
4.2. P. putida Cells Growth on Phenol or p-Cresol
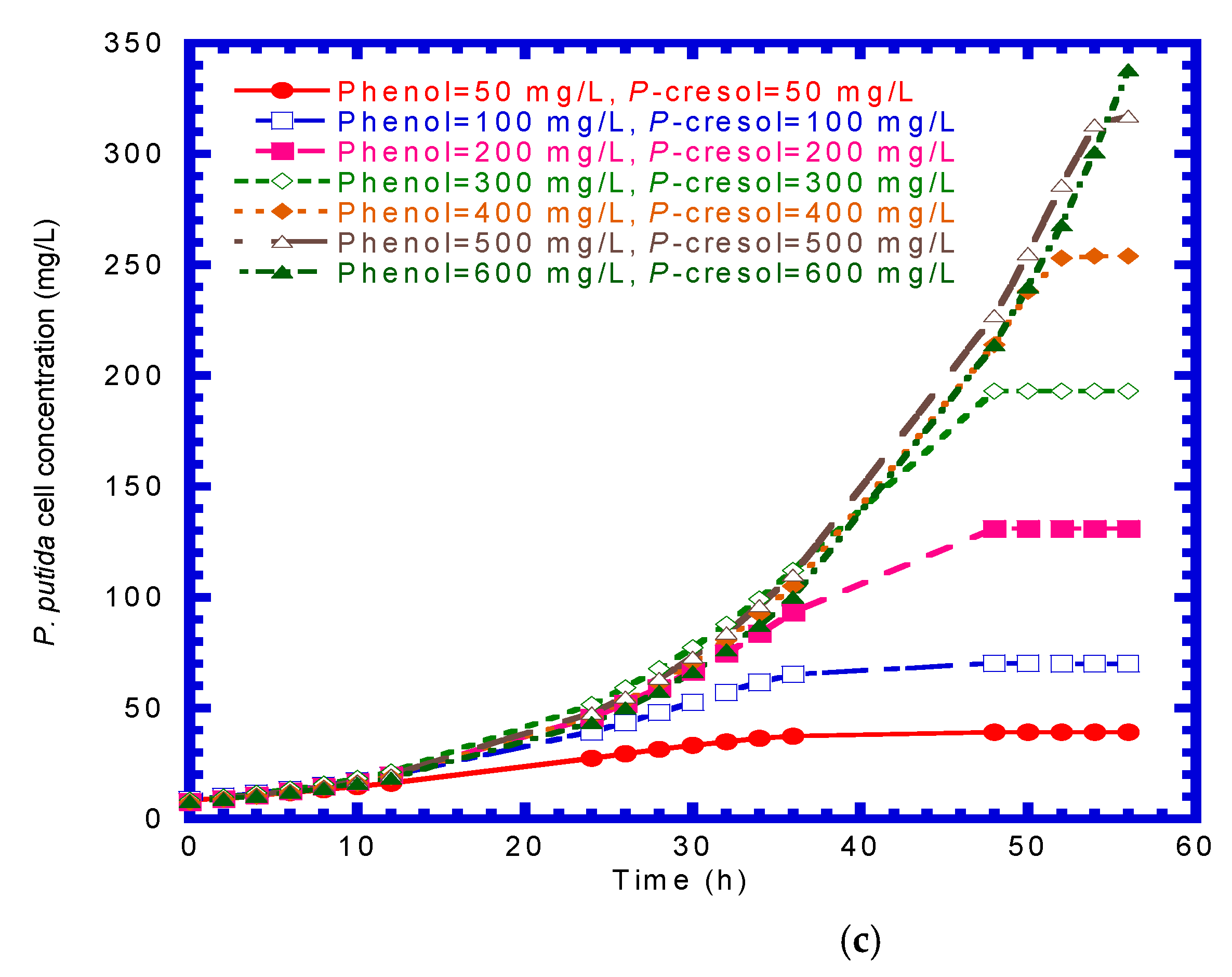
4.3. Phenol Plus p-Cresol in Binary Substrates System
4.4. Mass Transfer Coefficients
4.5. Biodegradation of Phenol and p-Cresol in Immobilized Cells
4.6. Immobilized Cells Growth
4.7. Flux into Gel Bead
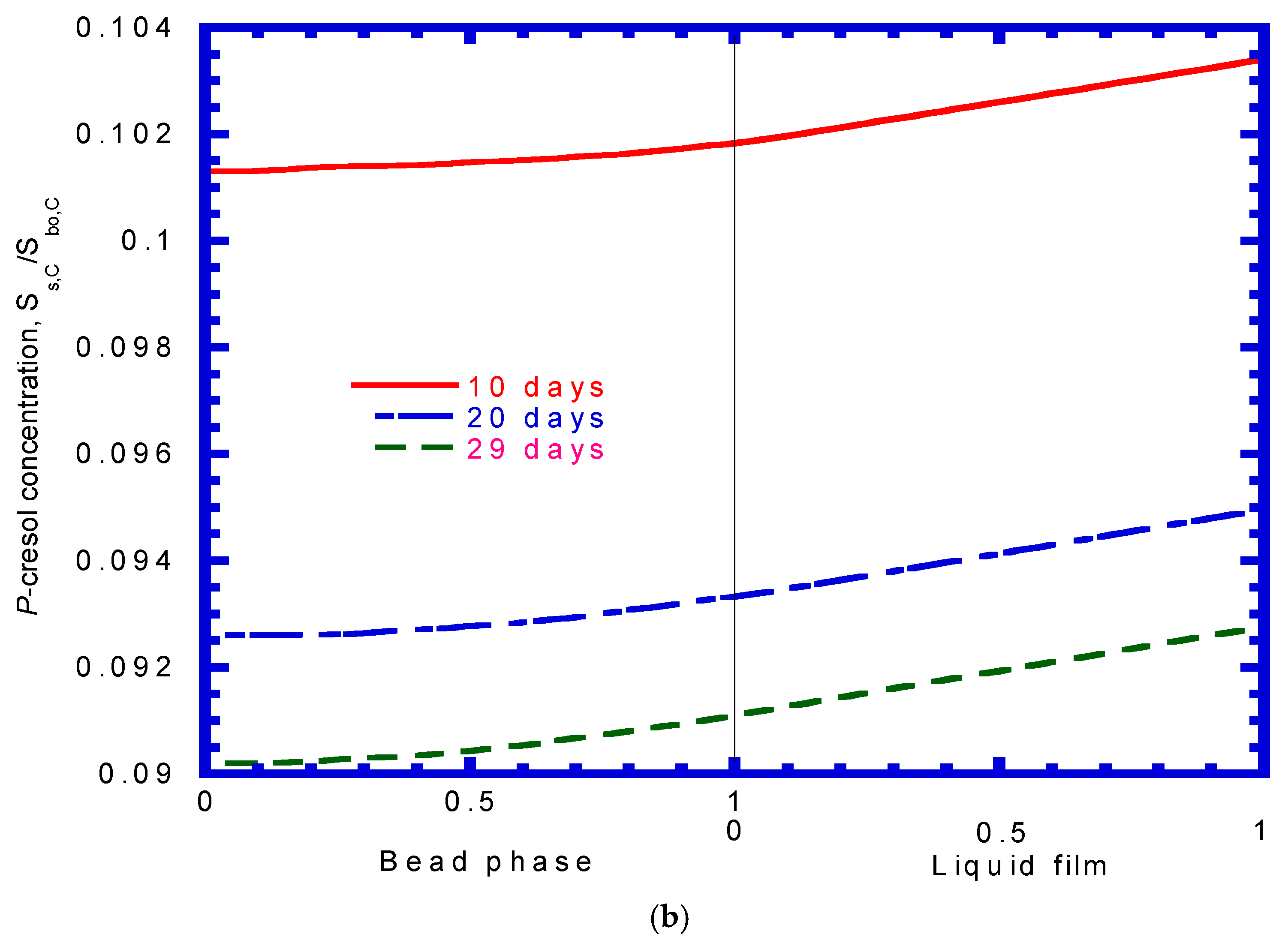
4.8. Phenol Concentration Profiles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, A.; Ghoshal, A.K. Phenol degradation performance by isolated Bacillus cereus immobilized in alginate. Int. Biodeterior. Biodegrad. 2011, 65, 1052–1060. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Pakshirajan, K.; Ghosh, P.K. Enhancing the biodegradation of 4-chlorophenol by Arthrobacter chlorophenolics A6 via medium development. Int. Biodeterior. Biodegrad. 2010, 64, 474–480. [Google Scholar] [CrossRef]
- Mollaei, M.; Abdollahpour, S.; Atashgahi, S.; Abbasi, H.; Masoomi, F.; Rad, I.; Lotfi, A.S.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Enhanced phenol degradation by pseudomonas sp. SA01: Gaining insight into the novel single and hybrid immobilizations. J. Hazard. Mater. 2010, 175, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Chaudhari, A. Biodegradation of p-nitro phenol by P. putida. Bioresour. Technol. 2006, 97, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Pakshirajan, K.; Chugh, D.; Saravanan, P. Feasibility of m-cresol degradation using an indigenous mixed microbial culture with glucose as co-substrate. Clean Technol. Environ. Policy 2007, 10, 303–308. [Google Scholar] [CrossRef]
- Bai, J.; Wen, J.P.; Li, H.M.; Jiang, Y. Kinetic modeling of growth and biodegradation of phenol and m-cresol using Alcaligenes faecalis. Process Biochem. 2007, 42, 510–517. [Google Scholar] [CrossRef]
- Masque, C.; Nolla, M.; Bordons, A. Selection and adaptation of a phenol degrading strain of Pseudomonas. Biotechnol. Lett. 1987, 9, 655–660. [Google Scholar] [CrossRef]
- Soda, S.; Ike, M.; Fujita, M. Effects of inoculation of a sequencing batch activated sludge process treating phenol. J. Ferment. Bioeng. 1998, 86, 90–96. [Google Scholar] [CrossRef]
- Aksu, Z.; Bülbül, G. Determination of the effective diffusion coefficient of phenol in Ca-alginate-immobilized P. putida beads. Enzym. Microb. Technol. 1999, 25, 344–348. [Google Scholar] [CrossRef]
- Loh, K.C.; Chung, T.S.; Wei-Fern, A. Immobilized-cell membrane bioreactor for high-strength phenol wastewater. J. Environ. Eng. 2000, 126, 75–79. [Google Scholar] [CrossRef]
- Jiangliang, X.; Yanan, W.; Shi, K.; Xiao, X.; Gao, Y.; Li, L.; Qiao, Y. Study on the degradation performance and kinetics of immobilized cells in straw-alginate beads in marine environment. Bioresour. Technol. 2019, 280, 88–94. [Google Scholar]
- Parvanova-Mancheva, T.; Vasileva, E.; Beschkov, V.; Gerginova, M.; Stoilova-Disheva, M.; Alexieva, Z. Biodegradation potential of Pseudomonas putida to phenol compared to Xanthobacter autotrophicus GJ10 and Pseudomonas denitrificans strains. J. Chem. Technol. Metall. 2020, 55, 23–27. [Google Scholar]
- González, G.; Herrera, G.; García, M.T.; Peña, M. Biodegradation of phenolic industrial wastewater in a fluidized bed bioreactor with immobilized cells of Pseudomonas putida. Bioresour. Technol. 2001, 80, 137–142. [Google Scholar] [CrossRef]
- Yu, Y.G.; Loh, K.C. Inhibition of p-cresol on aerobic biodegradation of carbazole and sodium salicylate by Pseudomonas putida. Water Res. 2002, 36, 1794–1802. [Google Scholar] [CrossRef]
- Loh, K.C.; Ranganath, S. External-loop fluidized bed airlift bioreactor (EFBAB) for the cometabolic biotransformation of 4-chlorophenol (4-cp) in the presence of phenol. Chem. Eng. Sci. 2005, 60, 6313–6319. [Google Scholar] [CrossRef]
- Singh, U.; Arora, N.K.; Sachan, P. Simultaneous biodegradation of phenol and cyanide present in coke-oven effluent using immobilized Pseudomonas putida and Pseudomonas stutzeri. Brazil. J. Microbiol. 2018, 49, 38–44. [Google Scholar] [CrossRef]
- Ehrhardt, H.M.; Rehm, H.J. Semicontinuous and continuous degradation of phenol by Pseudomonas putida P8 adsorbed on activated carbon. Appl. Environ. Microbiol. 1989, 30, 312–317. [Google Scholar] [CrossRef]
- Chen, K.C.; Lin, Y.H.; Chen, W.H.; Liu, Y.C. Degradation of phenol by PAA-immobilized Candida tropicalis. Enzym. Microb. Technol. 2002, 31, 490–497. [Google Scholar] [CrossRef]
- Chung, T.P.; Wu, C.Y.; Juang, R.S. Improved dynamic analysis on cell growth with substrate inhibition using two-phase models. Biochem. Eng. J. 2005, 25, 209–217. [Google Scholar] [CrossRef]
- Bera, S.; Kauser, H.; Mohanty, K. Optimization of p-cresol biodegradation using novel bacterial strains from petroleum hydrocarbon fallout. J. Water Process Eng. 2019, 31, 1–6. [Google Scholar] [CrossRef]
- Arya, D.; Kumar, S.; Kumar, S. Biodegradation dynamics and cell maintenance for the treatment of resorcinol and p-cresol by filamentous fungus Gliomastix indicus. J. Hazard. Mater. 2011, 198, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Klinzing, G.; Blanch, H.W. Competition for mixed substrates by microbial populations. Biotechnol. Bioeng. 1977, 19, 1193–1210. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.T.; Huang, Y.Y.; Ye, J.; Guo, Y.H. Study on the kinetic characteristics of the asymmetric production of R-(−)-mandelic acid with immobilized Saccharomyces cerevisiae FD11b. Biochem. Eng. J. 2008, 39, 311–318. [Google Scholar] [CrossRef]
- González, G.; Herrera, M.G.; García, M.T.; Peña, M.M. Biodegradation of phenol in a continuous process: Comparative study of stirred tank and fluidized-bed bioreactors. Bioresour. Technol. 2001, 76, 245–251. [Google Scholar] [CrossRef]
- Wang, S.W.; Loh, K.C.; Chua, S.S. Prediction of critical cell growth behavior of Pseudomonas putida to maximize the cometabolism of 4-chlorophenol with phenol and sodium glutamate as carbon source. Enzym. Microb. Technol. 2003, 32, 422–430. [Google Scholar] [CrossRef]
- Panigrahy, N.; Barik, M.; Sahoo, R.K.; Sahoo, N.K. Metabolic profile analysis and kinetics of p-cresol biodegradation by an indigenous Pseudomonas citronellolis NS1 isolated from coke oven wastewater. Int. Biodeterior. Biodegrad. 2020, 147, 1–9. [Google Scholar] [CrossRef]
- Juang, R.S.; Tsai, S.Y. Growth kinetics of Pseudomonas putida in the biodegradation of single and mixed phenol and sodium salicylate. Biochem. Eng. J. 2006, 31, 133–140. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kumar, S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- Wang, S.J.; Loh, K.C. Modeling the role of metabolic intermediates in kinetics of phenol biodegradation. Enzym. Microb. Technol. 1999, 25, 177–184. [Google Scholar] [CrossRef]
- Chung, T.P.; Tseng, H.Y.; Juang, R.S. Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochem. 2003, 38, 1497–1507. [Google Scholar] [CrossRef]
- Monteiro, A.A.; Boaventura, R.A.; Rodrigues, A.E. Phenol biodegradation by Pseudomonas putida DSM 548 in a batch reactor. Biochem. Eng. J. 2000, 6, 45–49. [Google Scholar] [CrossRef]
- Banerjee, I.; Modak, J.M.; Bandopadhyay, K.; Das, D.; Maiti, B.R. Mathematical model for evaluation of mass transfer limitations in phenol biodegradation by immobilized Pseudomonas putida. J. Biotechnol. 2001, 87, 211–223. [Google Scholar] [CrossRef]
- Abuhamed, T.; Bayraktar, E.; Mehmetoğlu, T.; Mehmetoğlu, Ü. Kinetics model for growth of Pseudomonas putida F1 during benzene, toluene and phenol biodegradation. Process Biochem. 2004, 39, 983–988. [Google Scholar] [CrossRef]
- Saravanan, P.; Pakshirajan, K.; Saha, P. Biodegradation of phenol and m-cresol in a batch and fed batch operated internal loop airlift bioreactor by indigenous mixed microbial culture predominantly Pseudomonas sp. Bioresour. Technol. 2008, 99, 8553–8558. [Google Scholar] [CrossRef] [PubMed]
- Wakao, N.; Smith, J.M. Diffusion and reaction in porous catalysts. Ind. Eng. Chem. Fund. 1964, 2, 123–127. [Google Scholar] [CrossRef]
- Korgel, B.A.; Rotem, A.; Monbonquett, H.G. Effective diffusivity of galactose in calcium alginate gels containing immobilized Zymonmonas mobilis. Biotechnol. Prog. 1992, 8, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.K.; Ho, C.S. Correlation of cell volume fractions with cell concentrations in fermentation media. Biotechnol. Bioeng. 1988, 32, 95–99. [Google Scholar] [CrossRef]
- Wilke, C.E.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Brian, P.L.T.; Hales, H.B. Effect of transpiration and changing diameter on heat and mass transfer to sphere. AIChE J. 1969, 15, 419–425. [Google Scholar] [CrossRef]
- Yadzir, Z.H.M.; Shukor, M.Y.; Ahmad, A.; Nazir, M.S.; Shah, S.M.U.; Abdullah, M.A. Phenol removal by newly isolated Acinetobacter baumannii strain Serdang 1 in a packed-bed column reactor. Desalination Water Treat. 2016, 57, 13307–13317. [Google Scholar] [CrossRef]
- Basak, B.; Jeon, B.H.; Kurade, M.B.; Saratale, G.D.; Bhunia, B.; Chatterjee, P.K.; Dey, A. Biodegradation of high concentration phenol using sugarcane bagasse immobilized Candida tropicalis PHB5 in a packed-bed column reactor. Ecotoxicol. Environ. Saf. 2019, 180, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Removal of phenol from aqueous solution using acid-modified Pseudomonas putida-sepiolite/ZIF-8 bio-nanocomposites. Chemosphere 2020, 239, 124708. [Google Scholar] [CrossRef] [PubMed]






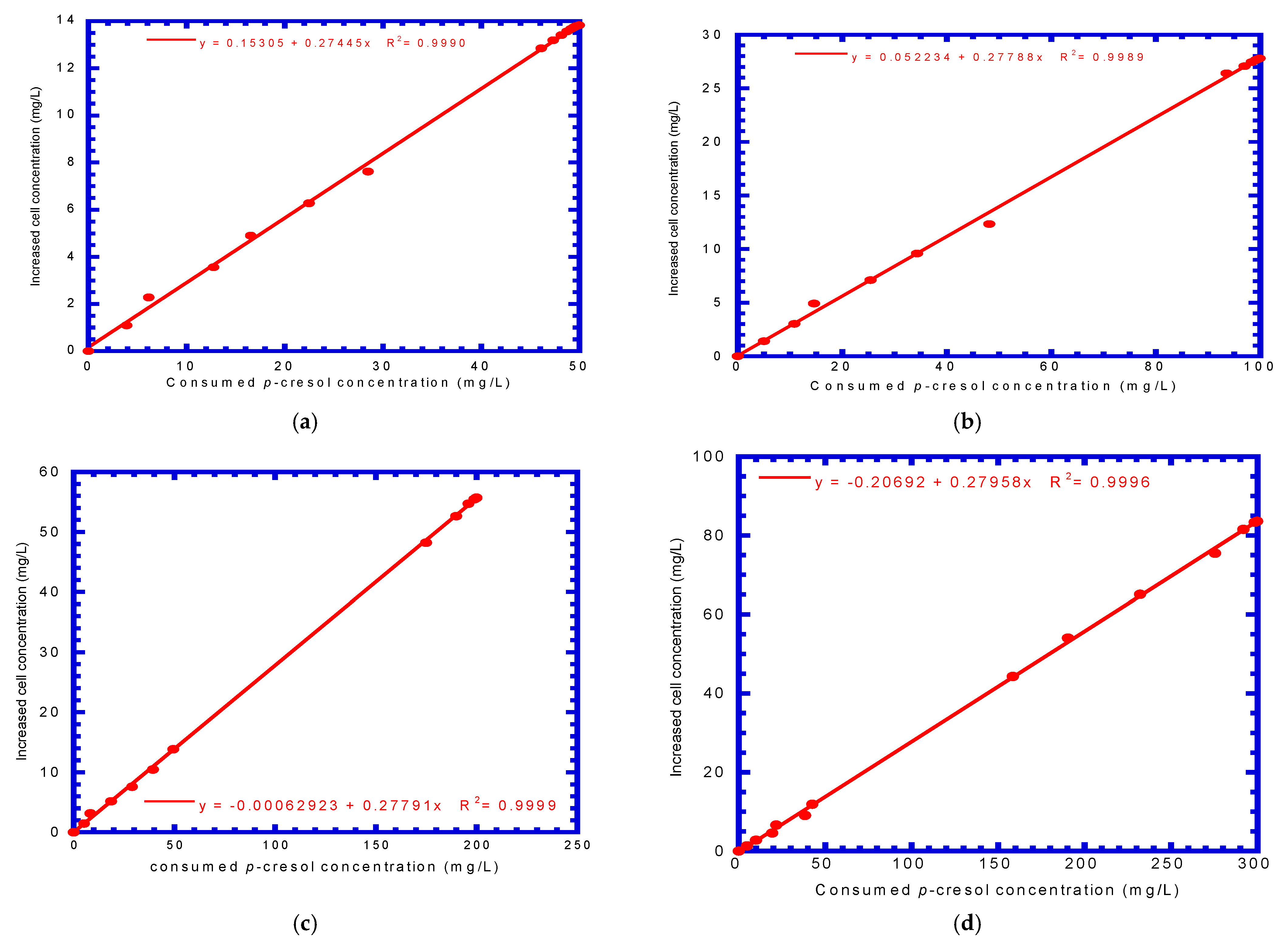
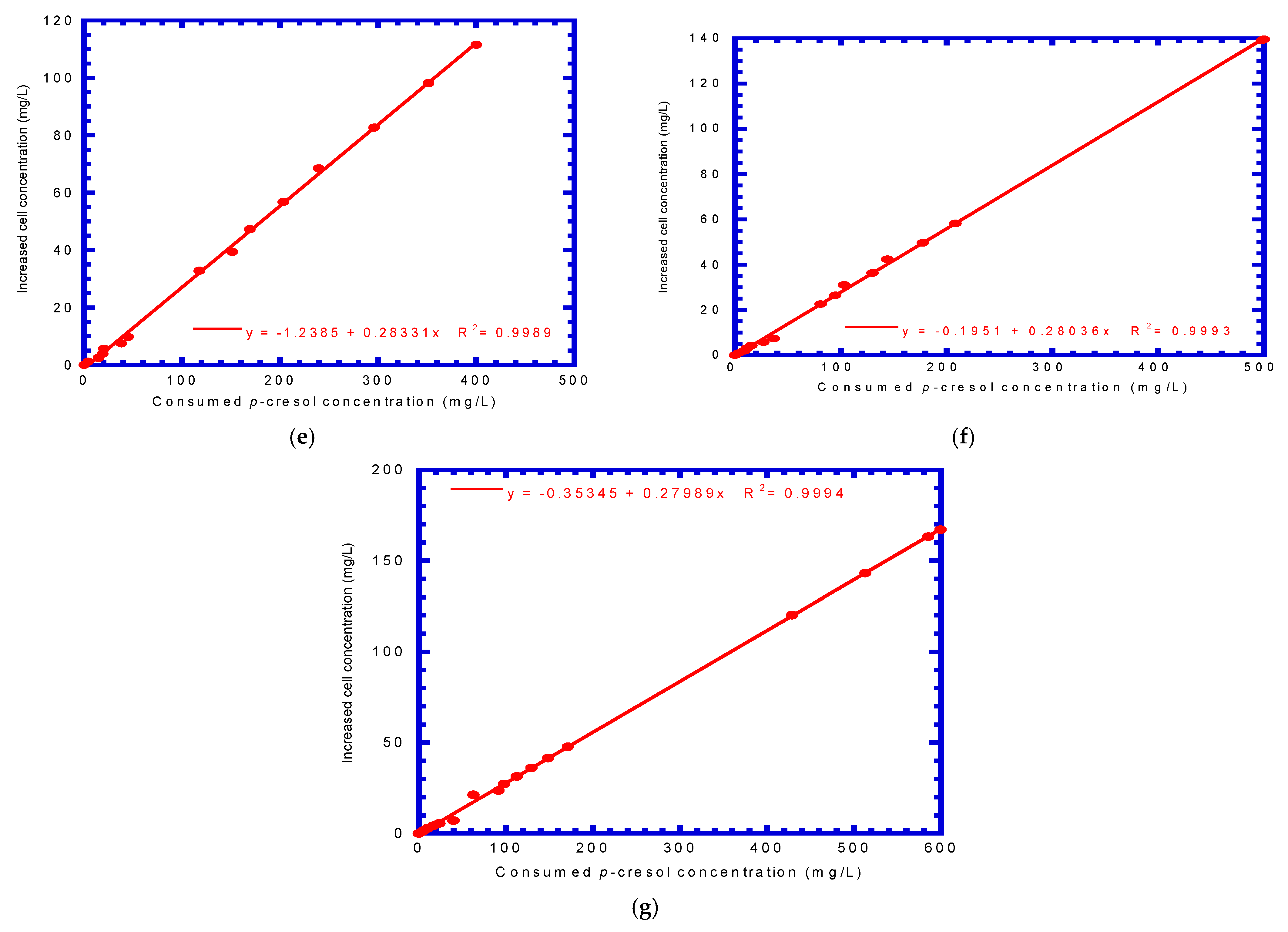




| Run No. | Initial Phenol Concentration (mg L−1) | Initial p-Cresol Concentration (mg L−1) | Bio-Kinetic Parameters | |
|---|---|---|---|---|
| YP (mg mg−1) | YC (mg mg−1) | |||
| 1 | 50 | 50 | 0.340 | 0.274 |
| 2 | 100 | 100 | 0.338 | 0.278 |
| 3 | 200 | 200 | 0.343 | 0.278 |
| 4 | 300 | 300 | 0.342 | 0.280 |
| 5 | 400 | 400 | 0.340 | 0.283 |
| 6 | 500 | 500 | 0.337 | 0.280 |
| 7 | 600 | 600 | 0.339 | 0.280 |
| mean | - | - | 0.340 | 0.279 |
| standard deviation | - | - | 2.116 × 10−3 | 2.769 × 10−3 |
| Symbol | Parameters Description (Unit) | Value | Remarks |
|---|---|---|---|
| ε | reactor porosity (dimensionless) | 0.72 | measured |
| A | total surface area of gel beads (cm2) | 3.522 × 104 | calculated |
| DeP | effective diffusivity of phenol in the gel bead (cm2 d−1) | 0.945 | calculated |
| DeC | effective diffusivity of p-cresol in the gel bead (cm2 d−1) | 0.853 | calculated |
| kfP | mass-transfer coefficient of phenol (cm d−1) | 105.91 | calculated |
| kfC | mass-transfer coefficient of p-cresol (cm d−1) | 98.87 | calculated |
| Ki,P | inhibition constant of phenol (mg L−1) | 310.5 | measured |
| Ki,C | inhibition constant of p-cresol (mg L−1) | 243.56 | measured |
| Ks,P | saturation constant of phenol (mg L−1) | 221.4 | measured |
| Ks,C | saturation constant of p-cresol (mg L−1) | 65.1 | measured |
| IC,P | inhibition of cell growth on phenol due to the presence of p-cresol (dimensionless) | 4.8 | measured |
| IP,C | inhibition of cell growth on p-cresol due to the presence of phenol (dimensionless) | 12.7 | measured |
| Q | influent flow rate (mL d−1) | 6.272 × 103 | measured |
| Sb0,P | concentration of phenol in feed (mg L−1) | 125.0 | measured |
| Sb0,C | concentration of p-cresol in feed (mg L−1) | 125.0 | measured |
| V | effective working volume (mL) | 1.568 × 103 | measured |
| YP | growth yield of cell on phenol (mg cell [mg phenol]−1) | 0.340 | measured |
| YC | growth yield of cell on p-cresol (mg cell [mg p-cresol]−1) | 0.279 | measured |
| μmax,P | maximum specific growth rate of cell on phenol (h−1) | 0.45 | measured |
| μmax,C | maximum specific growth rate of cell on p-cresol (h−1) | 0.185 | measured |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Gu, Y.-J. Biodegradation Kinetic Studies of Phenol and p-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells. Processes 2021, 9, 133. https://doi.org/10.3390/pr9010133
Lin Y-H, Gu Y-J. Biodegradation Kinetic Studies of Phenol and p-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells. Processes. 2021; 9(1):133. https://doi.org/10.3390/pr9010133
Chicago/Turabian StyleLin, Yen-Hui, and Yi-Jie Gu. 2021. "Biodegradation Kinetic Studies of Phenol and p-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells" Processes 9, no. 1: 133. https://doi.org/10.3390/pr9010133
APA StyleLin, Y.-H., & Gu, Y.-J. (2021). Biodegradation Kinetic Studies of Phenol and p-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells. Processes, 9(1), 133. https://doi.org/10.3390/pr9010133






