1. Introduction
Boilers for the production of hot water or water vapor are widespread devices in the residential and industrial sectors [
1,
2,
3,
4]. The most crucial element is the burner, where the combustion in air of a suitable fuel transforms the chemical energy in thermal energy [
5]. The latter is then employed in heat exchangers to transfer the thermal energy to a suitable fluid carrier, by involving mainly radiative (flame) [
6] and convective (flue gases) thermal exchanges [
7]. So, given a type of fuel, the environmental quality of these devices depends on the combustion features [
8], the magnitude of the emitted pollutants [
9] and the actual heat exchanger efficiency that, in turn, affects the fuel consumption to release a precise thermal power [
10]. Modern devices are modulating units that are able to regulate the produced thermal power in order to meet exactly the user needs, without detect an efficiency worsening [
11]. As a consequence, the risk of a negative environmental impact due to the thermal power surpluses, and the correspondent fuel consumption growth, is largely overcame [
12]. Quantitatively, the environmental quality can be measured by determining the entropy production in the involved processes, because the irreversibilities can be related to the modification of the external environment [
13]. For this purpose, the entropic generation number (EGN), ranging between 0 and 1, defined in study can represent an appropriate index able to take into account all the irreversibilities connected with the device functioning [
14]. The larger the result, the worse the impact on the environment is. Regarding the combustion process, a first qualification index is defined exclusively to determine the quality of the chemical reaction. It is determined by modifying the EGNs of the chemical process with a suitable correction factor that weights the influence of the emitted pollutants in the external environment. In particular, the EGN measures the “quality” of the chemical reaction supposing a free combustion in a closed system between two equilibrium states, in order to quantify the “defect” in the chemical energy potential not used for the production of the combustion heat [
15]. Nevertheless, the EGN has to be modified opportunely involving the mass exchange between boiler and outward to account for the emission of combustion products in the external environment. So, the final results allow to measure the combustion effects, where values are negatively affected with the increase of emitted pollutants: a unitary result means that a complete process degradation occurs with no advantage in terms of the combustion reaction. Conversely, a null result corresponds to an ideal process (no entropy production) where all the chemical potential is transformed in combustion heat without emission of pollutants. Nevertheless, the impact of the combustion process on the external environment also depends on the actual boiler performances that could be different due to the different technology employed (for instance, condensing or traditional boilers) and the deterioration due to aging that could affect the boiler efficiency negatively [
16]. As a consequence, in order to take into account these aspects, a second EGN was introduced to determine the irreversibilities related to the heat transfer process during the device operation. In a simplified way, boilers can be considered as a heat exchanger in which an isobaric heat transfer process occurs, by neglecting the entropy generation due to the fluids pressure drops [
17]. Therefore, an additional entropic generation number was calculated starting from the Kay-London theory supposing the use of a counter-current heat exchanger [
18]. The latter, combined opportunely with the modified EGN related to the combustion, allows to quantify the environmental damage in different operational conditions. In particular, the environmental damage was quantified by a dimensionless Environmental Quality Index (EQI) defined as the product of the complementary of the two entropic generation numbers. A unitary result means an ideal device where no damages on the external environment are detected, conversely a null result is related to the maximum allowed damage. By means of a parametric study, different fuel typologies (natural gas, coal, diesel and hydrogen) and heat exchanger functioning conditions (varied by modifying fluid flow rates and temperatures) were considered, in order to determine the environmental performances of boilers in different situations. In light of this, a comparison between traditional fossil fuels and a renewable fuel as solar-hydrogen (produced, for instance, by electrolysis supplied by PV power [
15,
19]) was carried out. The aim of this document is the evaluation of the boilers environmental performances when fed by a precise fuel typology, furthermore the functioning conditions that allow the achievement of similar environmental impacts were identified supposing equivalent boilers supplied by other fuels.
3. Results
The results concerning the EGN of the combustion process are shown in
Table 2.
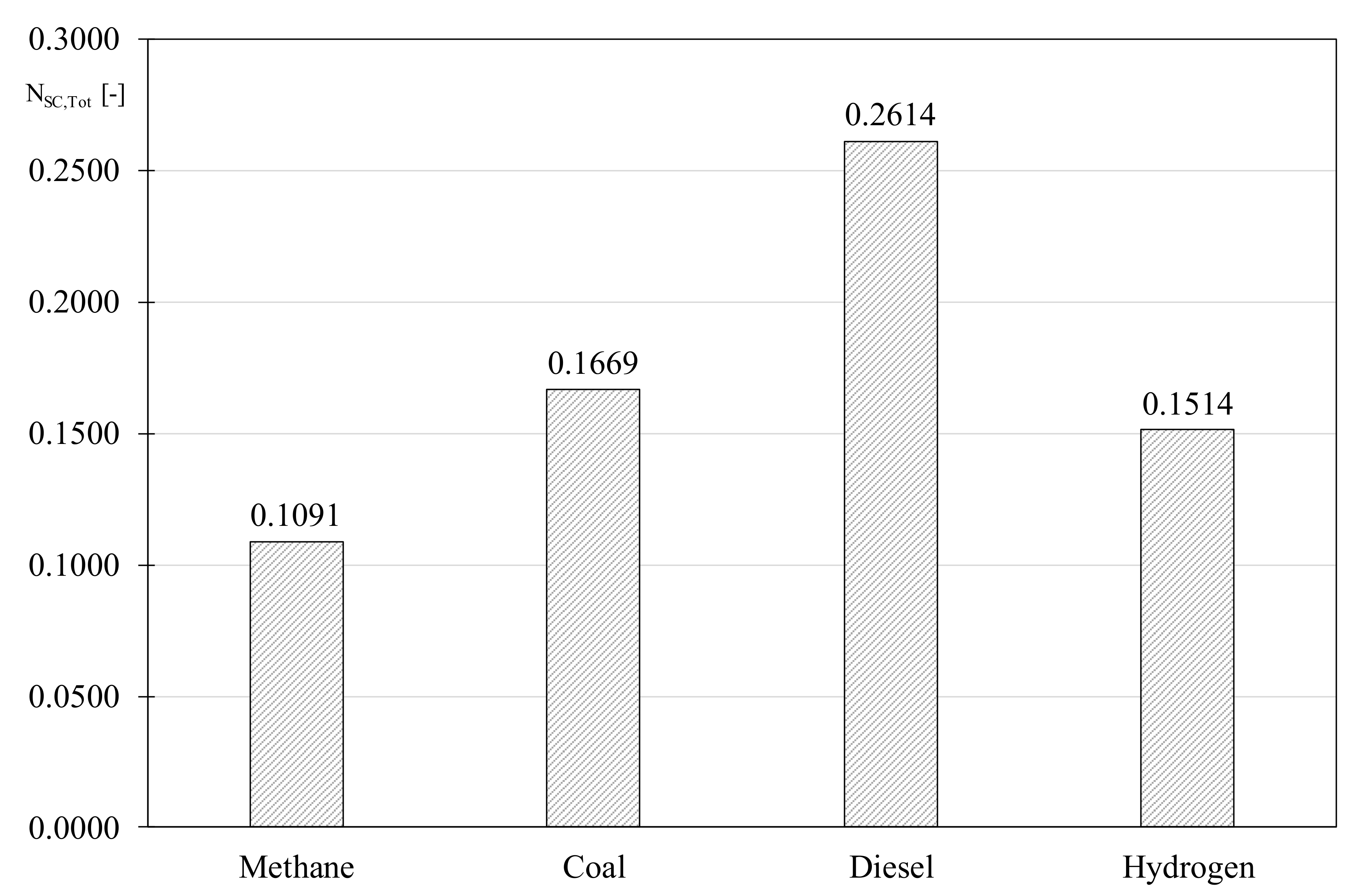
Results show the better behavior of natural gas followed by coal, hydrogen and diesel, respectively. It is worth nothing that, despite the low irreversibilities, the lowest molar enthalpy of hydrogen, compared to the other fuels, determined a high value of the index. Conversely, the Diesel, even presenting the highest unavailable Gibbs potential rate, two orders of magnitude greater than the other fuels, is counterbalanced by an elevated value of the correspondent molar enthalpy.
In order to weight the pollutants emission, the correction factor of Equation (9) is quantified for the considered fuels by using the mass fraction of the substances listed in
Table 3. These values refer to data provided by IPCC (International Panel for Climatic Changes) for carbon dioxide and the nitrogen oxides when fuels are employed in the residential sector [
9]. Sulfur dioxides refer to data concerning coal with a content of 2.6% of sulfur (CH
0.81N
0.013S
0.013O
0.057) [
23], natural gas with a sulfur concentration of 0.0024 per m
3 [
24] and diesel as naphtha for heating application [
25]. It is worth noting that, also in the case of hydrogen combustion, air was supposed to account for the production of nitrogen oxides [
26]. Incomplete combustion is an important issue when analyzing environmental performances of fuels and can determine the emission of additional pollutants. Even though the combustion process was not analyzed in detail in the paper, other significant emitted substances as particulate matter and unburnt were included in the calculation, to keep in account the real combustion process that occurs in actual devices [
27].
In
Table 4, the values of the partial correction factors
f of Equation (8) referring to the considered pollutants and global correction factor
F of Equation (9) associated with the combustion process of the considered fuel are listed. For the latter, it must be highlighted that a high value does not imply a minor pollutants emission, because these are weighted by the available combustion heat. As a consequence, the highest value of
F allows to determine the best compromise between available heat and correspondent emission of pollutants in the external environment. Results show that the lack of CO
2 emissions for hydrogen combustion allows the attainment of the best score. Despite the presence of other pollutants, coal and diesel benefit of their lower heating value, therefore the correction factor is prevalently influenced by the CO
2 mass concentration. An intermediate result was obtained for natural gas, because of the compromise between better thermodynamic properties and emission of pollutants when compared with the other fossil fuels.
The modified EGN determined by Equation (10) quantifies the combustion quality of the considered fuels including the effect of the pollutants emission, with results showed in
Figure 1. It is worth noting that the best score obtained by natural gas is due to the best combustion properties and it is not affected by the
F value noticeably. Conversely, despite the excellent environmental properties, hydrogen is penalized by the combustion properties, therefore the minor emission of pollutants is not able to compensate the gap in terms of available combustion heat. Regarding the other fossil fuels, coal shows a better score than diesel due to the more favorable value determined by Equation (6), even though a similar correction factor was determined despite the higher emissions of sulfur dioxide and particulate matter for coal. Indeed, the better thermodynamic properties of the latter allow for recovering the negative aspects concerning the higher mass concentration of the combustion products. Nevertheless, when considering the high score reached by coal it has to be underlined the favorable situation to consider it as composed only of a prevalent carbon monoxide, generating therefore an evident advantage when compared with Diesel, in terms of the EGN determined by Equation (6).
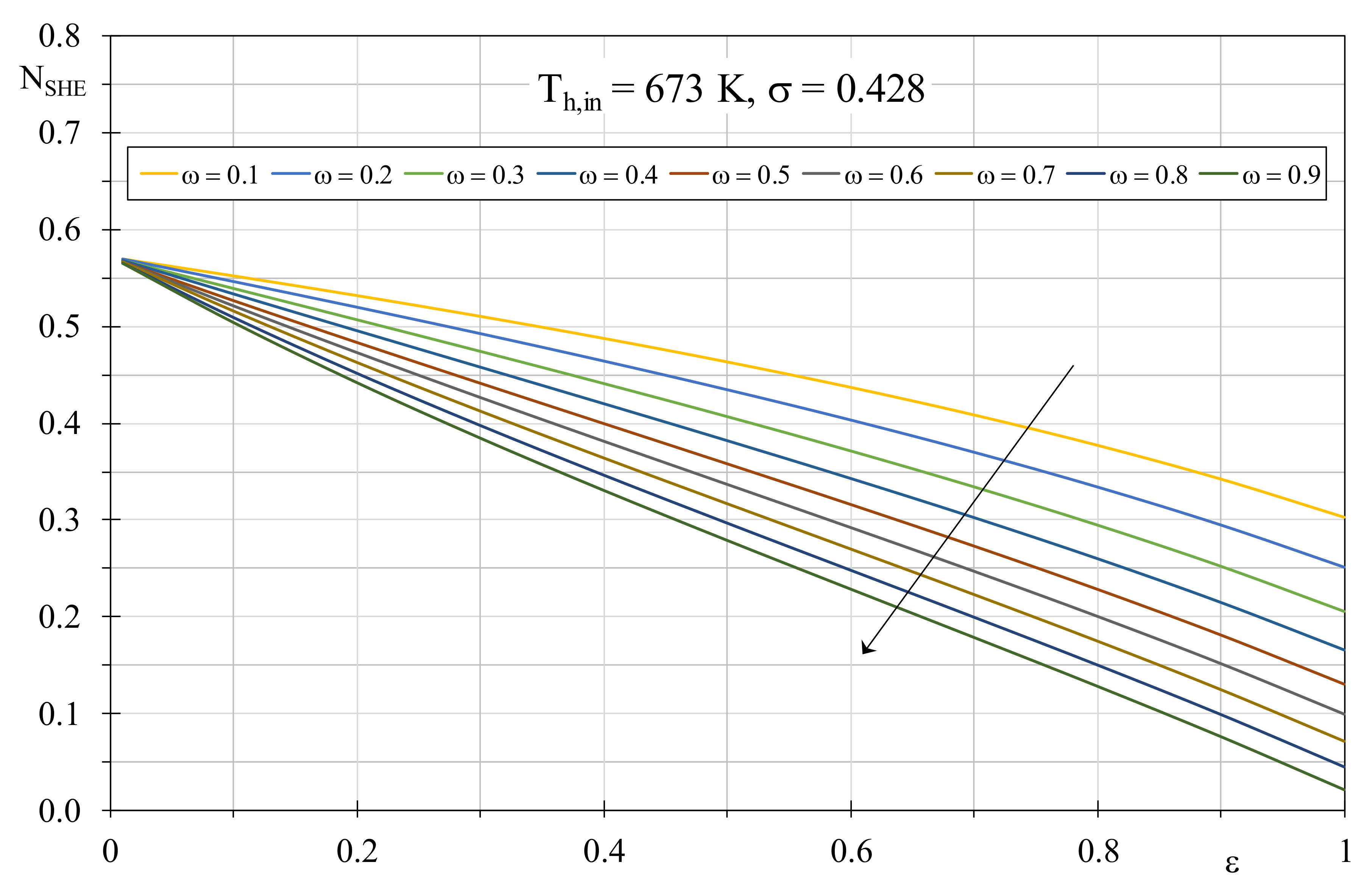
As regards the additional entropy generation due to the boiler heat exchanger, in
Figure 2 the EGN of Equation (15), determined by setting
Tc,in = 15 °C (cold water inlet inside the boiler) and
Th,in = 400 °C (flue gases) is shown by varying boiler efficiency and the fluid thermal capacity ratio, with a constant dimensionless temperature factor of 0.428. A unitary efficiency was considered for condensing units that, in ideal functioning conditions, require limited inlet temperature for the cold fluid. It is worth to highlight that, for every
ω value, the corresponding efficiency can be obtained by tuning the NTU value, in turn by acting on the global heat transfer coefficient or the exchange surface.
Obviously, the EGN related to the heat exchanger decreases with the efficiency and the thermal capacity ratio growth: in both cases, this trend is justified by the limitation of the temperature difference among the fluids that, in turn, determines a reduction of the thermal exchange irreversibilities. In particular, when
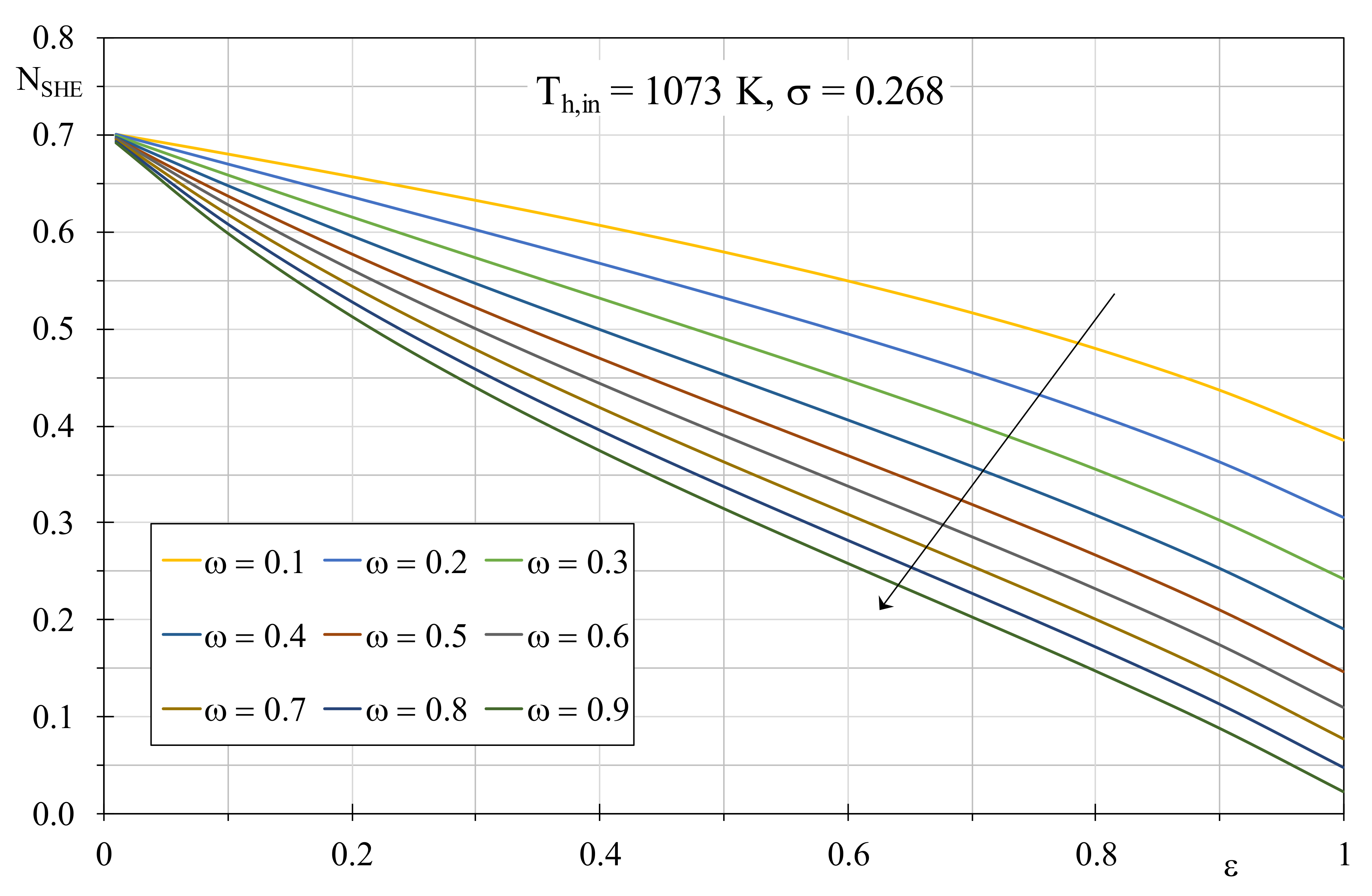
ω increases, an augment of the mass flow rate of the hot fluid can be associated and, at parity of transferred thermal power, the correspondent reduction of the difference temperature between inlet and outlet produces a limited entropy production, in accordance with Equation (12). To confirm this, in
Figure 3, the EGN trends are showed with a dimensionless temperature factor of 0.268, the latter achievable by setting the inlet hot and the cold fluid temperatures to 800 °C and 15 °C, respectively. Again, the evident increment of the EGN is due to the large temperature difference between the fluids that determines a consequent irreversibility growth. Another significant aspect concerns the change in the thermal capacity ratio, that in this case produces more visible deviances for the EGN, that tends to reduce with the
ω increment.
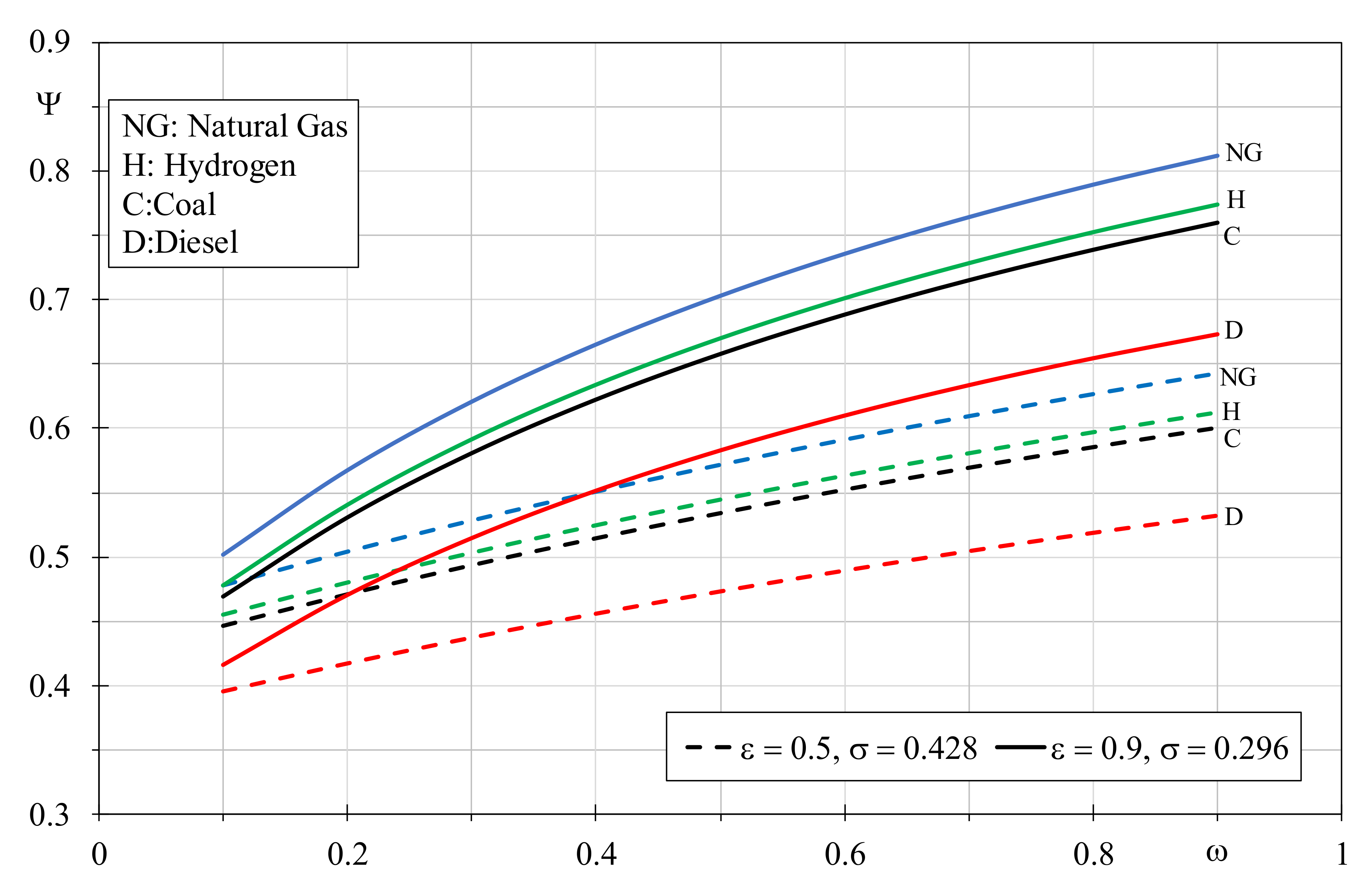
By combining the EGNs of the combustion and thermal transfer processes by Equation (16), at parity of thermal capacity ratio, dimensionless temperature factor and efficiency, the EQI will show similar trends to
Figure 1. Indeed, the thermal transfer irreversibilities will be the same for every fuel, so the final result will be prevalently influenced by the combustion process. Therefore, the worst EQI will be obtained for the diesel boiler with the lowest efficiency, thermal capacity ratio and dimensionless temperature factor. Conversely, natural gas will produce the highest score in presence of unitary efficiencies and with the highest thermal capacity ratio and dimensionless temperature factors. Nevertheless, if during the fuel comparison the parameters that characterize the heat exchanger operation are changed, it is possible to identify precise operating conditions that allow a better EQI even though some fuels are limited from the combustion point of view. Consequently, a natural gas boiler operating with lower thermal capacity ratio and limited dimensionless temperature factor will assume lower EQI when compared with other fuels in boilers operating with more favorable values of
ω and
σ. In
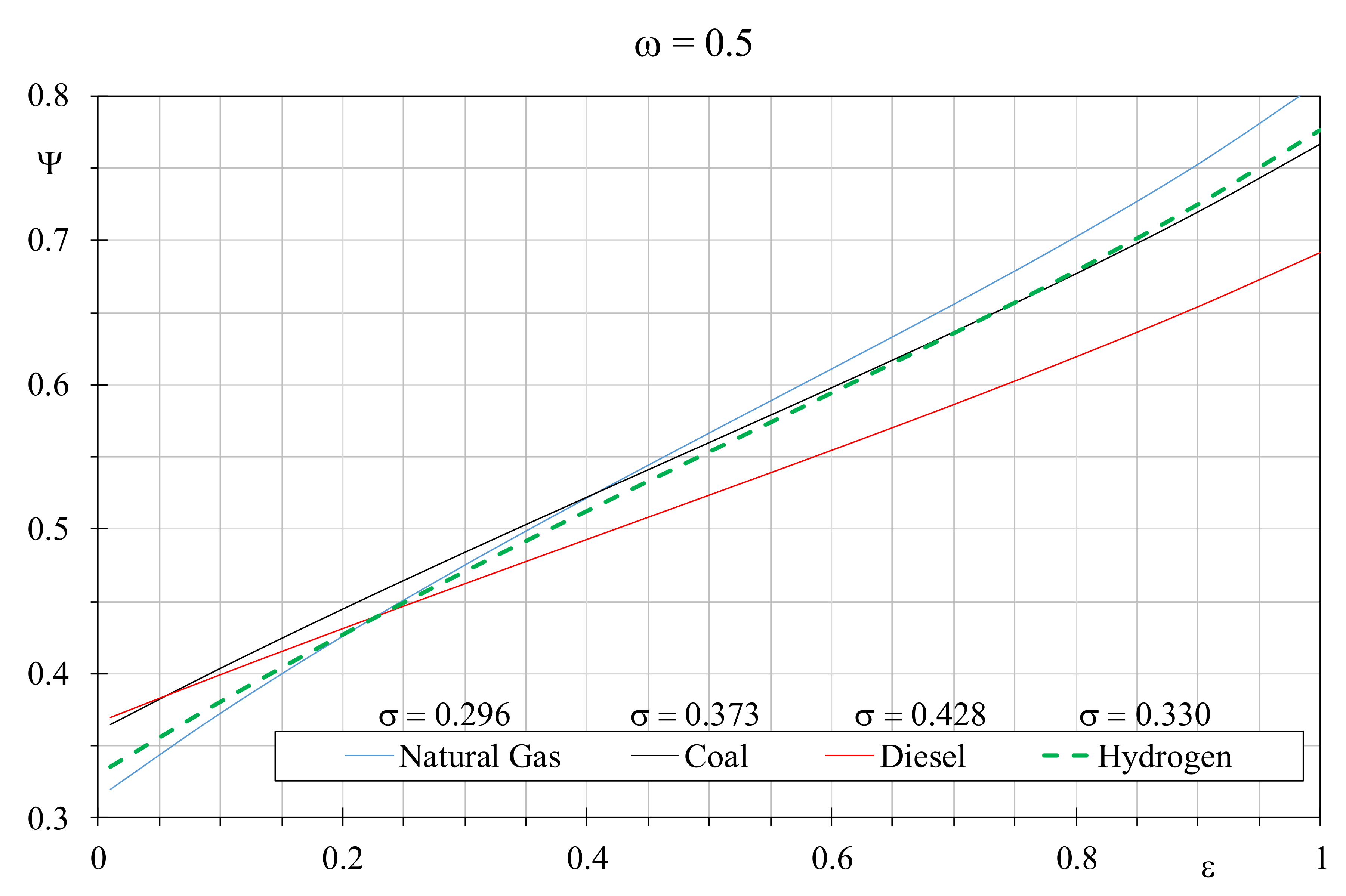
Figure 4, the EQI obtained by varying the boiler efficiency are depicted assuming different temperature dimensionless factors for the considered fuels, in particular 0.296 for natural gas, 0.330 for hydrogen, 0.373 for coal and 0.428 for diesel. Indeed, since the EGN of the heat exchanger decreases with the dimensionless temperature factor growth, the choice was to associate higher σ values when the fuel is negatively affected by the combustion EGN. By setting the constant temperature value of 15 °C for the cold flow rate, the employed dimensionless temperature factors correspond to inlet hot fluid temperatures of 700 °C, 600 °C, 500 °C and 400 °C, respectively. From
Figure 4, it can be appreciated how the lower environmental damage is obtained with natural gas when the boiler efficiency is greater than 0.45; however, it offers the worst performances compared with the other fuels when boiler efficiency is lower than 0.25. A dimensionless temperature factor of 0.330 for hydrogen allows to obtain better environmental properties than natural gas, when the latter is used in boilers with efficiency lower than 0.225. Coal with a dimensionless temperature factor of 0.373 produces similar hydrogen scenarios, however the latter is preferable when used in boilers with efficiency higher than 0.75. Diesel showed the lowest environmental performances, nevertheless it could be recommended if the other fuels are used in boilers with efficiency lower than 0.25. In
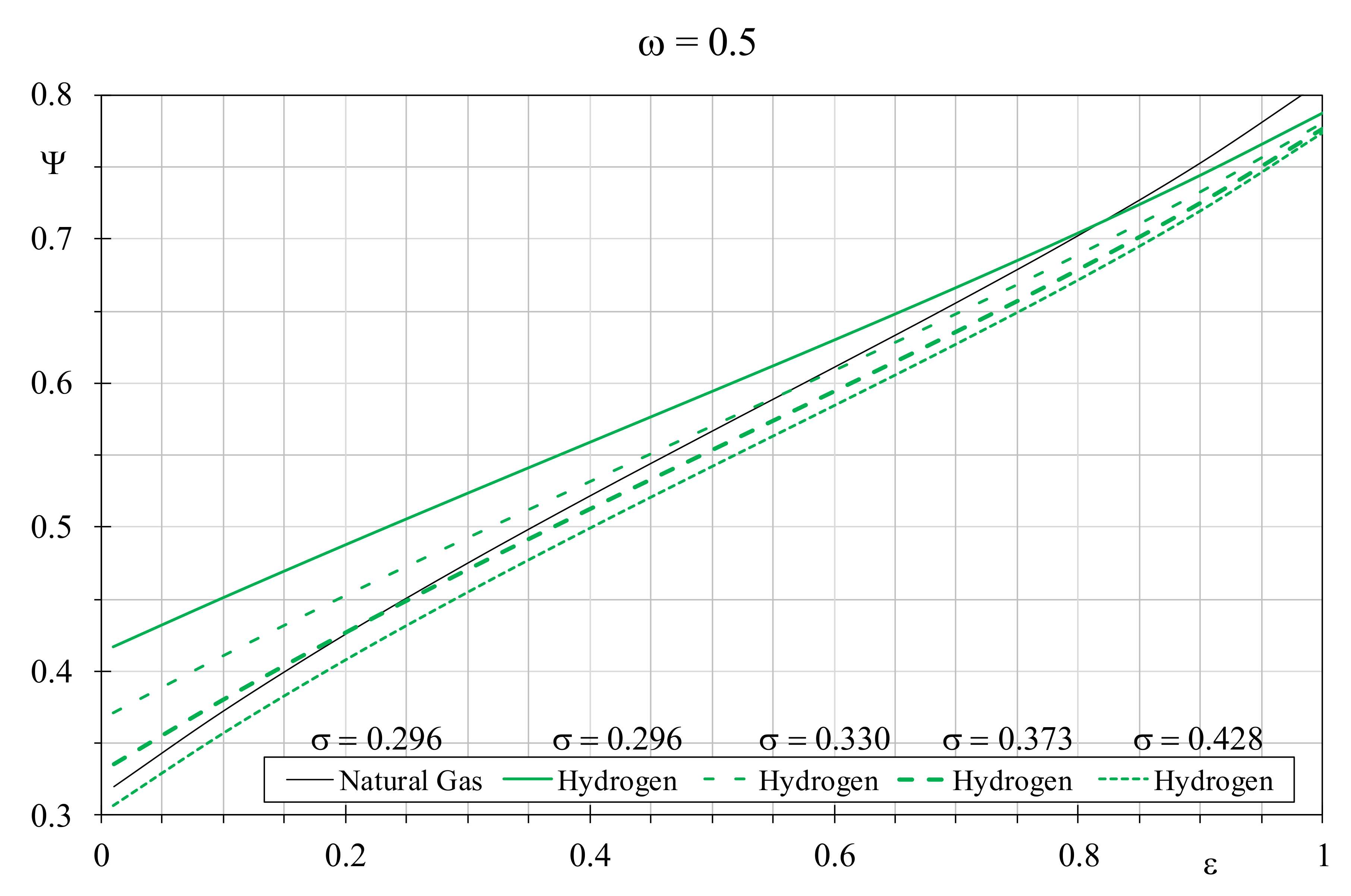
Figure 5, the best fossil fuel represented by natural gas with a constant dimensionless temperature factor of 0.296 is compared with hydrogen assuming the other σ values, by setting
ω = 0.5. When hydrogen is analyzed with the favorable σ value of 0.428, it provides better EQI than natural gas if the latter is used in boilers with efficiency lower than 0.825. When σ reduces to 0.373, the prior efficiency threshold falls to 0.55, and it drops down further to 0.2 assuming for hydrogen
σ = 0.330. Obviously, at parity of dimensionless temperature factor, hydrogen always leads to worse EQI because it cannot recover the gap in terms of combustion quality.
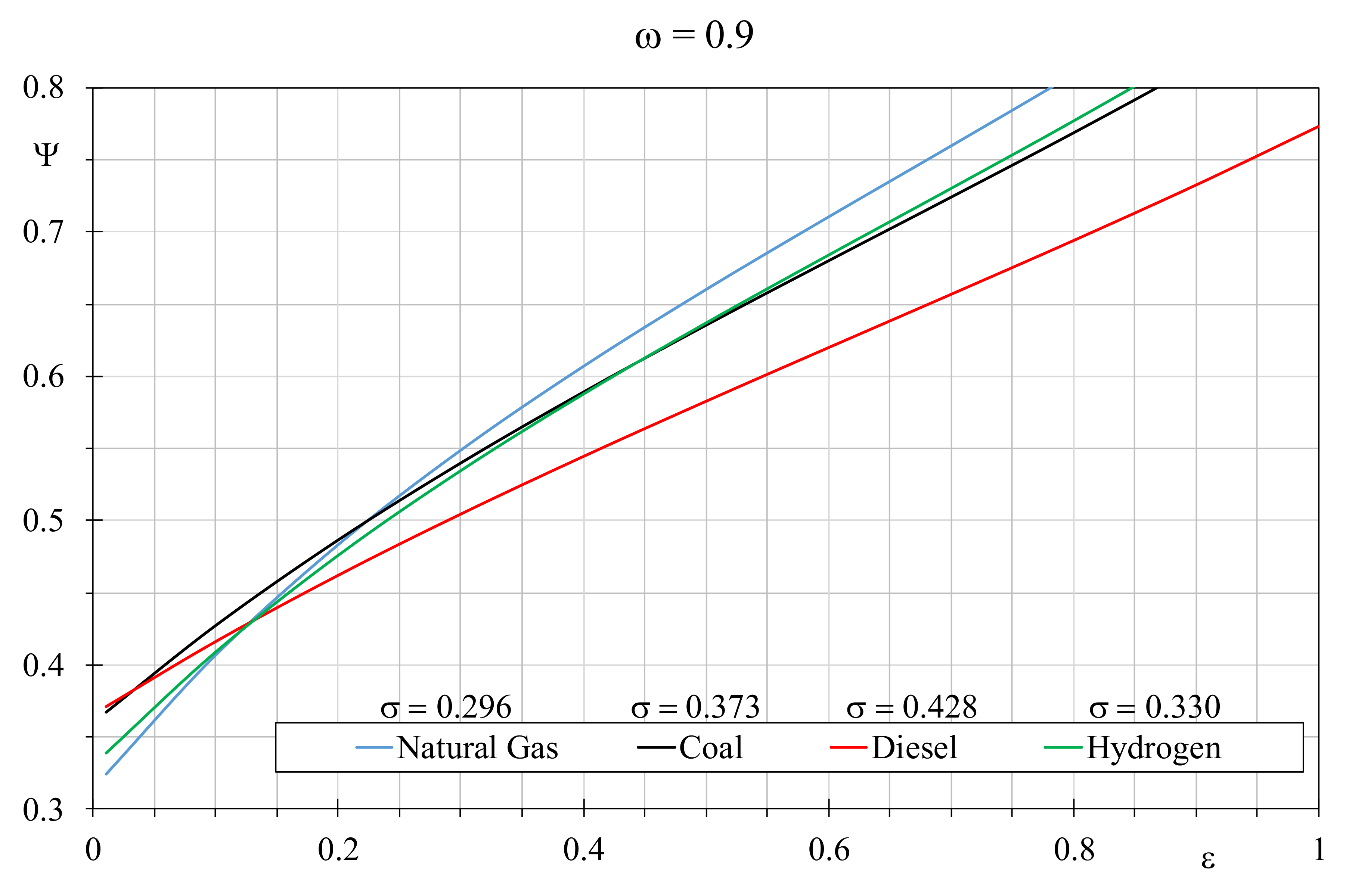
In order to evaluate the role of the thermal capacity ratio in the EQI trends,
Figure 6 depicts the comparison among the considered fuel by setting
ω = 0.9 and by assuming the usual
σ values of 0.296 for natural gas, 0.330 for hydrogen, 0.373 for coal and 0.428 for Diesel. It is appreciated that the increment of the thermal capacity ratio produces a wide variation of the EQIs, whose trends tend to rise quickly with the efficiency values than the case with
ω = 0.5. Again, natural gas offers the best EQI values but this time the boiler efficiency threshold falls to 0.25, under which the other fuels become more recommended. Furthermore, hydrogen is better than coal when used in boiler with efficiency greater than 0.425, even though their trends are slightly different. Diesel provides the worst EQI value, however it is preferable than natural gas and hydrogen when the latter are employed in boilers with efficiencies lower than 17%.
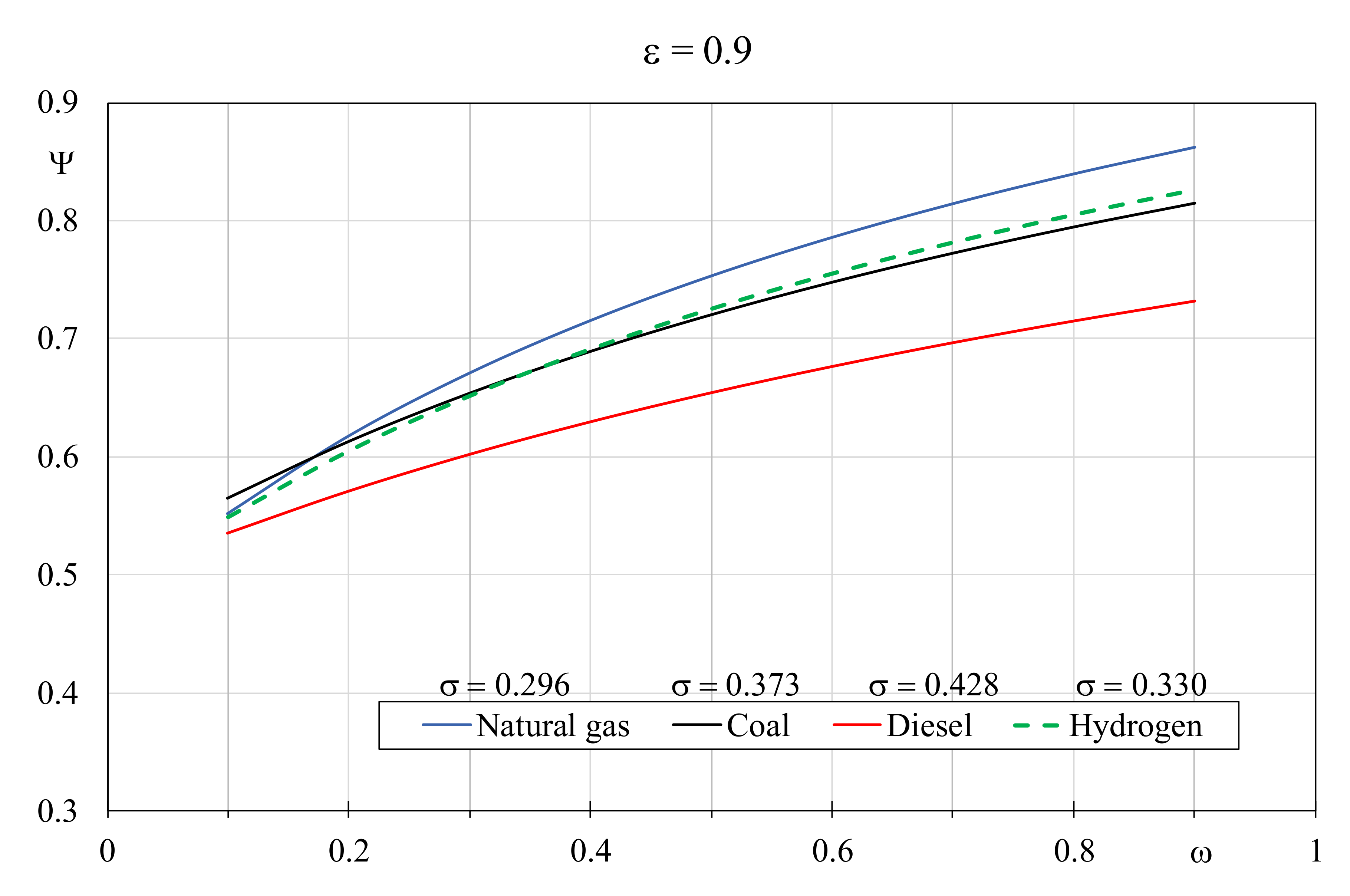
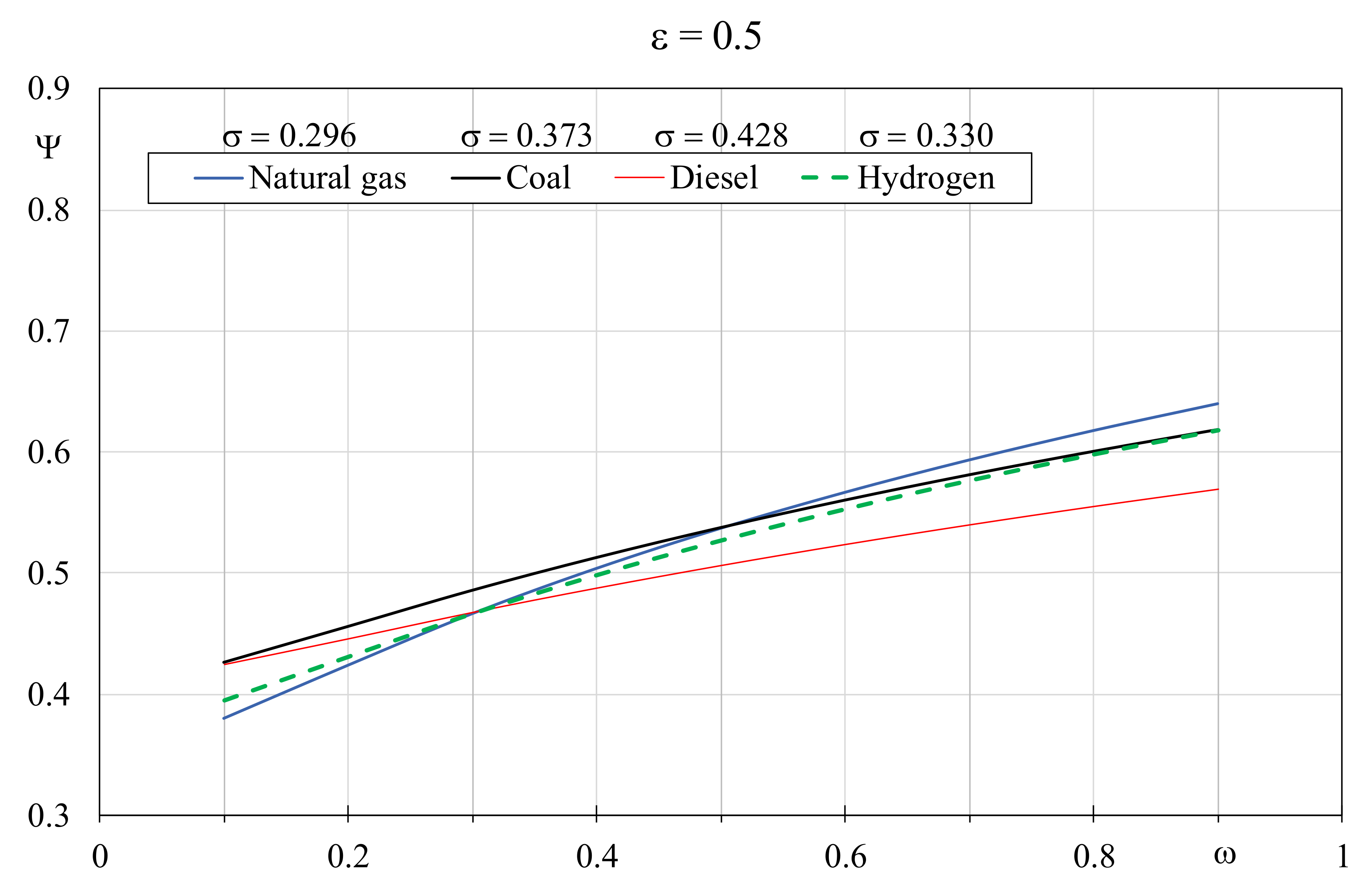
Figure 7 and
Figure 8 show the EQI determined by varying the thermal capacity ratio and the dimensionless temperature factor, by setting the boiler efficiency to 0.9 and 0.5, respectively. With the efficiency growth, a wide variation of the EQI values can be observed; it can be appreciated that the role of coal as alternative fuel improves especially in presence of limited thermal capacity ratio values and when the boiler efficiency is low. Conversely, despite that the more favorable dimensionless temperature factor was associated, Diesel is not indicated with high boiler efficiency, independently from the thermal capacity ratio value. In particular,
Figure 7 shows that natural gas is again the recommended fuel for larger efficiencies despite the unfavorable σ value, but only when
ω is greater than 0.2, below which coal with
σ = 0.373 produces better results. Hydrogen with
σ = 0.330 becomes better than coal when
ω is greater than 0.4. In the presence of lower efficiency boilers (
Figure 8), natural gas with
σ = 0.296 is again the fuel with the best EQI but this time
ω has to be greater than 0.5, otherwise coal, with
σ = 0.373, prevails. Furthermore, hydrogen with a
σ = 0.330 always produces results worse than coal whereas Diesel with σ = 0.428 becomes appreciable with
ω lower than 0.3.
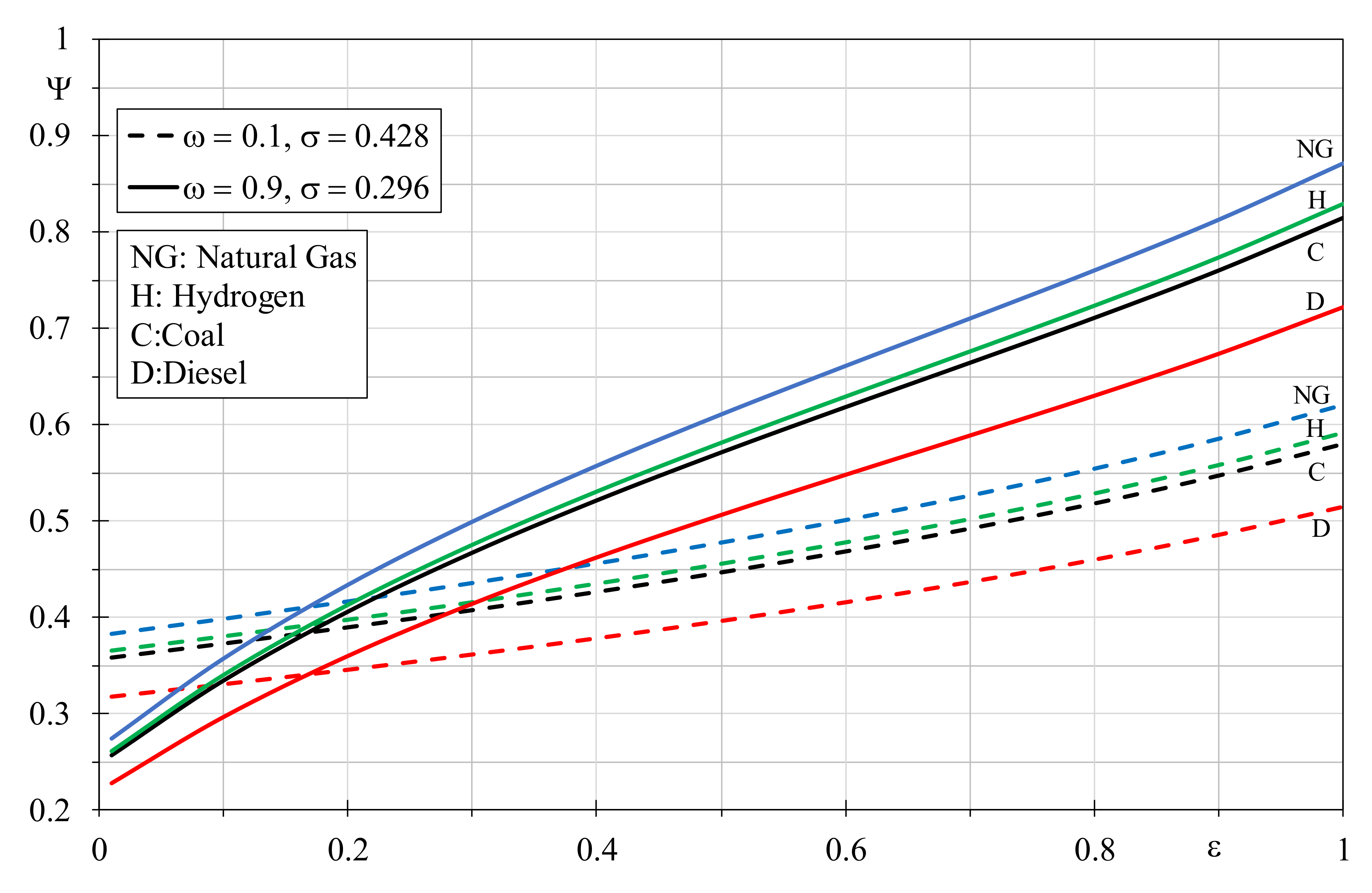
A sensitive analysis varying the parameters affecting EGN of the heat exchanger has showed the influence on the EQI trends was consucted. In particular, the environmental quality index was analyzed by varying the thermal capacity ratio, for different couples of efficiency and dimensionless temperature factor, and the obtained results are presented in
Figure 9. Similarly, in
Figure 10, the EQI dependence on the heat exchanger efficiency, for different couple of the thermal capacity ratio and of the dimensionless temperature factor, is shown. The couples have been chosen in order to confer simultaneously a favorable and an unfavorable value for the considered parameters. It is clear that the EQI increases quickly for high
ω values when the efficiency is greater than 0.5, whereas the dependence from the thermal capacity ratio shows a regular trend. Furthermore, by comparing the EQI of natural gas and hydrogen, the deviances tend to increase in favor of the natural gas with the thermal capacity ratio, especially in the presence of higher efficiencies and lower dimensionless temperature factors. From
Figure 10, the deviances increase is more evident with the efficiency growth, and these differences are marked with the thermal capacity ratio augment and the dimensionless temperature factor decrement. In
Figure 9 it is interesting to note that, by setting an advantageous
ε = 0.9 and an unfavorable
σ = 0.296, natural gas, hydrogen and coal offer better environmental performances than the other case with an unfavorable
ε = 0.5 and an advantageous σ = 0.428, for every thermal capacity ratio. Conversely, Diesel with an advantageous
ε = 0.9 and an unfavorable
σ = 0.296 is better than the other fuels that are employed in a boiler working with
ε = 0.5 and
σ = 0.428, but the thermal capacity ratio has to be higher than 0.4. With reference to the EQI dependence from the efficiency, again Diesel offers better values than other fuels when boilers operate with precise couples of parameters. In particular, for a heat exchanger efficiency greater than 0.4 with thermal capacity ratio and dimensionless temperature factor of 0.9 and 0.296, respectively, Diesel attains better environmental performances compared to the other fuels evaluated with an unfavorable
ε = 0.5 and an advantageous
σ = 0.428.
Globally, it can be appreciated that the role of the dimensionless temperature factor prevails on the heat exchanger efficiency when the thermal capacity ratio values are limited; furthermore, the role of the latter is more influent on the dimensionless temperature factor with the efficiency growth.
4. Discussion
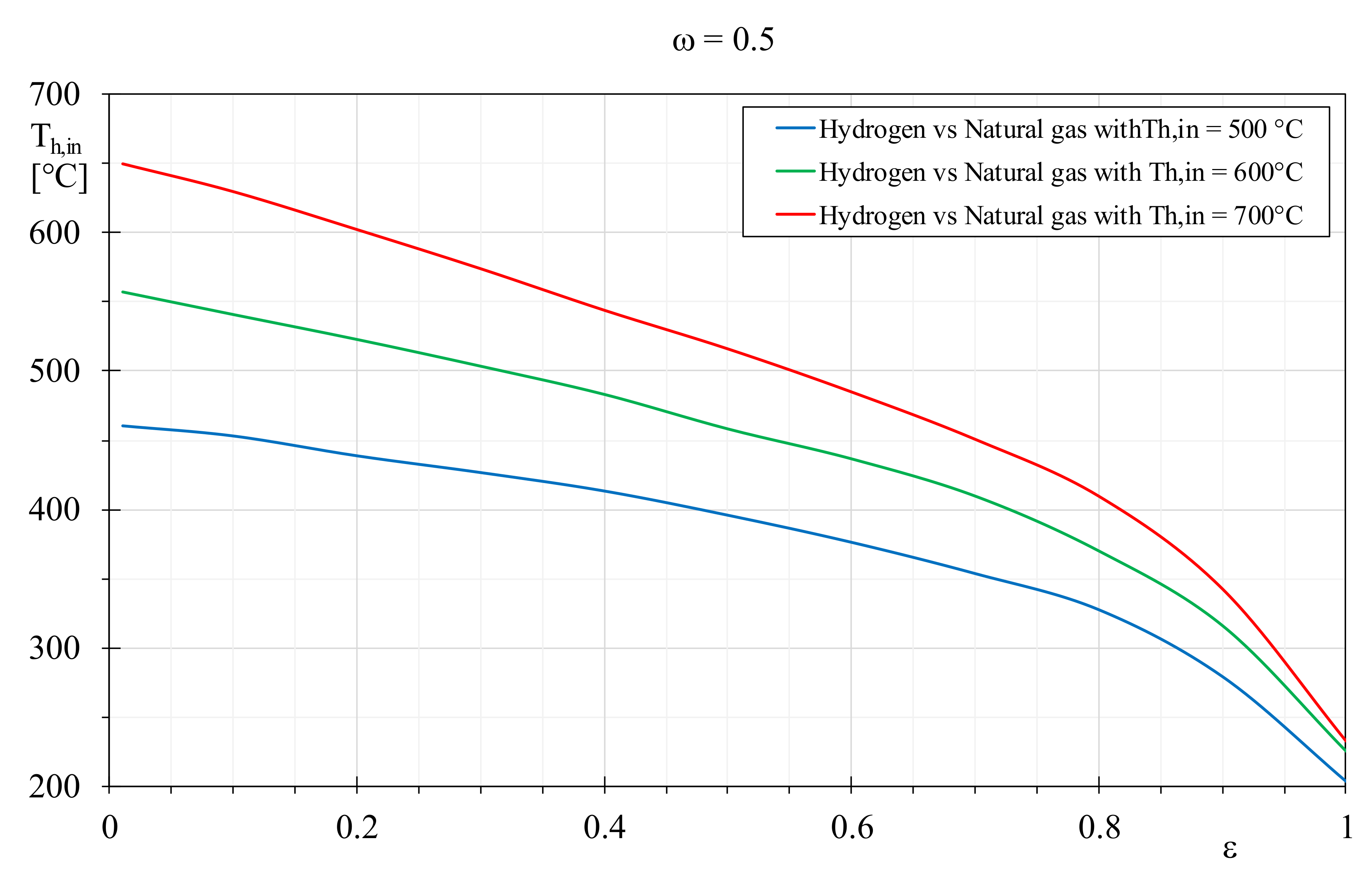
Equivalent EQI values detected among the considered fuels can be achieved by regulating opportunely the operating condition of the boiler. Therefore, the gap in terms of combustion quality can be filled by adjusting the parameters affecting the boiler operation, despite the detected different EGN of Equation (10). For instance, by decreasing the inlet temperature of the hydrogen flue gases, and consequently the thermal transfer irreversibilities, it is possible to attain better EGNs for the heat exchanger that, in turn, allows to achieve the same environmental indexes of natural gas producing flue gases at higher temperatures.
Figure 11 shows the temperatures that the hydrogen flue gases should assume to achieve the same EQIs of natural gas with combustion products at 500, 600 and 700 °C. The hydrogen flue gases temperatures are plotted against the heat exchanger efficiency by setting a constant thermal capacity ratio of 0.5. It is clear that the required decrement of the hydrogen combustion products temperature reduces with the heat exchanger efficiency growth, and this reduction is more accentuated when the efficiency is greater than 0.8. Furthermore, the temperature difference between natural gas and hydrogen flue gases decreases with the limitation of the temperature of the natural gas combustion products, especially in the presence of limited efficiencies. For instance, by setting
ε = 0.2, equivalent EQI can be achieved with hydrogen and natural gas combustion products at about 600 °C and 700 °C, respectively, with a deviance of about 100 °C. Conversely, assuming for the natural gas products a temperature of 500 °C, the correspondent temperature decrement required for the hydrogen flue gases reduces to 62 °C. With the heat exchanger efficiency growth, the temperature difference between natural gas and hydrogen flue gases increases. Indeed, by setting
ε = 0.9, equivalent EQI can be attained by considering natural gas products at 700 °C and hydrogen flue gas at 343 °C, with a temperature difference of 357 °C. With natural gas combustion products at a temperature of 500 °C, the required temperature for hydrogen flue gases is equal to 279 °C, with a deviance of 221 °C. Globally,
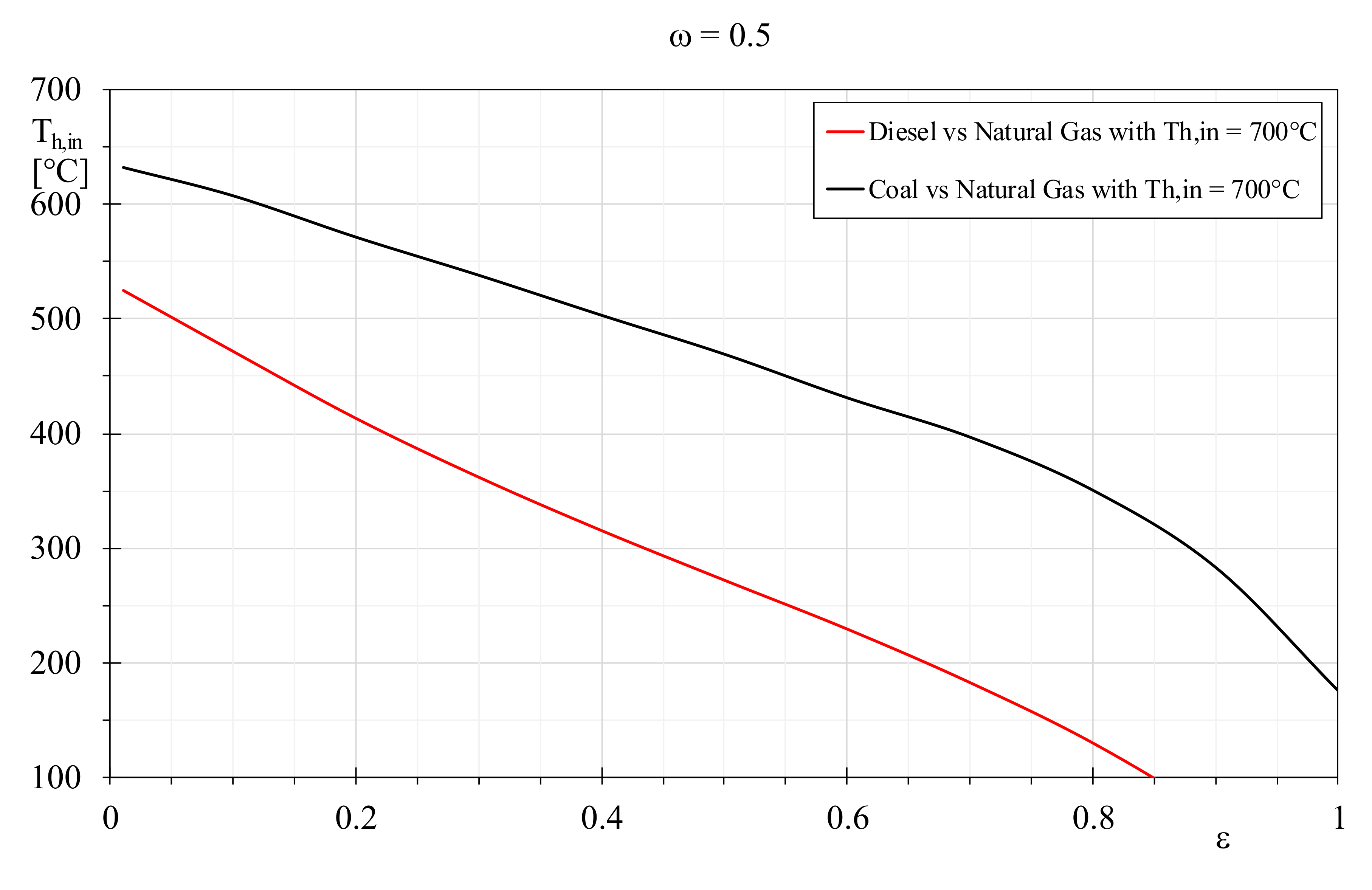
Figure 11 demonstrates that the same EQI between natural gas and hydrogen can be obtained more easily in presence of boiler with lower efficiency, alternatively and at parity of efficiency, when the natural gas flue gas temperature reduces. Conversely, for high boiler efficiencies or limited natural gas combustion products temperature, it is difficult to attain equivalent EQI for hydrogen, because its flue gases temperatures should be attenuated noticeably, and this solution seems scarcely practicable. The same comparison was carried out considering only the fossil fuels and the results showed in
Figure 12, highlighting the required flue gases temperatures that allow the achievement of equivalent EQIs. In particular, the plot depicts the combustion products temperatures of Coal and Diesel necessary to attain the same EQI of natural gas with flues gases at 700 °C, by varying the heat exchanger efficiency and by setting the thermal capacity ratio to 0.5. It is worth noting that equivalent EQIs for Coal require flue gases temperature that reduces linearly with the heat exchanger efficiency, but exclusively up to
ε = 0.8. For higher efficiency, these decrements drop noticeably, and the coal flue gases should be attenuated to impracticable values. For Diesel, the situation is even worse: it has to be highlighted that heat exchanger efficiency greater than 0.85 does not allow the attainment of natural gas equivalent EQIs. Furthermore, the required Diesel flue gas temperatures drops more quickly than coal trend, and only in the presence of low efficient heat exchangers could Diesel assume equivalent EQIs.
Another parameter that could be changed easily is the mass flow rate of the hot fluid (combustion products) achievable by varying the correspondent channel section geometry in the boiler. By setting the highest thermal capacity ratio and a constant mass flow rate for the cold fluid, the increase of the hot fluid mass flow rate produces an augment of the thermal capacity ratio that, in turn, provides a reduction of the EGN in accordance with
Figure 2 and
Figure 3. As a consequence, the gap in terms of EQI among the considered fuels can be recovered in accordance with Equation (16).
Indicating with
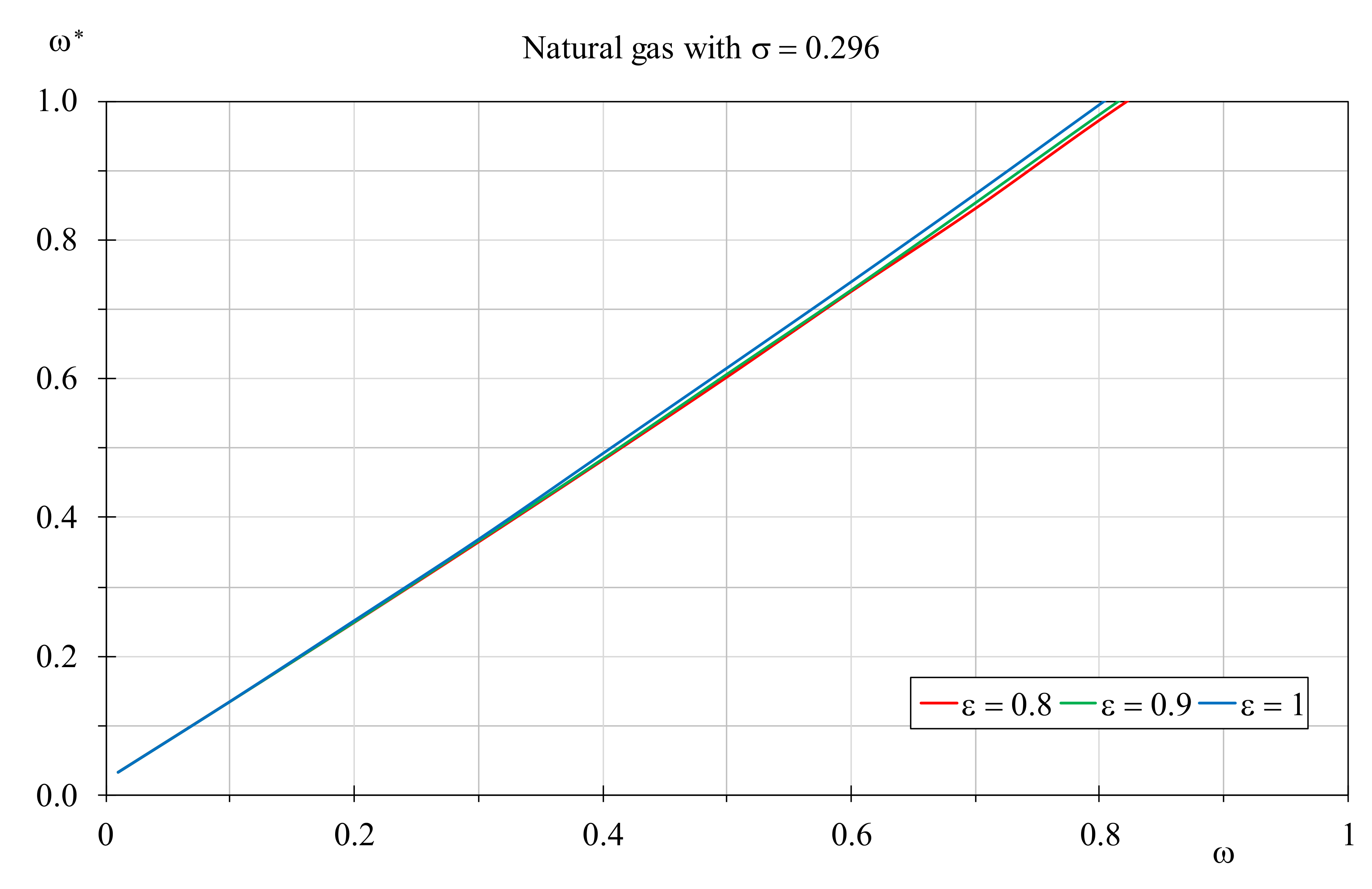
ω* the adjusted thermal capacity ratio for hydrogen,
Figure 13 shows its trend against the
ω values determined for boilers supplied by natural gas by setting
σ = 0.296, for three different values of the heat exchanger efficiency. Obviously, these trends are located over the graph bisector to highlight that an augment of the thermal capacity ratio is required for every considered case to fill the gap between hydrogen and natural gas EQIs. It is interesting to note that the trend is almost linear and is slightly affected by the heat exchanger efficiency. However, a hot fluid mass flow rate increase is requested mainly for limited value of the heat exchanger efficiency, and in the majority of the cases a percentage augment ranging between 120% and 130% is necessary. Nevertheless, this condition can be achieved with efficiency values up to 0.8, because over this threshold value the required mass flow rate increment makes the hot fluid with the highest thermal capacity ratio, invalidating the EGN determined by Equation (15).
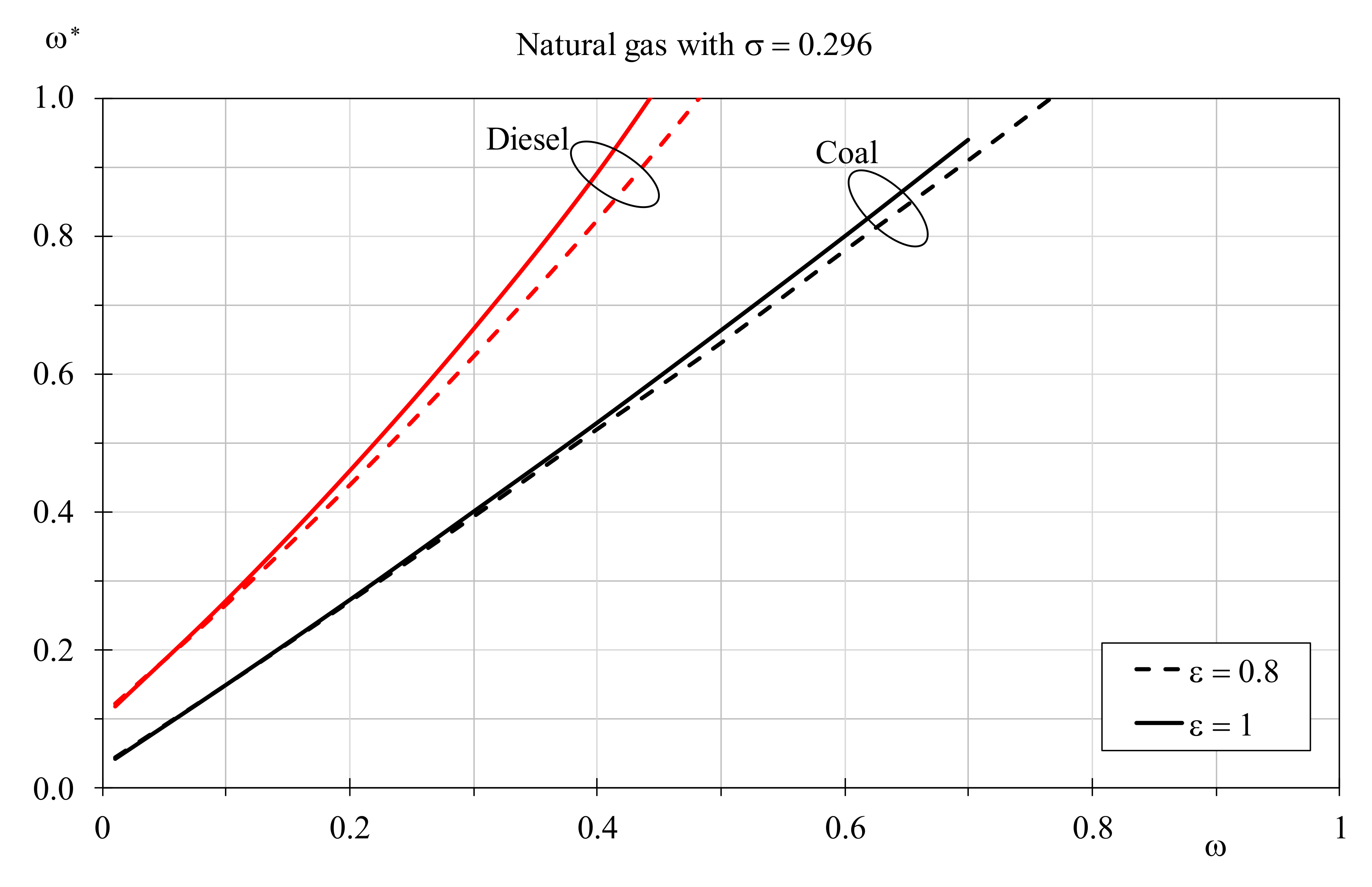
In
Figure 14, a similar graph was produced to determine the adjusted
ω* required for coal and diesel to reach the same EQI of natural gas in boilers that operate at different
ω values and with a constant dimensionless temperature factor of 0.296, assuming heat exchanger efficiency of 0.8 (traditional boiler) and 1.0 (condensing unit). For both, the required increment of the hot fluid mass flow rate increases with the boiler efficiency and the deviances are more evident than the cases analyzed in
Figure 13. It is interesting to note that coal shows a mass flow rate augment that increases linearly up to a thermal capacity ratio threshold of 0.7 ÷ 0.75 to guarantee the highest thermal capacity for the cold fluid. Conversely, the Diesel augment is more pronounced and the
ω threshold is lower than 0.5. Globally, the last two graphs confirm that equivalent EQIs can be achieved for every fuel when the thermal capacity ratio employed in natural gas boilers is not too elevated. The adjustment of the hot fluid mass flow rate is more practicable for hydrogen, less for coal and diesel. Nevertheless, if coal reaches the equivalent EQI of natural gas with an average flue gases flow rate increment of 130%, for diesel increments higher than 200% are necessary.