Abstract
Developing economical and robust catalysts for the highly selective and stable destruction of chlorinated volatile organic compounds (CVOCs) is a great challenge. Here, hollow nanosphere-like VOx/CeO2 catalysts with different V/Ce molar ratios were fabricated and adopted for the destruction of1,2–dichloroethane (1,2–DCE). The V0.05Ce catalyst possessed superior catalytic activity, reaction selectivity, and chlorine resistance owing to a large number of oxygen vacancies, excellent low-temperature redox ability, and chemically adsorbed oxygen (O− and O2−) species mobility. Typical chlorinated byproducts (CHCl3, CCl4, C2HCl3, and C2H3Cl3) derived from the cleavage of C–Cl and C–C bonds of 1,2–DCE were detected, which could be effectively inhibited by the abundant acid sites and the strong interactions of VOx species with CeO2. The presence of water vapor benefited the activation and deep destruction of 1,2–DCE over V0.05Ce owing to the efficient removal of Cl species from the catalyst surface.
1. Introduction
Chlorinated volatile organic compounds (CVOCs) mainly originate from industrial processes and result in great hazards to public health and the natural environment because of their long durability, poor reactivity, and high toxicity [1,2,3]. So far, several methods including absorption, photocatalytic degradation, catalytic oxidation, and biological processes have been used for CVOC elimination [4]. Among them, catalytic oxidation has been identified as the most efficient treatment measure due to its significant energy saving, adjustable reaction selectivity, low operation temperature, and green environment effect [5,6]. Up to now, various catalysts including transition metal oxides, zeolites/modified zeolites, and supported noble metals have been studied for CVOC destruction. Supported noble–metal catalysts exhibit outstanding catalytic performance, whereas their widespread application is greatly limited by their susceptibility to chlorine poisoning, the formation of chlorinated byproducts, and their high cost [7,8]. Although zeolites/modified zeolites exhibit good catalytic performance, they more easily suffer deactivation due to chlorine poisoning and coke deposition during the oxidation process [9,10].
Comparatively, transition metal oxides (e.g., Cr2O3, MnOx, and V2O5) are considered as a category of desired candidates to destroy CVOCs owing to their considerable catalytic performance, high availability, and low cost [6,11,12,13]. For example, VOx catalysts are usually used for CVOC destruction due to their excellent resistance to chlorine poisoning [13,14]. Recently, CeO2 catalysts attracted much attention for CVOC destruction attributed to their higher oxygen mobility, superior redox ability, and abundant oxygen vacancies. However, CeO2 catalysts are easily deactivated because of the strong adsorption of Cl species onto their active sites. Generally, the introduction of transition metal oxides is advantageous for promoting activity and stability. Dai et al. [15] presented that a 6 wt.% VOx/CeO2 catalyst displayed superior catalytic activity for 1,2–dichloroethane (1,2–DCE) degradation owing to the strong interaction of VOx species with CeO2 and the high valence of VOx. It was observed that the stability of VOx/CeO2 materials was significantly improved, attributed to the VOx species preventing Cl species from exchanging the surface lattice oxygen of CeO2 during chlorobenzene destruction [16]. Although VOx/CeO2 catalysts have made certain progress in CVOC destruction, there are many issues that should be further solved and clarified; for instance, the role of VOx and/or the synergistic effect of VOx/CeO2 in the production of chlorinated byproducts requires an in-depth study, as well as the effect of water vapor on catalytic performance and chlorinated byproduct distributions over VOx/CeO2 catalysts. It was reported that CeO2 material with a hollow nanosphere structure (CeO2–HS) had abundant oxygen vacancies and a large surface area, which is favorable for VOC oxidation [17]. Meanwhile, CeO2–HS with high stability and water resistance is a potential carrier for fabricating effective CVOC oxidation materials.
Herein, hollow nanosphere–like VOx/CeO2 catalysts with well–dispersed VOx species, abundant surface acid sites, and outstanding low–temperature reducibility were fabricated via a simple wetness impregnation process. The structural properties, reducibility, oxygen mobility, and acidity of prepared materials were deeply analyzed using various techniques such as field emission scanning electron microscopy (FE–SEM), high-resolution transmission electron microscopy (HR–TEM), the hydrogen temperature programmed reduction (H2–TPR), X-ray photoelectron spectroscopy (XPS), the temperature programmed desorption of O2 (O2–TPD) and the temperature programmed desorption of NH3 (NH3–TPD). Catalytic activity and stability, yields of CO and CO2, and the distribution of chlorinated byproducts over prepared materials were studied in detail. In particular, the influence of water vapor on catalytic performance and the vital factors inhibiting the generation of chlorinated byproducts were explored. Accordingly, the destruction mechanism of 1,2–DCE over VOx/CeO2 samples was further proposed.
2. Materials and Methods
2.1. Catalyst Preparation
Hollow nanosphere–like CeO2 was prepared using a hydrothermal route [18]. Specifically, 5.0 g of Ce(NO3)3·6H2O and 5 mL of CH3COOH (36 wt.%) were added into 150 mL of a mixed solution (ethylene glycol (100 mL) and deionized water (50 mL)) under vigorous stirring for 0.5 h. After that, 4.0 g of polyvinyl pyrrolidone (PVP; K–30) was added, with continued stirring at 60 °C for 1 h. The solution was then placed into a 200 mL Teflon–lined stainless–steel autoclave and kept at 180 °C for 6 h. Finally, the precursor was centrifuged, washed, dried, and calcined at 500 °C for 4 h in air.
Hollow nanosphere–like VOx/CeO2 materials were prepared via a wetness impregnation approach. Typically, 3.0 g of the above–prepared CeO2 was dispersed into 150 mL of deionized water, and an appropriate amount of NH4VO3 with the molar ratio of nNH4VO3:nCeO2 = 0.025:1, 0.05:1, 0.1:1, or 0.2:1 was then mixed in the above solution. Subsequently, the corresponding molar amount of oxalic acid with the molar ratio of nNH4VO3:noxalic acid = 1:2 was added to the suspension of CeO2. After that, the solution was vigorously stirred at 80 °C until excess deionized water was completely evaporated. The final products were treated under the same conditions as for CeO2 preparation. The obtained materials were designated as V0.025Ce, V0.05Ce, V0.1Ce, and V0.2Ce, respectively.
In comparison, VOx was also prepared using a hydrothermal method [19]. The prepared powder was also treated as in the CeO2 preparation process to obtain the bulk VOx. Additionally, commercial CeO2 (Aladdin Reagent Co., Ltd., Shanghai, China, 99.5%) named CeO2–C was also investigated for 1,2–DCE oxidation.
2.2. Catalyst Characterizations
The prepared materials were characterized by X-ray diffraction (XRD), Fourier transform infrared spectra (FT–IR), low–temperature N2 adsorption/desorption, FE–SEM, HR–TEM, XPS, H2–TPR, O2–TPD, NH3–TPD, FT–IR spectra of NH3 adsorption (NH3–IR), and in situ diffuse reflectance infrared spectroscopy (DRIFTS). The detailed information can be found in the Supplementary Materials.
2.3. Catalytic Activity
The 1,2–DCE destruction experiments were employed in a continuous–flow fixed–bed quartz tube reactor with 10.0 mm inner diameter. Typically, the prepared catalyst (500 mg; 40–60 mesh) was loaded into the isothermal region of the tube reactor. The reactant mixture containing 1000 ppm of 1,2–DCE, 79% N2, and 21% O2, with a total flow rate of 250 mL·min−1 (gas hourly space velocity (GHSV) = 30,000 mL·g−1·h−1) was obtained through the reactor. The concentrations of 1,2–DCE, chlorinated byproducts, and COx (CO and CO2) were measured using an online gas chromatograph (GC9890, China) with Electron Capture Detector (ECD). Furthermore, an online Cl2 and HCl detector (PN–2000, China) was used to analyze the concentrations of Cl2 and HCl.
The 1,2–DCE conversion (X1,2-DCE) was calculated using Equation (1).
where Cin and Cout refer to the inlet and outlet 1,2–DCE concentrations, respectively.
The CO and CO2 yields (YCO and YCO2) were calculated using Equations (2) and (3), respectively.
where CCO and CCO2 refer to the outlet concentrations of CO and CO2, respectively.
The stability of the prepared catalysts was evaluated at different temperatures corresponding to 90% conversion of 1,2–DCE under the same conditions as the activity experiments. Moreover, the stability of the highest–activity V0.05Ce sample for 1,2–DCE oxidation was studied in the absence and presence of water vapor. Different concentrations of water vapor (1, 2, and 5 vol.%) were injected into the reactant mixture containing 1000 ppm of 1,2–DCE using an automatic sample injector.
3. Results
3.1. Structural and Textural Properties
Figure 1a and Figure S1 (Supplementary Materials) exhibit the XRD patterns of prepared catalysts. The peaks located at 28.4°, 33.1°, 47.4°, 56.3°, 59.1°, 69.6°, 76.6°, and 79.2° were attributed to the (111), (200), (220), (311), (222), (400), (331), and (420) crystal planes of CeO2, respectively [20]. The characteristic peaks of VOx (V2O5) were located at 15.3°, 20.2°, 21.7°, 26.1°, 31.0°, 32.4°, 34.3°, and 47.4° [21]. Notably, no characteristic peaks corresponding to VOx and CeVO4 species (2θ = 24.0° and 32.5°) could be detected over VOx/CeO2 catalysts, suggesting that VOx species were highly dispersed on the surface or in the form of a solid solution [15,22]. The position of the main peak of CeO2 (ca. 28.4°) was well maintained over VOx/CeO2 materials, suggesting that VOx neither altered the CeO2 crystallization nor was incorporated into the CeO2 crystalline phase, forming a solid solution [15,23]. Hence, VOx species existed in a highly dispersed form.
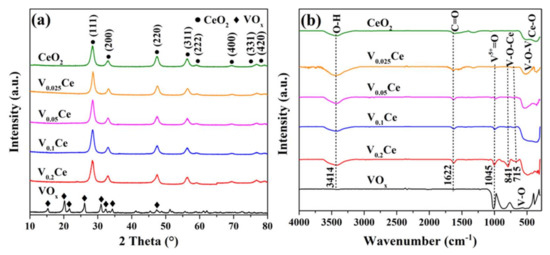
Figure 1.
(a) X-ray diffraction (XRD) profiles and (b) Fourier transform infrared spectra (FT–IR) of prepared catalysts.
As shown in Figure 1b, the broad IR peaks over all catalysts between 3500 and 3000 cm−1 corresponded to the stretching vibration of surface hydroxyl groups, while the peaks presented at 1622 cm−1 were indexed as C=O stretching vibration [24]. For CeO2, the peaks located at wavenumbers lower than 1000 cm−1 were associated with the vibration of Ce–O [25]. For VOx, the peaks located in the 1100–400 cm−1 region were related to the V–O stretching vibration [26]. The peaks centered at 1045 and 841 cm−1 were consistent with the symmetric stretching vibration of V5+=O and stretching vibration of O–(V)3, respectively. It can be observed that V5+=O vibration located at 1045 cm−1 was detected over VOx and VOx/CeO2 catalysts [26]. The two peaks over VOx/CeO2 catalysts at 715 and 841 cm−1 could be regarded as the V–O–Ce modes [27]. In addition, the symmetric and antisymmetric stretching vibration of V–O–V could be detected in the 700–400 cm−1 range over VOx and VOx/CeO2 catalysts [26].
FE-SEM images of prepared catalysts are shown in Figure 2a–x. CeO2 exhibited a regular nanosphere–like morphology with different diameters (ca. 245–600 nm), as displayed in Figure 2a,b. The V0.05Ce catalyst was made up of nanospheres with a diameter of ca. 100–200 nm (Figure 2e,f). Compared with the V0.05Ce catalyst, V0.025Ce (Figure 2c,d), V0.1Ce (Figure 2g,h) and V0.2Ce catalysts (Figure 2i,j) consisted of larger nanosphere–like particles with a diameter of ca. 300–400 nm. Moreover, VOx showed an irregular nanosphere–like morphology with size varying from ca. 200 to 300 nm (Figure 2k,l). Figure 2m–x and Figure S2 (Supplementary Materials) exhibit the transmission electron microscope (TEM) and high-angle annular dark-field imaging in scanning transmission electron microscopy (HAADF–STEM) images of prepared catalysts. It was revealed that CeO2 and VOx/CeO2 catalysts possessed a well–defined hollow nanosphere–like structure with diameter in the range of ca. 200–400 nm, in agreement with the FE–SEM results. The results exhibited that VOx species were highly dispersed and did not change CeO2 morphology [28]. VOx had an irregular nanosphere–like morphology with a lattice spacing of 0.34 nm, ascribed to the (101) plane of V2O5 (Figure 2w,x) [29]. The measured lattice distances of 0.31, 0.27, and 0.19 nm were ascribed to the (111), (200), and (220) lattice planes of CeO2 nanospheres (JCPDS #34–0394), respectively (Figure 2n). Figure 2p,r,t and Figure S2 (Supplementary Materials) show that V0.025Ce, V0.05Ce, V0.1Ce, and CeO2–C catalysts possessed the (200) and (111) lattice planes of CeO2 with lattice spacings of 0.27 and 0.31 nm, respectively. For V0.2Ce, the lattice spacings of 0.27 and 0.19 nm were associated with the (200) and (220) crystal planes of CeO2, respectively (Figure 2v). No VOx species on the VOx/CeO2 catalysts could be detected in HAADF–STEM images (Figure 2p,r,t,v), which could be attributed to the rough surface of CeO2 or the generation of VOx films from the layer structure [15].
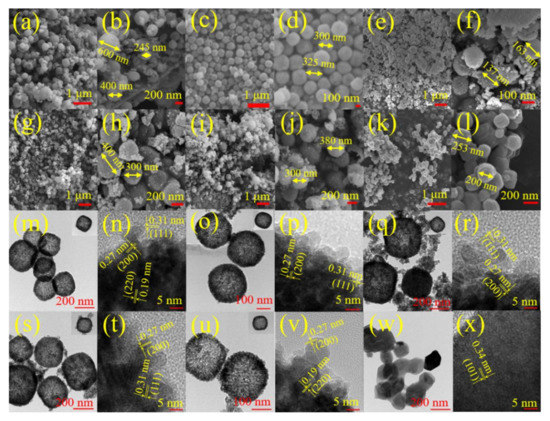
Figure 2.
Field emission scanning electron microscopy (FE–SEM) images of (a,b) CeO2, (c,d) V0.025Ce, (e,f) V0.05Ce, (g,h) V0.1Ce, (i,j) V0.2Ce, and (k,l) VOx; high-resolution transmission electron microscopy (HR–TEM) patterns of (m,n) CeO2, (o,p) V0.025Ce, (q,r) V0.05Ce, (s,t) V0.1Ce, (u,v) V0.2Ce, and (w,x) VOx.
N2 adsorption/desorption isotherms of the prepared materials are presented in Figure 3. CeO2 and VOx/CeO2 catalysts showed a type II isotherm curve with H3–type hysteresis loops at the relative pressure (P/P0) range of 0.7–1.0 (Figure 3a), indicating that meso- and macropore structures co–existed in CeO2 and VOx/CeO2 catalysts [30,31]. VOx exhibited a type III isotherm curve with H3–type hysteresis loops (P/P0 = 0.9–1.0), illustrating that the presence of textural pores facilitated the filling of interparticle spaces. It was demonstrated that the pore size distribution of prepared catalysts was centered at approximately 25.4, 11.7, 19.4, 37.6, 27.0, and 17.4 nm, respectively (Figure 3b), indicating that all catalysts possessed a large number of mesopores. The pore size distribution of materials in the 100–500 nm region could be attributed to the existence of a macroporous structure. The specific surface area, average pore diameter, and pore volume of materials are documented in Table 1, and the average pore diameter followed the order of VOx (63.1 nm) > V0.1Ce (30.1 nm) > V0.025Ce (18.0 nm) > V0.2Ce (14.8 nm) > CeO2 (13.2 nm) > V0.05Ce (10.7 nm). The specific surface area of CeO2 and VOx/CeO2 was much higher than that of VOx, which obeyed the sequence of CeO2 (110.9 m2·g−1) > V0.025Ce (101.0 m2·g−1) > V0.05Ce (98.6 m2·g−1) > V0.1Ce (93.4 m2·g−1) > V0.2Ce (76.1 m2·g−1) >> VOx (11.3 m2·g−1). It was revealed that the specific surface area of VOx/CeO2 catalysts decreased along with the increase in VOx loading because of the partial blockage of CeO2poresby VOx species [28].
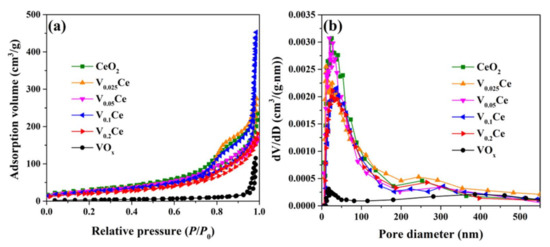
Figure 3.
(a) Nitrogen adsorption/desorption isotherms and (b) pore size distribution of prepared catalysts.

Table 1.
Textural properties and catalytic performance of prepared catalysts.
3.2. Surface Composition and Element Status
The surface elemental composition and oxidation status of prepared materials were characterized through XPS, as illustrated in Figure 4 and Table 2. The C 1s, O 1s, V 2p, and Ce 3d peaks of prepared materials could be detected, as shown in Figure 4a. As shown in Figure 4b, O 1s XPS spectra were classified into three sub-peaks centered at 529.3–529.9, 531.5–531.9, and 533.0–533.4 eV, which were attributed to lattice oxygen (Oα; O2−), surface oxygen species adsorbed on oxygen vacancies (Oβ; O22−, O−, OH−, and CO32−), and carbonates and/or water (Oγ), respectively [32,33]. The Oβ/(Oα + Oβ) ratios of prepared catalysts followed the sequence of V0.05Ce (0.61) > V0.1Ce (0.57) > V0.025Ce (0.54) > CeO2 (0.48) > V0.2Ce (0.46) > VOx (0.45) (Table 2), suggesting that the V0.05Ce catalyst possessed the largest number of surface oxygen species, facilitating the destruction of 1,2–DCE [5]. Figure 4c shows the V 2p XPS spectra of VOx and VOx/CeO2 catalysts. Only V5+ species could be detected over VOx, corresponding to the presence of the V2O5 phase. The peaks at 517.1–517.4 and 515.0–515.4 eV represented V5+ and V4+, respectively, suggesting that V5+ and V4+ species co–existed on VOx/CeO2 catalysts [34]. Moreover, the peak–fitting results demonstrated that VOx/CeO2 catalysts were mainly composed ofV5+ species corresponding to the V2O5 phase [21,34]. As shown in Table 2, the ratios of V5+/(V4++ V5+) for V0.025Ce, V0.05Ce, V0.1Ce, and V0.2Ce catalysts were 0.84, 0.87, 0.85, and 0.83, respectively, indicating that the V0.05Ce material possessed high ratios of V5+/(V4++ V5+), favorable for 1,2–DCE oxidation. The Ce 3d XPS spectra were split into eight sub–peaks, as depicted in Figure 4d. Six peaks of Ce 3d labeled by V, V″, V‴, U, U″, and U‴ were assigned to Ce4+ species and the other two peaks labeled by U′ and V′ belonged to Ce3+ species [34]. The Ce3+/(Ce3+ + Ce4+) ratios of prepared catalysts were calculated according to the corresponding peak areas, as documented in Table 2. Generally, surface oxygen vacancies were formed by the removal of lattice oxygen species (Oα) in CeO2, causing the formation of Ce3+ species (Ce4+ + e−→Ce3+ + ◇) [35]. According to Table 2, Ce3+/(Ce3+ + Ce4+) ratios of prepared materials followed the order of V0.05Ce (0.19) > V0.1Ce (0.18) > V0.025Ce (0.17) > CeO2(0.15) > V0.2Ce (0.13), suggesting that V0.05Ce owned the largest amount of Ce3+ species. It was reported that a large amount of Ce3+ species contributes to the formation of oxygen vacancies, which can effectively accelerate the mobility of active oxygen species and consequently promote the catalytic activity [35]. This result also suggested that VOx species interaction with CeO2 was strongest over V0.05Ce, leading to the formation of more low–valence Ce3+ species [28].
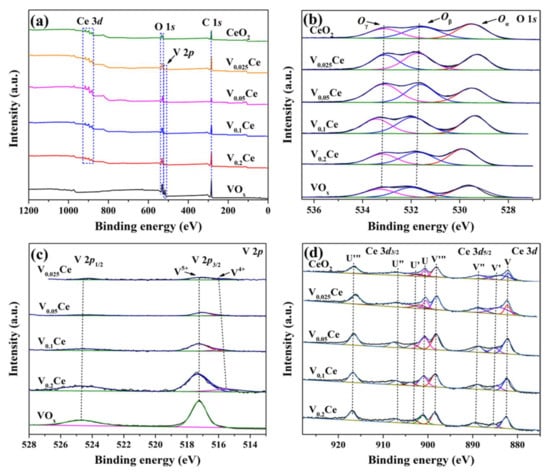
Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of prepared catalysts: (a) C 1s, (b) O 1s, (c) V 2p, and (d) Ce 3d.

Table 2.
XPS results of prepared catalysts.
3.3. Reducibility and Oxygen Species Mobility
An H2–TPR experiment was employed to test the redox properties of all samples, as depicted in Figure 5a. For CeO2, the reduction peaks presented at ca. 524 and 785 °C were respectively associated with the reduction of surface Ce4+ to Ce3+ and bulk CeO2 [28]. The introduction of VOx could effectively enhance the redox capacity of CeO2. For VOx/CeO2 catalysts, the reduction peaks located at 518, 500, 507, and 526 °C were indexed to the reduction of VOx species (V5+→V4+), while the peaks at 436, 454, 466, and 492 °C were indexed to the reduction of surface CeO2 (Ce4+→Ce3+) [22,28], indicating the co–existence of Ce4+/Ce3+ and V5+/V4+ species, in accordance with the XPS results (Figure 4). It is illustrated that the reduction temperature of VOx species in VOx/CeO2 (except V0.025Ce) catalysts apparently shifted to higher temperatures along with the increase in VOx loading due to theCeO2 interaction with VOx species affecting the redox performance of surface CeO2 [22]. The total H2 consumption of prepared materials below 600 °C followed the order of V0.05Ce (9.86 mmol·g−1) > V0.1Ce (9.53 mmol·g−1) > V0.025Ce (8.64 mmol·g−1) >CeO2 (8.02 mmol·g−1) > V0.2Ce (7.63 mmol·g−1), suggesting that V0.05Ce possessed superior reducibility (Table 3). Additionally, the initial H2 consumption (less than 25% H2 consumption for the first reduction peak) rate was also employed to evaluate the low-temperature reducibility of all catalysts [24]. Figure 5b shows that the initial H2 consumption rates of the catalysts followed the sequence of V0.05Ce > V0.1Ce > V0.025Ce > CeO2> V0.2Ce, suggesting that V0.05Ce had superior low-temperature reducibility.
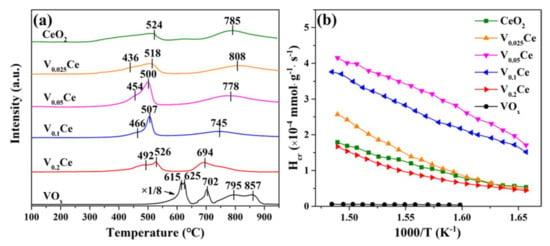
Figure 5.
(a) Hydrogen temperature programmed reduction (H2–TPR) and (b) the initial H2 consumption rate of prepared catalysts.

Table 3.
H2–TPR, O2–TPD, and NH3–TPD results of prepared catalysts.
The O2–TPD results of prepared materials are displayed in Figure 6. Generally, the peaks located at <250, 250–550, and 550–750 °C could be regarded as the physically adsorbed oxygen species, chemically adsorbed oxygen (O− and O2−) species, and weak surface lattice oxygen (O2−) (bulk lattice oxygen at above 750 °C), respectively [36,37]. For CeO2 and VOx/CeO2 catalysts, the desorption peaks located at 169, 160, 187, 193, and 176 °C were assigned to the physically adsorbed oxygen (O− and O2−) species. The desorption peaks in the region of 250–550 °C corresponded to the chemically adsorbed oxygen species. Additionally, the desorption peaks located at 642, 747, 706, 644, and 565 °C could be associated with the weak surface lattice oxygen (O2−) [16,38]. Generally, the lattice oxygen diffused from bulk to surface, which further promoted the reduction of V5+ to V4+ and Ce4+ to Ce3+ [38]. For the VOx catalyst, the desorption peaks presented at 629 and 684 °C were consistent with the weak surface lattice oxygen (O2−). As displayed in Table 3, the desorption amount of chemically adsorbed oxygen (O− and O2−) species followed the sequence of V0.05Ce > V0.1Ce > V0.025Ce > CeO2> V0.2Ce, suggesting that V0.05Ce possessed excellent chemically adsorbed oxygen (O− and O2−) species mobility, favorable for 1,2–DCE oxidation.

Figure 6.
Temperature programmed desorption of O2 (O2–TPD) profiles of prepared catalysts.
3.4. Surface Acidity
The acidity properties of the prepared catalysts were investigated through NH3–TPD, as displayed in Figure 7a. Generally, the NH3desorption spectra of prepared catalysts were divided into three peaks corresponding to weak acidity (<200 °C), moderate acidity (200–400 °C), and strong acidity (>400 °C) [24]. CeO2 displayed two peaks presented at 198 and 365 °C, which were related to the weak and moderate acid sites, respectively. The broad peaks over V0.05Ce (232 °C) and V0.1Ce (246 °C) catalysts were attributed to the moderate acid sites. Similarly, V0.025Ce, V0.2Ce, and VOx catalysts also exhibited wide peaks at 160, 187, and 157 °C, which corresponded to the weak acid sites. The total NH3desorption amount of the prepared catalysts followed the order of V0.05Ce > V0.1Ce > V0.025Ce > CeO2 > V0.2Ce >> VOx (Table 3), which revealed that the opportune introduction of VOx could enhance the total acidity of CeO2. V0.05Ce possessed the largest number of acid sites, crucial for promoting the adsorption and activation of 1,2–DCE molecules [39].
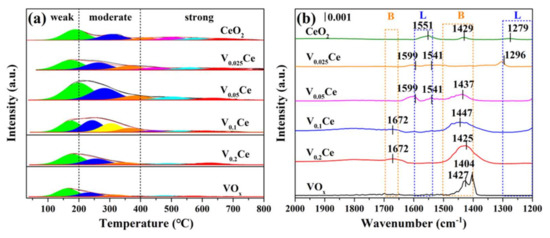
Figure 7.
(a) Temperature programmed desorption of NH3 (NH3–TPD) and (b) FT–IR spectra of NH3 adsorption (NH3–IR) profiles of prepared catalysts.
An NH3–IR analysis of prepared catalysts was further conducted to characterize the acid site types, as shown in Figure 7b. Generally, the bands in the regions of 1500–1400 and 1690–1660 cm−1 were associated with the symmetric and antisymmetric stretching vibrations of NH4+ species adsorbed on Brønsted acid (designed as B) sites [40,41]. The bands at 1300–1200 and 1600–1540 cm−1 were related to the symmetric and antisymmetric stretching vibration of NH3 adsorbed on Lewis acid (designed as L) sites [30,40]. For CeO2, the band located at 1429 cm−1 was related to the B acid sites, and the bands centered at 1279 and 1551 cm−1 could be connected to the L acid sites. The bands at 1296, 1541, and 1599 cm−1 associated with L acid sites over V0.025Ce could be detected. For V0.05Ce, the band located at 1437 cm−1 corresponded to the B acid sites, while the bands centered at 1541 and 1599 cm−1 corresponded to the L acid sites. Compared with CeO2, more B and L acid sites could be observed over V0.05Ce. For V0.1Ce and V0.2Ce, the bands at 1404, 1427, and 1672 cm−1 could be ascribed to the B acid sites. However, no L acid sites could be detected over V0.1Ce and V0.2Ce catalysts, indicating that more L acid sites were occupied with the increase in V loading [30]. The bands at 1404 and 1427 cm−1 over VOx corresponded to the B acid sites.
4. Discussion
4.1. Catalytic Activity and Stability
All prepared materials were evaluated in 1,2-DCE destruction, as exhibited in Figure 8a. According to T90 (the temperature for 90% conversion of 1,2–DCE), the activity sequence of synthesized materials was in the order of V0.05Ce (347 °C) > V0.1Ce (356 °C) > V0.025Ce (367 °C) > CeO2 (381 °C) > V0.2Ce (413 °C) > CeO2-C (> 450 °C) >> VOx. The catalytic activity of CeO2–C was significantly lower than that of hollow nanosphere–like CeO2, indicating that the designed hollow nanosphere structure for CeO2 had an advantage in 1,2–DCE degradation. Furthermore, it could be found that the activity of all catalysts increased slowly (<240 °C) and then enhanced rapidly with the increase in temperature as 1,2–DCE was firstly adsorbed at relatively low temperature and then activated with increasing temperature. Compared with CeO2, the activity of V0.025Ce, V0.05Ce, and V0.1Ce catalysts was obviously improved because the strong interaction of VOx species with CeO2couldpromote the efficient removal of Cl species on the catalyst surface [16]. On the other hand, the catalytic activity of V0.2Ce was lower than that of CeO2, which was related to the excessive VOx content covering some oxygen vacancies or active sites of CeO2 and inhibiting the removal of Cl species [42]. According to the XPS results in Figure 4d, V0.05Ce possessed the highest amount of Ce3+, indicating the presence of more oxygen vacancies in CeO2, which could be advantageous to improving the mobility of active oxygen species and further promoting the oxidation of 1,2–DCE. According to H2–TPR and O2/NH3–TPD results (Figure 5, Figure 6 and Figure 7), it was demonstrated that V0.05Ce possessed superior low–temperature reducibility, excellent chemically adsorbed oxygen (O− and O2−) species mobility, and a large number of acid sites, facilitating the adsorption and oxidation of 1,2–DCE. Moreover, the strong interaction of VOx species with CeO2 could also efficiently enhance the activity of V0.05Ce.
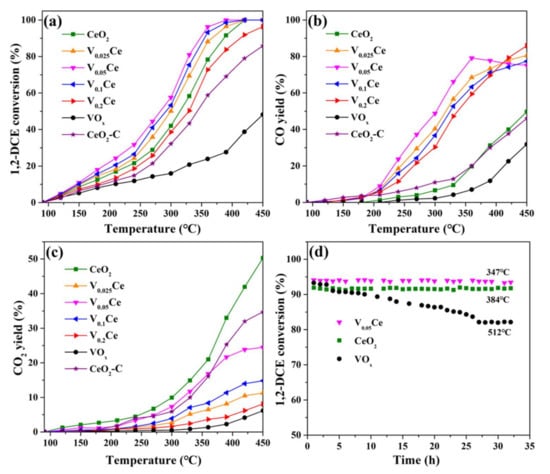
Figure 8.
(a) Catalytic activity, (b) CO yield, (c) CO2 yield, and (d) stability of prepared catalysts for 1,2–DCE destruction.
The yields of CO and CO2 as a function of temperature were analyzed in Figure 8b,c. It was revealed that the yields of CO and CO2 over CeO2 and CeO2–C catalysts increased with increasing temperature; however, much different behaviors could be found over VOx/CeO2. The yields of CO and CO2 over CeO2 only had a tiny gap and could reach approximately 50% at 450 °C, whereas the yields of CO (46%) and CO2 (35%) over CeO2–C were lower than that of hollow nanosphere–like CeO2. It could be found that the yield of CO significantly increased when VOx species were loaded on the surface of CeO2. In addition, the yields of CO and CO2 over V0.025Ce, V0.1Ce, and V0.2Ce catalysts increased with increasing temperature. However, the yield of CO over V0.05Ce catalyst initially increased and then decreased with increasing temperature, attributed to the further oxidation of CO (>360 °C). It was reported that the final product mainly containing carbon species is CO over VOx/CeO2 catalysts during 1,2–DCE destruction, and the high yield of CO is presumed to be connected to the surface lattice oxygen of VOx [15].
Reaction stability is one of the most important indices to evaluate the application prospect of a catalyst. The stabilities of V0.05Ce, CeO2, and VOx were studied at approximatelyT90 temperatures, as shown in Figure 8d. The V0.05Ce catalyst possessed outstanding catalytic stability and resistance to chlorine poisoning, maintaining approximately 94% of 1,2–DCE conversion at 347 °C for 32 h. Similarly, the catalytic stability of CeO2 was maintained at approximately 92% at 384 °C in 32 h. However, the conversion of 1,2–DCE over VOx decreased distinctly from 93% to 83% in the first 27 h, which could be ascribed to the attack of Cl species, leading to the loss of active sites.
4.2. Chlorinated Intermediate Species Distribution and Proposed Reaction Mechanism
CO2, CO, HCl, and Cl2 are the ideal products for CVOC destruction; however, chlorinated byproducts are usually inevitably formed. In this work, 1,1,2–trichloroethane (C2H3Cl3), vinyl chloride (C2H3Cl), trichloroethylene (C2HCl3), dichloromethane (CH2Cl2), trichloromethane (CHCl3), and perchloromethane (CCl4) were observed during the oxidation of 1,2–DCE. CCl4, CHCl3, C2HCl3, and C2H3Cl3 as primary chlorinated byproducts collected at different reaction temperatures. Figure 9 presents the remarkable differences in the concentration of chlorinated byproducts detected over all catalysts. The concentrations of CCl4, CHCl3, C2HCl3, and C2H3Cl3 over VOx/CeO2 catalysts were obviously lower than those over CeO2 and VOx. Additionally, it was observed that the concentrations of chlorinated byproducts over CeO2 and VOx/CeO2 catalysts firstly increased and then decreased with increasing test temperature (>250 °C). The appropriate introduction of VOx species (V0.025Ce, V0.05Ce, and V0.1Ce) in CeO2 was advantageous for the catalytic activity of CeO2 and remarkably inhibited the formation of chlorinated byproducts, ascribed to the strong interaction between VOx species and CeO2 and the superior removal ability of Cl species during 1,2-DCE oxidation [30,43].
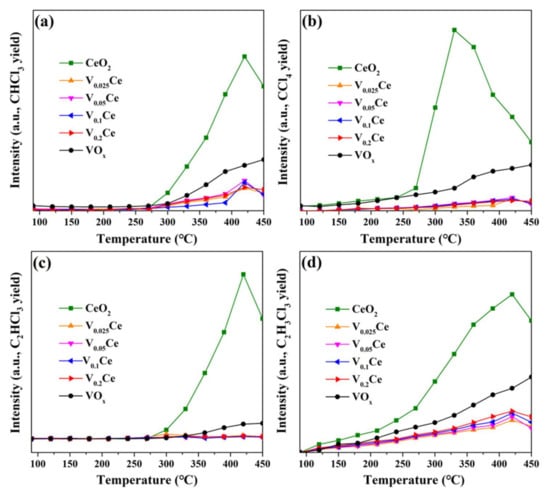
Figure 9.
Chlorinated byproducts distribution in 1,2–DCE destruction over prepared catalysts: (a) CHCl3, (b) CCl4, (c) C2HCl3, and (d) C2H3Cl3.
The reaction process of 1,2-DCE over prepared materials was deeply studied using an in situ DRIFTS experiment, as displayed in Figure 10. The broad bands presented at 3100–4000 cm−1 were indexed to the stretching vibrations of hydrogen–bonded OH [15,44]. Additionally, no bands corresponding to VOx species were detected over VOx/CeO2 catalysts because of the highly dispersed VOx species on the CeO2 surface [44].
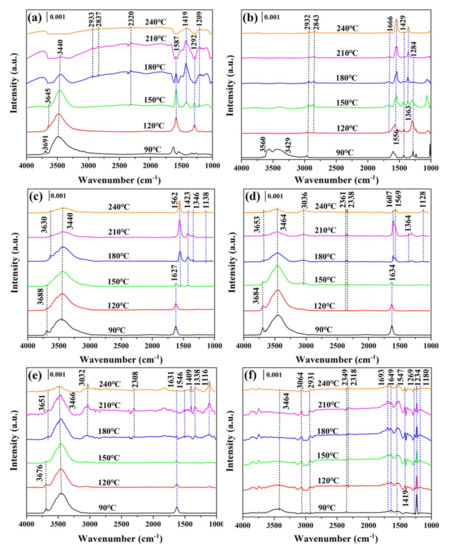
Figure 10.
In situ diffuse reflectance infrared spectroscopy (DRIFTS) of 1,2–DCE catalytic oxidation: (a) CeO2, (b) V0.025Ce, (c) V0.05Ce, (d) V0.1Ce, (e) V0.2Ce, and (f) VOx.
The bands presented at 2932–2933 and 2837–2843 cm−1 attributed to the asymmetric and symmetric stretching of methyl (CH3–) could be observed over CeO2 and V0.025Ce, respectively, which was attributed to the generation of CH3CHO and CH3COOH intermediates during the 1,2–DCE oxidation process [45]. On the other hand, no bands could be detected over the V0.05Ce catalyst in the above wavenumber range. The weak bands located at 3036–3032 cm−1 were detected over V0.1Ce (>150 °C) and V0.2Ce (>180 °C) catalysts, which corresponded with the asymmetric stretching of methylene species (–CH2–) [15,45]. VOx exhibited two weak bands at 3064 and 2931 cm−1 corresponding to antisymmetric stretching of methylene species and antisymmetric stretching of methyl (CH2–), respectively [45]. Generally, the vibration of –CH– stretching (v(CH), such as CH2– and CH3– groups) is related to the adsorption of 1,2–DCE on the catalyst surface [46]. The bands present between 2400 and 2300 cm−1 were associated withCO2 adsorption, while the bands located in the 2300–2000 cm−1 region were indexed to CO adsorption [15,43]. As shown in Figure 10b,c, no bands corresponding to adsorbed CO2 were detected over V0.025Ce and V0.05Ce catalysts, and the bands corresponding to adsorbed CO could not be observed in all catalysts. The bands at 1700–1200 cm−1 could be classified as the vibration of surface formates, carbonate species, and hydrocarbons, which were associated with the formation of CO2 and CO during 1,2–DCE oxidation [24].
For all catalysts, the bands present at 1666–1610 cm−1 were considered as C=C stretching vibration [30]. The intensity of the stretching vibration of C=C over V0.1Ce and V0.2Ce catalysts gradually decreased with increasing temperature and finally disappeared at relative high temperature. The band deemed as C=O stretching vibration (ca. 1693 cm−1) could be found over VOx [43]. The characteristic bands appearing at 1587–1532 cm−1 corresponding to the carboxylate v(COO−) asymmetric stretching of acetate species could be detected over all catalysts [47]. The bands located at 1429–1409 cm−1 associated with the formation of –CH2– and –CH– groups (e.g., δ(CH2Cl), δ(HCCl), and δ(CCH)) could be detected over all catalysts (except V0.1Ce), illustrating that 1,2-DCE was adsorbed and activated [15,43]. The intensity of bands over these catalysts primarily increased and then decreased with escalating temperature, inferring that 1,2–DCE oxidation occurred [15]. Bands located at 1332–1364 cm−1 over VOx/CeO2 catalysts could be detected, which were ascribed to the partial oxidized surface species as acetates, maleate species, and formats [47]. The bands presented at 1138–1120 cm−1 were indexed to the vibration stretching of –CH–. The bands at 1284–1292 cm−1 over CeO2 and V0.025Ce catalysts could be attributed to –CH– vibration groups [48]. The band present at 1209 cm−1 could be indexed to phenolate (v(CH)/v(CO)) over CeO2. Moreover, the bands over VOx located at 1269, 1234, and 1180 cm−1 could be identified as the formation of –CH2– and –CH– groups, suggesting that 1,2–DCE was adsorbed and activated over the catalyst.
Combining the results of in situ DRIFTS and byproduct distribution (Figure 9 and Figure 10), a destruction mechanism of 1,2–DCE over prepared materials was proposed, as shown in Scheme 1. Generally, the catalytic oxidation of chlorinated alkanes (CA) proceeds according to the procedure of CA adsorption →Cl dissociation →C–C/C–Cl cleavage →Cl desorption/accumulation [49,50]. As such, 1,2–DCE is initially adsorbed on the surface of catalysts, which is mainly ascribed with the adsorption and dissociated of Cl bonds on Lewis acid sites (V5+/4+ and Ce4+/3+) [15]; The C–Cl (Path 1) and C–C (Path 2) bonds in 1,2–DCE are activated and cleaved, forming chloroethane, vinyl chloride, and chloromethane intermediates. Afterward, the polychlorinated byproducts (CHCl3, CCl4, C2HCl3,and C2H3Cl3) are mainly produced via dehydrochlorination and chlorination. Then, the reaction of dissociative Cl from polychlorinated byproducts and surface hydroxy groups can form HCl, and HCl would be partially oxidized to form Cl2 (the Deacon reaction) [15,49]. Additionally, the intermediate acetaldehyde (from the activation of the C–H bond and the transformation of hydrogen species on VOx species [15]) would be easily and rapidly oxidized to oxygenate species (carbonate bidentate) and partially oxidized to maleates, acetates, formates, and so on, which are usually considered the main source for the generation of CO and CO2 [24,51]. The above oxygenate species are further oxidized to form the final products (CO, CO2, Cl2, and HCl).
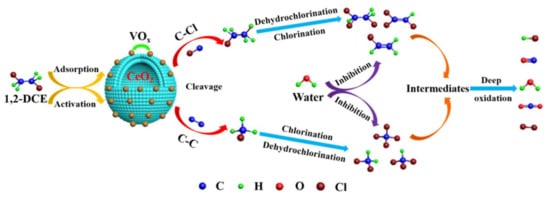
Scheme 1.
Proposed 1,2–DCE destruction mechanism over prepared catalysts.
4.3. Effect of Water Vapor
It is of great importance to study the influence of water vapor on the reaction activity of samples for CVOC destruction. Here, water vapor with different concentrations (1–5 vol.%) was introduced to study the influence of water vapor on the activity of the V0.05Ce catalyst in 1,2–DCE oxidation (Figure 11a). The conversion of 1,2–DCE was maintained at approximately94% without water vapor. Interestingly, when 1, 2, or 5 vol.% of water vapor was injected into the atmosphere, 1,2–DCE conversion over the V0.05Ce catalyst increased slightly and was maintained at approximately 97%, 96%, or 95%, respectively, owing to that low concentration of water molecules efficiently removing Cl species from the catalyst surface [52]. However, the promotion effect of water vapor gradually decreased with increasing concentration because of the competitive adsorption or blockage of water molecules on active sites [17,52]. In addition, the catalytic activity of V0.05Ce was restored to the initial level when water vapor was removed from the reaction atmosphere, indicating that the V0.05Ce catalyst had good water resistance and stability under the conditions with/without water vapor during 1,2–DCE destruction. The effect of water vapor on chlorinated byproduct distribution over the V0.05Ce catalyst was further investigated, as exhibited in Figure 10b–d. It was found that the presence of water vapor did not change the type of chlorinated byproducts; however, the yields of CHCl3, CCl4, and C2HCl3 over the V0.05Ce catalyst were apparently lower than those in the absence of water vapor because the reactivity of Cl2 was inhibited by water molecules as a hydrogen source [7,49]. Moreover, the yield of chlorinated byproducts decreased with increasing water vapor concentration, suggesting that the presence of water vapor could efficiently inhibit the generation of chlorinated byproducts during 1,2–DCE oxidation.
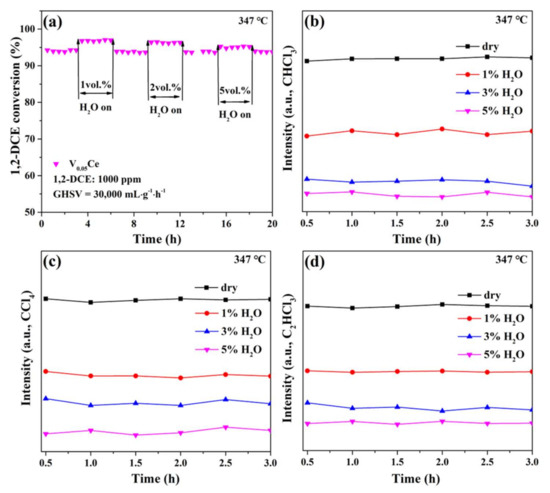
Figure 11.
(a) Effects of water on 1,2–DCE conversion over V0.05Ce catalyst; (b) CHCl3, (c) CCl4, and (d) C2HCl3 distribution during the stability test.
4.4. Key Factors Inhibiting the Formation of Chlorinated Intermediate Species
Figure 9 reveals that the yields of chlorinated byproducts (CHCl3, CCl4, C2HCl3, and C2H3Cl3) over VOx/CeO2 were significantly lower than those of CeO2 and VOx during the 1,2–DCE oxidation process, ascribed to the high surface Cl removal ability of composite catalysts. Feng et al. [24] reported that the CoCrOx catalyst can effectively inhibit the yield of chlorinated byproducts during 1,2–DCE destruction, and it possesses better product selectivity than Cr2O3 and Co3O4 due to the abundant B and L acid sites and higher acid strength. Therefore, the relationship between chlorinated byproducts yield and total acidity over VOx/CeO2 catalysts was analyzed. As shown in Figure 12a, CHCl3, CCl4, C2HCl3, and C2H3Cl3 yields versus total acidity maintained linear relationships (R2 = 0.996, 0.991, 0.993, and 0.997, respectively). Compared with V0.025Ce, V0.1Ce, and V0.2Ce catalysts, V0.05Ce possessed higher total acidity and lower chlorinated byproduct (CHCl3, CCl4, C2HCl3, and C2H3Cl3) yields, indicating that the yields of chlorinated byproducts decreased with increasing total acidity, which apparently reduced the occurrence of the chlorination reaction [53]. In addition, the catalytic activity of VOx/CeO2 catalysts (T90) versus total acidity maintained a good linear relationship (R2 = 0.995), as shown in Figure 12b. The above results indicate that the total acidity of catalysts played a vital role in inhibiting the production of chlorinated byproducts. Therefore, it can be reasonably explained that the low chlorinated byproduct yields could be ascribed to the higher total acidity and good synergy between CeO2 and VOx species during the 1,2–DCE oxidation process.
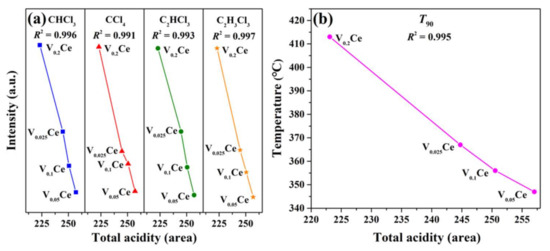
Figure 12.
(a) Relationship between chlorinated byproduct yields and total acidity over VOx/CeO2 catalysts; (b) relationship between T90 of VOx/CeO2 catalysts and total acidity.
4.5. Characterization of the Used Catalysts
XRD and XPS analyses of the used V0.05Ce catalyst were carried out, and the results are summarized in Figure 13 and Table S1 (Supplementary Materials). Compared to the fresh V0.05Ce catalyst, the crystal phase of CeO2 of the used V0.05Ce catalyst remained unchanged, indicating that the phase of CeO2 was not destroyed by the attack of Cl species, further confirming that the V0.05Ce catalyst possessed excellent resistance to chlorine poisoning (Figure 13a). Figure 13b shows that the lattice oxygen (Oα) species abundance of the used V0.05Ce catalyst was lower than that of the fresh V0.05Ce catalyst, indicating that a large number of lattice oxygen species were removed, giving rise to the greater formation of Ce3+ species (Table S1, Supplementary Materials) [35]. We discovered that the Ce3+ species abundance of the used V0.05Ce catalyst was remarkably higher than that of the fresh V0.05Ce catalyst, confirming that the lattice oxygen species was removed leading to the greater formation of Ce3+ species (Figure 13c and Table S1, Supplementary Materials). Additionally, greaterCe3+ species abundance led to greater formation of surface oxygen vacancies, with surface oxygen species being adsorbed onto oxygen vacancies over time, showing that the V0.05Ce catalyst owned superior catalytic performance (Figure 13b and Table S1, Supplementary Materials). No V4+ species over the used V0.05Ce catalyst could be detected, suggesting that the V4+ species were completely transformed into V5+ species (V4+→V5+), accelerating the oxidation of 1,2–DCE (Figure 13d). According to the above results, it was confirmed that the Ce3+ species and surface oxygen (Oβ) species are important factors in improving the catalytic performance of the V0.05Ce catalyst.
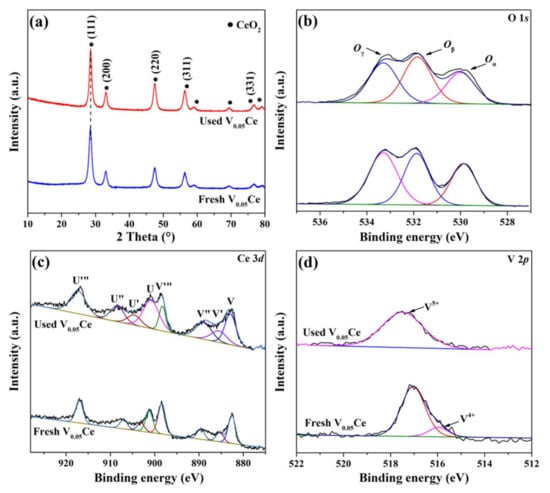
Figure 13.
(a) XRD of the used V0.05Ce catalyst; XPS spectra of the used V0.05Ce catalyst: (b) O 1s, (c) Ce 3d, and (d) V 2p.
The Cl 2p spectra of all used catalysts were also further employed to investigate the chlorine deposit over catalysts, as displayed in Figure S5 (Supplementary Materials). The peaks at 198.1–198.5 eV were assigned to HCl and/or Cl2, while the peaks at 198.1–198.5 eV were ascribed to the adsorption of 1,2–DCE on the catalyst surface [36]. It was revealed that CeO2 had two peaks respectively ascribed to the HCl (and/or Cl2) species and the adsorption of 1,2–DCE on the catalyst surface. However, it the adsorption peak of 1,2–DCE on the catalyst surface could not be observed over CeO2–C. It was speculated that the 1,2–DCE did not directly contact the surface of the catalyst during the reaction process, which could explain the poor catalytic performance of the CeO2–C catalyst. HCl (and/or Cl2) species and the adsorption of 1,2–DCE on the catalyst surface over VOx/CeO2 catalysts (except V0.05Ce) could be detected. Unexpectedly, no peaks could be discovered over V0.2Ce and VOx catalysts, potentially due to the formation of chlorinated oxide rather than HCl (and/or Cl2) species.
4.6. Key Factors for 1,2-DCE Oxidation
Combining the results of the surface properties (XRD and XPS) of the used V0.05Ce catalyst, it was discovered that the lattice oxygen (Oα) species abundance of the used V0.05Ce catalyst was lower than that of the fresh V0.05Ce catalyst and the Ce3+ species abundance of the used V0.05Ce catalyst was remarkably higher than that of the fresh V0.05Ce catalyst. Moreover, the surface oxygen species abundance adsorbed on the oxygen vacancies of the V0.05Ce catalyst increased after the stability test. Therefore, the large number of Ce3+ species could enhance the formation of oxygen vacancies, which could effectively accelerate the mobility of active oxygen species and consequently promote the catalytic activity. According to the results of H2–TPR, it was revealed that both the total H2 consumption amount and the initial H2 consumption rate of catalysts followed the sequence of V0.05Ce > V0.1Ce > V0.025Ce > CeO2> V0.2Ce, suggesting that V0.05Ce had superior low–temperature reducibility, effectively promoting the low–temperature reaction of 1,2–dichloroethane. The relationship between chlorinated byproduct yields and total acidity over VOx/CeO2 catalysts was also analyzed. It was found that CHCl3, CCl4, C2HCl3, and C2H3Cl3 yields versus total acidity maintained linear relationships (R2 = 0.996, 0.991, 0.993, and 0.997, respectively). Compared with V0.025Ce, V0.1Ce, and V0.2Ce catalysts, V0.05Ce possessed higher total acidity and lower chlorinated byproduct (CHCl3, CCl4, C2HCl3, and C2H3Cl3) yields, indicating that the yields of chlorinated byproducts decreased with increasing total acidity. Moreover, the catalytic activity of VOx/CeO2 catalysts (T90) versus total acidity maintained a good linear relationship (R2 = 0.995). The above results indicated that the low chlorinated byproduct yields could be ascribed to higher total acidity during 1,2–DCE oxidation process. The influence of water vapor on the formation of chlorinated byproducts was also studied. It was displayed that the presence of water vapor did not change the type of chlorinated byproducts; however, the yields of CHCl3, CCl4, and C2HCl3 over the V0.05Ce catalyst were apparently lower than those in the absence of water vapor because the reactivity of Cl2 was inhibited by water molecules as a hydrogen source. Moreover, the yield of chlorinated byproducts decreased with increasing water vapor concentration, suggesting that the presence of water vapor could efficiently inhibit the generation of chlorinated byproducts during 1,2–DCE oxidation.
5. Conclusions
In this work, hollow nanosphere–like VOx/CeO2 materials were prepared using a wetness impregnation route and tested for 1,2–DCE destruction. The superior low–temperature reducibility and the mobility of O− and O2− species (chemically adsorbed oxygen species) ensured the superior catalytic activity, catalytic stability, and superior resistance to chlorine poisoning of the V0.05Ce catalyst for 1,2–DCE oxidation. The generation of chlorinated byproducts (e.g., CHCl3, CCl4, C2HCl3, and C2H3Cl3) derived from the cleavage of C–C and C–Cl bonds of 1,2–DCE was inhibited owing to the abundant acid sites and synergy between VOx and CeO2. Water vapor (1–5 vol.%) had a promoting effect on the activity of V0.05Ce, which was ascribed to the efficient removal of Cl species on the surface.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/9/1/119/s1: Figure S1. XRD profiles of CeO2–C catalyst; Figure S2. HAADF–STEM images of CeO2–C catalyst; Figure S3. Nitrogen adsorption/desorption isotherms of CeO2–C catalyst; Figure S4. Pore size distribution of CeO2–C catalyst; Figure S5. Cl 2p XPS spectra of the used catalysts; Figure S6. COx selectivity of prepared catalysts; Table S1. XPS results of used V0.05Ce catalyst.
Author Contributions
Y.H. and S.F. presided over the methodology and writing—original draft; M.T. assisted with the investigation and resources; Y.W. helped with the visualization of graphs; Z.J. was in charge of data validation; C.H. conceptualized the project and was responsible for administration and writing—review and editing. All authors read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
“Not applicable” for studies not involving humans or animals.
Informed Consent Statement
“Not applicable” for studies not involving humans.
Data Availability Statement
All data, models, and code generated or used during the study appear in the submitted article.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21922606, 21876139, 21677114), the Key R&D Program of Shaanxi Province (2019SF-244, 2019ZDLSF05-05-02), and the Shaanxi Natural Science Fundamental Shaanxi Coal Chemical Joint Fund (2019JLM-14) The authors gratefully acknowledge the support of the K.C. Wong Education Foundation and Instrumental Analysis Center of Xi’an Jiaotong University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, P.; Yang, S.S.; Shi, Z.N.; Tao, F.; Guo, X.L.; Zhou, R.X. Accelerating effect of ZrO2 doping on catalytic performance and thermal stability of CeO2–CrOx mixed oxide for 1,2-dichloroethane elimination. Chem. Eng. J. 2016, 285, 544–553. [Google Scholar] [CrossRef]
- Gu, Y.F.; Cai, T.; Gao, X.H.; Xia, H.Q.; Sun, W.; Zhao, J.; Dai, Q.G.; Wang, X.Y. Catalytic combustion of chlorinated aromatics over WOx/CeO2 catalysts at low temperature. Appl. Catal. B Environ. 2019, 248, 264–276. [Google Scholar] [CrossRef]
- Lin, F.W.; Zhang, Z.M.; Li, N.; Yan, B.B.; He, C.; Hao, Z.P.; Chen, G.Y. How to achieve complete elimination of Cl-VOCs: A critical review on byproducts formation and inhibition strategies during catalytic oxidation. Chem. Eng. J. 2021, 404, 126534. [Google Scholar] [CrossRef]
- Lin, F.W.; Xiang, L.; Zhang, Z.M.; Li, N.; Yan, B.B.; He, C.; Hao, Z.P.; Chen, G.Y. Comprehensive review on catalytic degradation of Cl-VOCs under the practical application conditions. Crit. Rev. Environ. Sci. Technol. 2020, 1–45. [Google Scholar] [CrossRef]
- Jiao, Y.M.; Chen, X.; He, F.; Liu, S.T. Simple preparation of uniformly distributed mesoporous Cr/TiO2 microspheres for low-temperature catalytic combustion of chlorobenzene. Chem. Eng. J. 2019, 372, 107–117. [Google Scholar] [CrossRef]
- Ye, N.; Li, Y.; Yang, Z.; Zheng, J.; Zuo, S.F. Rare earth modified kaolin-based Cr2O3 catalysts for catalytic combustion of chlorobenzene. Appl. Catal. A Gen. 2019, 579, 44–51. [Google Scholar] [CrossRef]
- Dai, Q.G.; Bai, S.X.; Wang, Z.Y.; Wang, X.Y.; Lu, G.Z. Catalytic combustion of chlorobenzene over Ru-doped ceria catalysts. Appl. Catal. B Environ. 2012, 126, 64–75. [Google Scholar] [CrossRef]
- Topka, P.; Delaigle, R.; Kaluža, L.; Gaigneaux, E.M. Performance of platinum and gold catalysts supported on ceria–zirconia mixed oxide in the oxidation of chlorobenzene. Catal. Today 2015, 253, 172–177. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Divakar, D.; Aranzabal, A.; González-Velasco, J.R.; González-Marcos, J.A. Catalytic oxidation of trichloroethylene over Fe-ZSM-5: Influence of the preparation method on the iron species and the catalytic behavior. Appl. Catal. B Environ. 2016, 180, 210–218. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Yao, W.Y.; Wu, Z.B. Catalytic Combustion of Dichloromethane over HZSM-5-Supported Typical Transition Metal (Cr, Fe, and Cu) Oxide Catalysts: A Stability Study. J. Phys. Chem. C 2016, 120, 18046–18054. [Google Scholar] [CrossRef]
- Wang, X.Y.; Kang, Q.; Li, D. Catalytic combustion of chlorobenzene over MnOx–CeO2 mixed oxide catalysts. Appl. Catal. B Environ. 2009, 86, 166–175. [Google Scholar]
- Yang, Z.Y.; Yi, H.H.; Tang, X.L.; Zhao, S.Z.; Huang, Y.H.; Xie, X.Z.; Song, L.L.; Zhang, Y.Y. Study of reaction mechanism based on further promotion of low temperature degradation of toluene using nano-CeO2/Co3O4 under microwave radiation for cleaner production in spraying processing. J. Hazard. Mater. 2019, 373, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Gannoun, C.; Turki, A.; Kochkar, H.; Delaigle, R.; Eloy, P.; Ghorbel, A.; Gaigneaux, E.M. Elaboration and characterization of sulfated and unsulfated V2O5/TiO2 nanotubes catalysts for chlorobenzene total oxidation. Appl. Catal. B Environ. 2014, 147, 58–64. [Google Scholar] [CrossRef]
- Finocchio, E.; Ramis, G.; Busca, G. A study on catalytic combustion of chlorobenzenes. Catal. Today 2011, 169, 3–9. [Google Scholar] [CrossRef]
- Dai, Q.G.; Bai, S.X.; Li, H.; Liu, W.; Wang, X.Y.; Lu, G.Z. Catalytic total oxidation of 1,2-dichloroethane over highly dispersed vanadia supported on CeO2 nanobelts. Appl. Catal. B Environ. 2015, 168–169, 141–155. [Google Scholar] [CrossRef]
- Huang, H.; Gu, Y.F.; Zhao, J.; Wang, X.Y. Catalytic combustion of chlorobenzene over VOx/CeO2 catalysts. J. Catal. 2015, 326, 54–68. [Google Scholar] [CrossRef]
- Feng, Z.T.; Ren, Q.M.; Peng, R.S.; Mo, S.P.; Zhang, M.Y.; Fu, M.L.; Chen, L.M.; Ye, D.Q. Effect of CeO2 morphologies on toluene catalytic combustion. Catal. Today 2019, 332, 177–182. [Google Scholar] [CrossRef]
- Jiang, X.L.; Chen, R.Q.; Zhang, J.; Yu, L.; Xu, X. Synthesis of mono-dispersed ceria hollow nanospheres by a hydrothermal method. Micro Nano Lett. 2016, 11, 137–141. [Google Scholar] [CrossRef]
- Le, T.K.; Kang, M.; Kim, S.W. Morphology engineering, room-temperature photoluminescence behavior, and sunlight photocatalytic activity of V2O5nanostructures. Mater. Charact. 2019, 153, 52–59. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Ce3+-ion, Surface Oxygen Vacancy, and Visible Light-induced Photocatalytic Dye Degradation and Photocapacitive Performance of CeO2-Graphene Nanostructures. Sci. Rep. 2017, 7, 5928. [Google Scholar] [CrossRef]
- Le, T.K.; Kang, M.; Tran, V.T.; Kim, S.W. Relation of photoluminescence and sunlight photocatalytic activities of pure V2O5nanohollows and V2O5/RGO nanocomposites. Mat. Sci. Semico. Proc. 2019, 100, 159–166. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.H.; Gao, F.; Kovarik, L.; Peden, C.H.F.; Wang, Y. Effects of CeO2 support facets on VOx/CeO2 catalysts in oxidative dehydrogenation of methanol. J. Catal. 2014, 315, 15–24. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.Z.; Li, J.H. Structure–activity relationship of VOx/CeO2 nanorod for NO removal with ammonia. Appl. Catal. B Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Feng, X.B.; Tian, M.J.; He, C.; Li, L.; Shi, J.-W.; Yu, Y.K.; Cheng, J. Yolk-shell-like mesoporous CoCrOx with superior activity and chlorine resistance in dichloromethane destruction. Appl. Catal. B Environ. 2020, 264, 118493. [Google Scholar] [CrossRef]
- Kang, D.J.; Yu, X.L.; Ge, M.F. Morphology-dependent properties and adsorption performance of CeO2 for fluoride removal. Chem. Eng. J. 2017, 330, 36–43. [Google Scholar] [CrossRef]
- Jing, X.Y.; Zhang, Y.F.; Jiang, H.M.; Cheng, Y.; Xing, N.; Meng, C.G. Facile template-free fabrication of hierarchical V2O5 hollow spheres with excellent charge storage performance for symmetric and hybrid supercapacitor devices. J. Alloys Compd. 2018, 763, 180–191. [Google Scholar] [CrossRef]
- Ji, F.R.; Li, C.X.; Wang, J.Q.; Wang, J.; Shen, M.Q. New insights into the role of vanadia species as active sites for selective catalytic reduction of NO with ammonia over VOx/CeO2 catalysts. J. Rare Earths 2020, 38, 719–724. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, H.Z.; Li, K.Z.; Peng, Y.; Li, X.; Li, J.H. Different exposed facets VOx/CeO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2018, 349, 184–191. [Google Scholar] [CrossRef]
- Zeleke, M.A.; Kuo, D.-H. Synthesis and application of V2O5-CeO2 nanocomposite catalyst for enhanced degradation of methylene blue under visible light illumination. Chemosphere 2019, 235, 935–944. [Google Scholar] [CrossRef]
- Tian, M.J.; Guo, X.; Dong, R.; Guo, Z.; Shi, J.W.; Yu, Y.K.; Cheng, M.X.; Albilali, R.; He, C. Insight into the boosted catalytic performance and chlorine resistance of nanosphere-like meso-macroporous CrOx/MnCo3Ox for 1,2-dichloroethane destruction. Appl. Catal. B Environ. 2019, 259, 118018. [Google Scholar] [CrossRef]
- Xiao, P.; Zhu, J.J.; Li, H.L.; Jiang, W.; Wang, T.; Zhu, Y.J.; Zhao, Y.X.; Li, J.L. Effect of Textural Structure on the Catalytic Performance of LaCoO3 for CO Oxidation. ChemCatChem 2014, 6, 1774–1781. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.Y.; Chen, L.Q.; Yuan, F.L.; Zhu, Y.J. Soot Combustion over Nanostructured Ceria with Different Morphologies. Sci. Rep. 2016, 6, 29062. [Google Scholar] [CrossRef]
- Feng, X.B.; Chen, C.W.; He, C.; Chai, S.N.; Yu, Y.K.; Cheng, J. Non-thermal plasma coupled with MOF-74 derived Mn-Co-Ni-O porous composite oxide for toluene efficient degradation. J. Hazard. Mater. 2020, 383, 121143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.H.; Li, Y.L.; Zhang, L.Y.; Shen, L.J.; Xiao, Y.H.; Zhang, Y.F.; Au, C.; Jiang, L.L. Insight into the effect of morphology on catalytic performance of porous CeO2 nanocrystals for H2S selective oxidation. Appl. Catal. B Environ. 2019, 252, 98–110. [Google Scholar] [CrossRef]
- Dong, F.; Meng, Y.; Han, W.L.; Zhao, H.J.; Tang, Z.C. Morphology effects on surface chemical properties and lattice defects of Cu/CeO2 catalysts applied for low-temperature CO oxidation. Sci. Rep. 2019, 9, 12056. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.J.; Jian, Y.F.; Ma, M.D.; He, C.; Chen, C.W.; Liu, C.; Shi, J.-W. Rational design of CrOx/LaSrMnCoO6 composite catalysts with superior chlorine tolerance and stability for 1,2-dichloroethane deep destruction. Appl. Catal. A Gen. 2019, 570, 62–72. [Google Scholar] [CrossRef]
- Liang, H.; Hong, Y.X.; Zhu, C.Q.; Li, S.H.; Chen, Y.; Liu, Z.L.; Ye, D.Q. Influence of partial Mn-substitution on surface oxygen species of LaCoO3 catalysts. Catal. Today 2013, 201, 98–102. [Google Scholar] [CrossRef]
- Lin, X.T.; Li, S.J.; He, H.; Wu, Z.; Wu, J.L.; Chen, L.M.; Ye, D.Q.; Fu, M.L. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B Environ. 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Zhang, X.-m.; Deng, Y.-Q.; Tian, P.F.; Shang, H.-h.; Xu, J.; Han, Y.-F. Dynamic active sites over binary oxide catalysts: In situ/operando spectroscopic study of low-temperature CO oxidation over MnOx-CeO2 catalysts. Appl. Catal. B Environ. 2016, 191, 179–191. [Google Scholar] [CrossRef]
- Casapu, M.; Kroecher, O.; Mehring, M.; Nachtegaal, M.; Borca, C.; Harfouche, M.; Grolimund, D. Characterization of Nb-Containing MnOx-CeO2 Catalyst for Low-Temperature Selective Catalytic Reduction of NO with NH3. J. Phys. Chem. C 2010, 114, 9791–9801. [Google Scholar] [CrossRef]
- Su, J.; Yao, W.Y.; Liu, Y.; Wu, Z.B. The impact of CrOx loading on reaction behaviors of dichloromethane (DCM) catalytic combustion over Cr-O/HZSM-5 catalysts. Appl. Surf. Sci. 2017, 396, 1026–1033. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.Y.; Dai, Q.G.; Li, D. Effect of Ce and La on the structure and activity of MnOx catalyst in catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2012, 111–112, 141–149. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Dai, Q.G.; Wang, X.Y. Fe doped CeO2 nanosheets for catalytic oxidation of 1,2-dichloroethane: Effect of preparation method. Chem. Eng. J. 2017, 307, 1037–1046. [Google Scholar] [CrossRef]
- Gannoun, C.; Delaigle, R.; Debecker, D.P.; Eloy, P.; Ghorbel, A.; Gaigneaux, E.M. Effect of support on V2O5 catalytic activity in chlorobenzene oxidation. Appl. Catal. A Gen. 2012, 447–448, 1–6. [Google Scholar] [CrossRef]
- Mei, J.; Ke, Y.; Yu, Z.J.; Hu, X.F.; Qu, Z.; Yan, N.Q. Morphology-dependent properties of Co3O4/CeO2 catalysts for low temperature dibromomethane (CH2Br2) oxidation. Chem. Eng. J. 2017, 320, 124–134. [Google Scholar] [CrossRef]
- Yang, P.; Meng, Z.H.; Yang, S.S.; Shi, Z.N.; Zhou, R.X. Highly active behaviors of CeO2–CrOx mixed oxide catalysts in deep oxidation of 1,2-dichloroethane. J. Mol. Catal. A-Chem. 2014, 393, 75–83. [Google Scholar] [CrossRef]
- Cai, T.; Huang, H.; Deng, W.; Dai, Q.G.; Liu, W.; Wang, X.Y. Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl. Catal. B Environ. 2015, 166–167, 393–405. [Google Scholar] [CrossRef]
- Tian, M.J.; He, C.; Yu, Y.K.; Pan, H.; Smith, L.; Jiang, Z.Y.; Gao, N.B.; Jian, Y.F.; Hao, Z.P.; Zhu, Q. Catalytic oxidation of 1,2-dichloroethane over three-dimensional ordered meso-macroporous Co3O4/La0.7Sr0.3Fe0.5Co0.5O3: Destruction route and mechanism. Appl. Catal. A Gen. 2018, 553, 1–14. [Google Scholar] [CrossRef]
- Dai, Q.G.; Yin, L.-L.; Bai, S.X.; Wang, W.; Wang, X.Y.; Gong, X.-Q.; Lu, G.Z. Catalytic total oxidation of 1,2-dichloroethane over VOx/CeO2 catalysts: Further insights via isotopic tracer techniques. Appl. Catal. B Environ. 2016, 182, 598–610. [Google Scholar] [CrossRef]
- Dai, Q.G.; Wu, J.Y.; Deng, W.; Hu, J.S.; Wu, Q.Q.; Guo, L.M.; Sun, W.; Zhan, W.C.; Wang, X.Y. Comparative studies of P/CeO2 and Ru/CeO2 catalysts for catalytic combustion of dichloromethane: From effects of H2O to distribution of chlorinated by-products. Appl. Catal. B Environ. 2019, 249, 9–18. [Google Scholar] [CrossRef]
- Deng, W.; Dai, Q.G.; Lao, Y.J.; Shi, B.B.; Wang, X.Y. Low temperature catalytic combustion of 1,2-dichlorobenzene over CeO2–TiO2 mixed oxide catalysts. Appl. Catal. B Environ. 2016, 181, 848–861. [Google Scholar] [CrossRef]
- Hetrick, C.E.; Patcas, F.; Amiridis, M.D. Effect of water on the oxidation of dichlorobenzene over V2O5/TiO2 catalysts. Appl. Catal. B Environ. 2011, 101, 622–628. [Google Scholar] [CrossRef]
- Li, N.; Cheng, J.; Xing, X.; Sun, Y.G.; Hao, Z.P. Distribution and formation mechanisms of polychlorinated organic by-products upon the catalytic oxidation of 1,2-dichlorobenzene with palladium-loaded catalysts. J. Hazard. Mater. 2020, 393, 122412. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).