Freeze-Drying with Structured Sublimation Fronts—Visualization with Neutron Imaging
Abstract
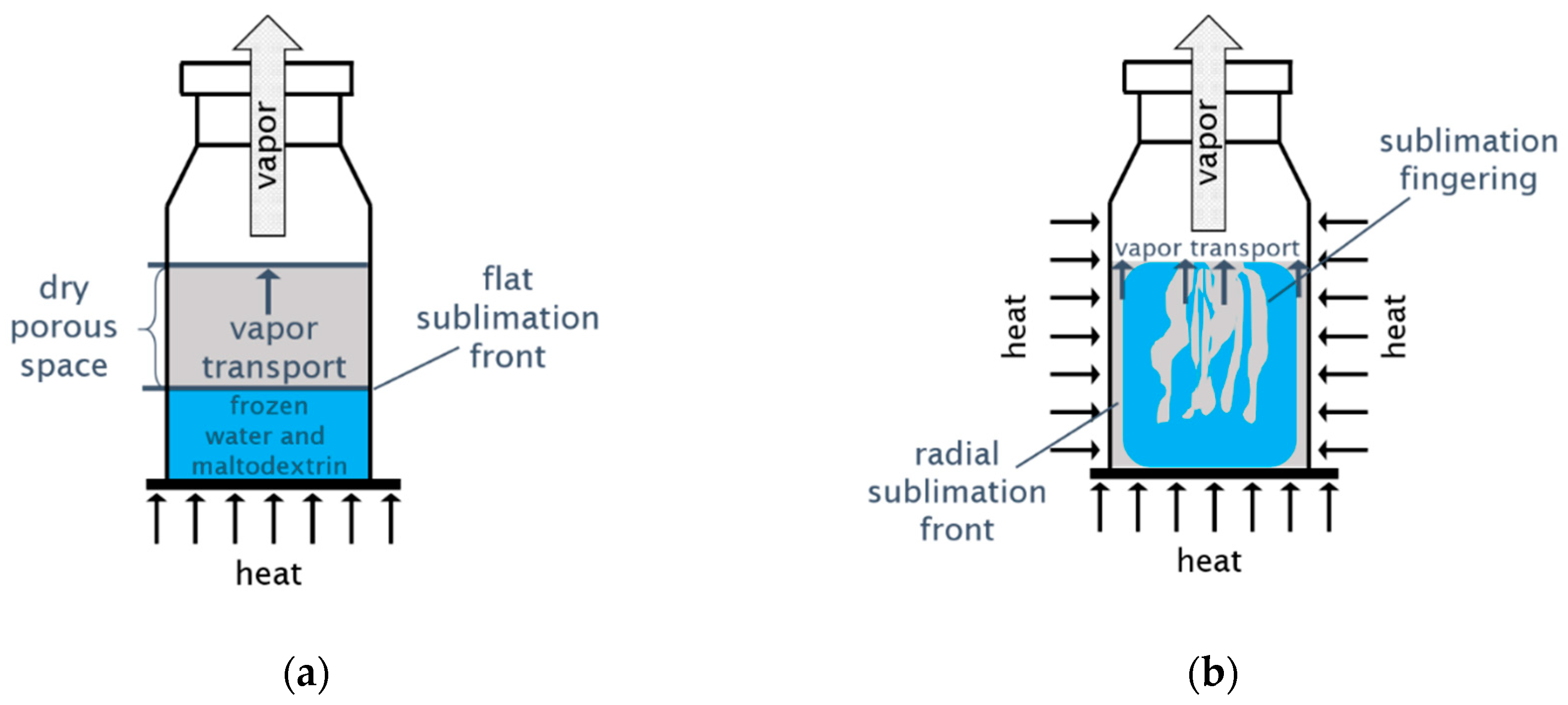
1. Introduction
2. Materials and Methods
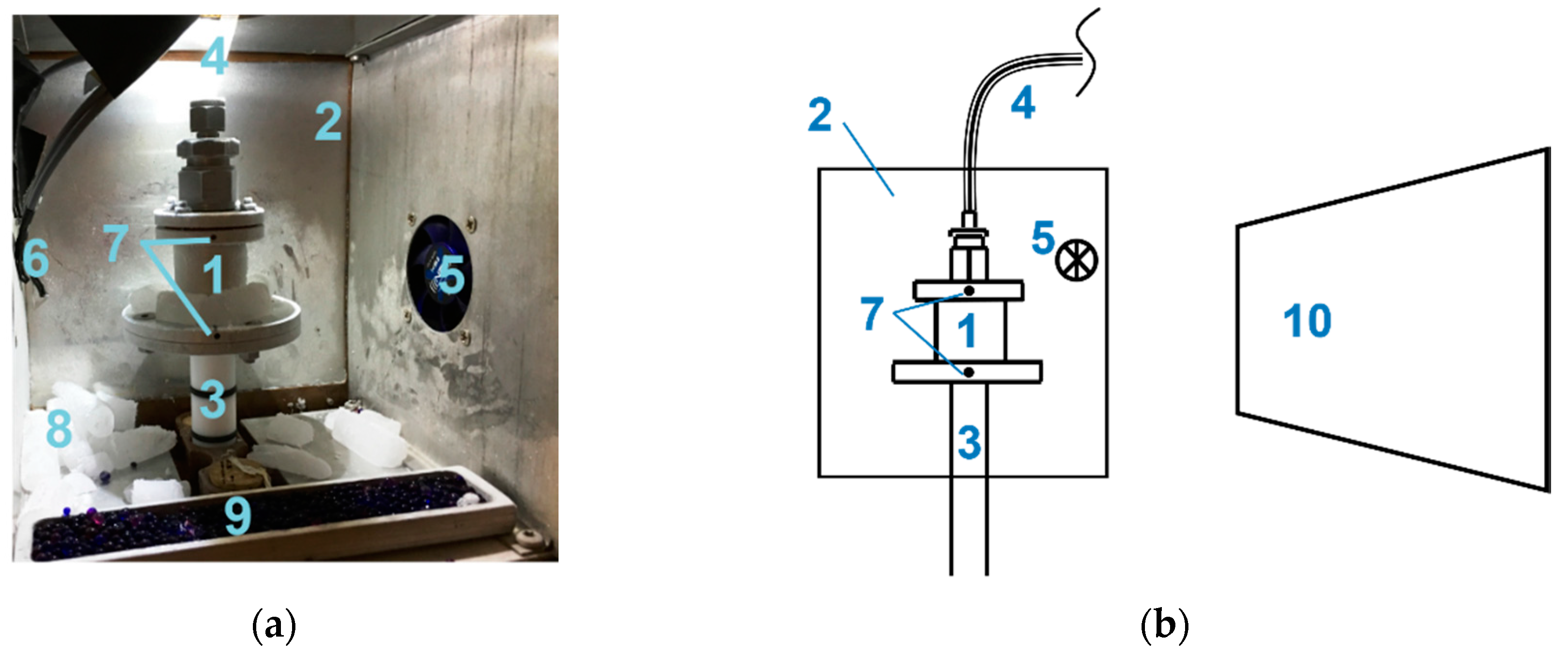
2.1. Experimental Equipment and Parameters
2.2. Sample Preparation
2.3. Neutron Facility
2.4. Tomography
3. Results and Discussion
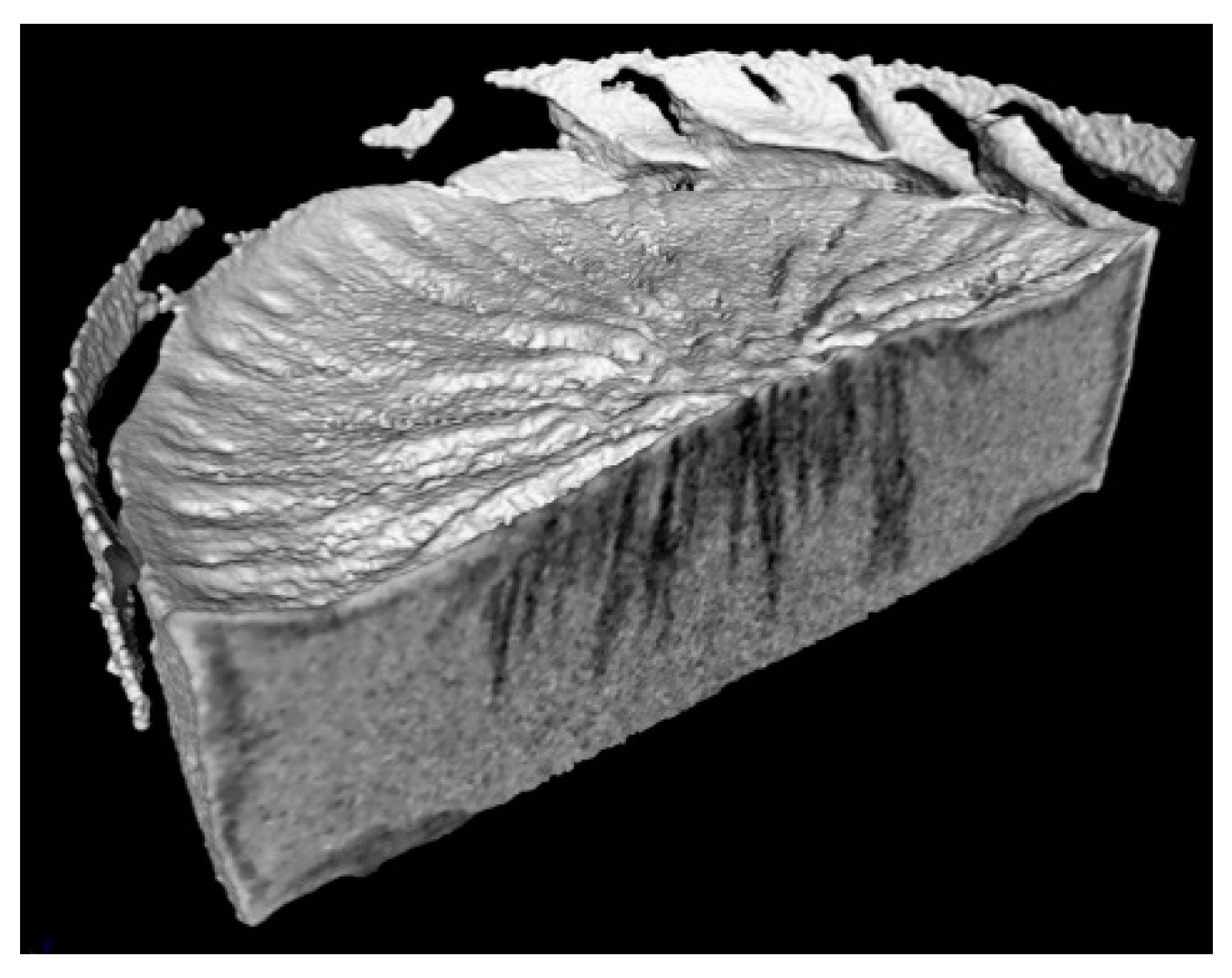
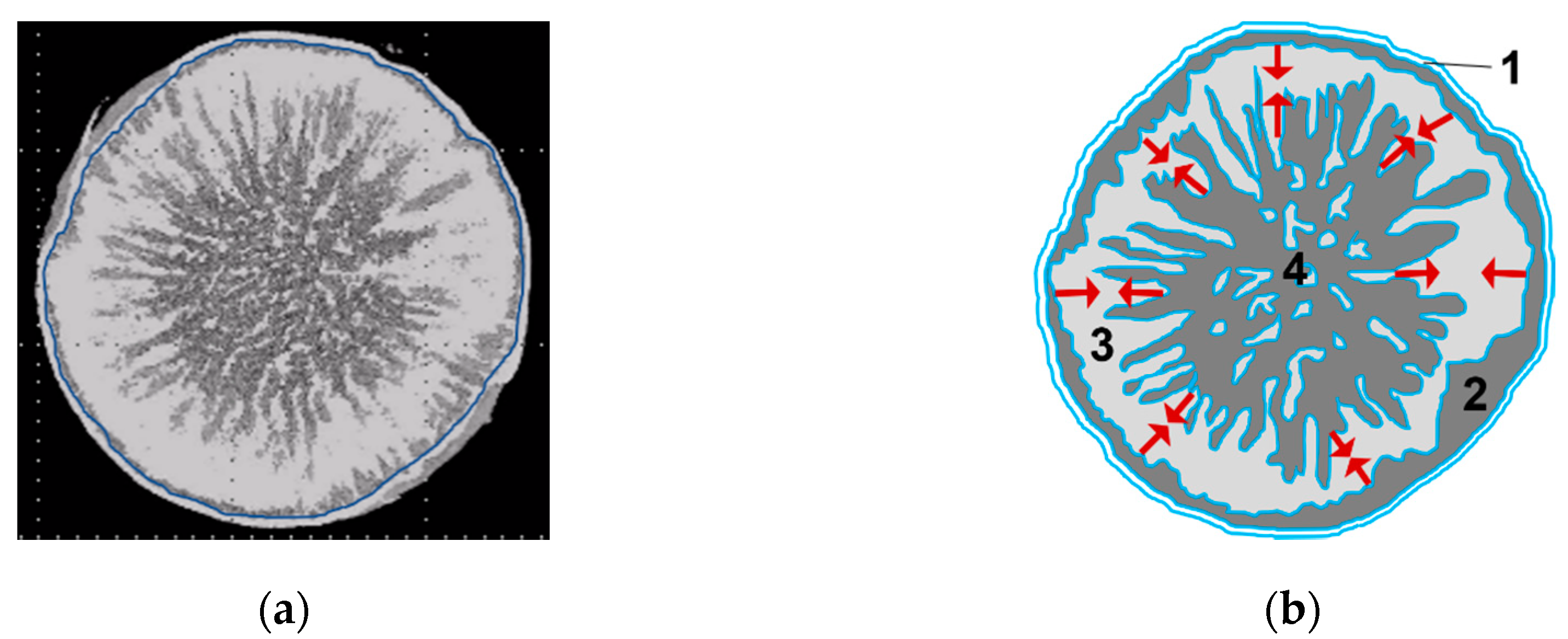
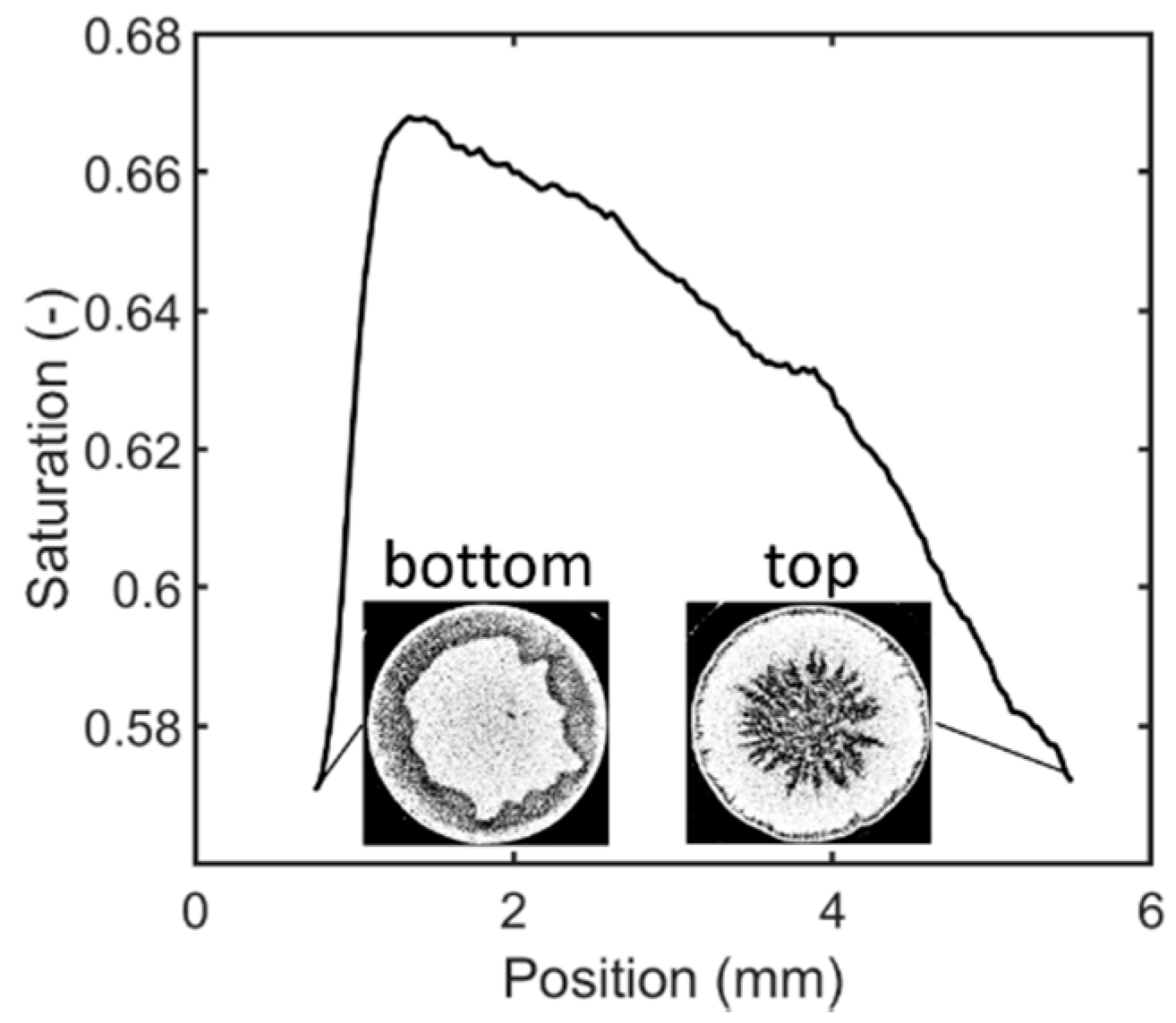
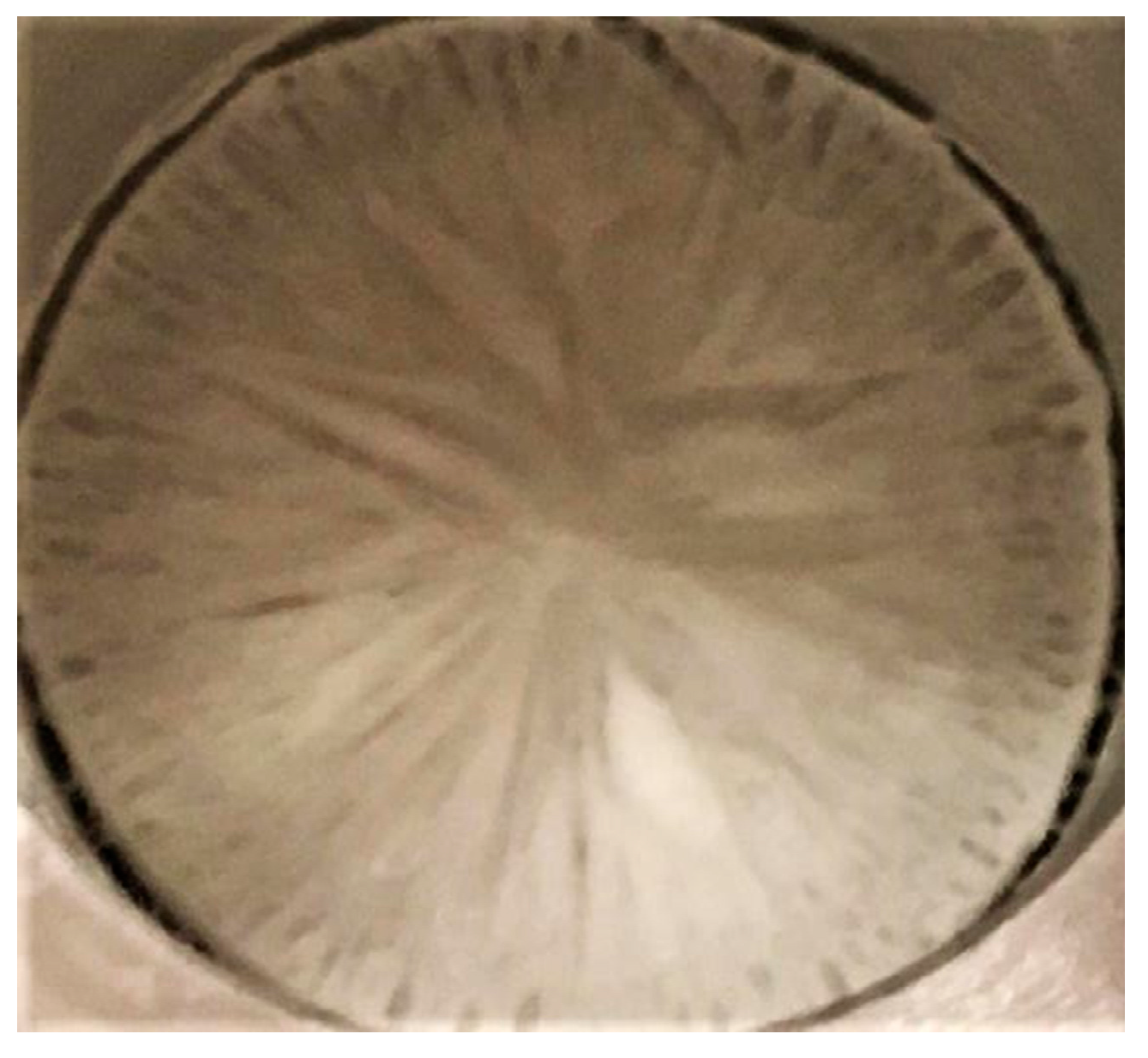
3.1. Structure of the Sublimation Front
3.2. Discussion
4. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haseley, P.; Oetjen, G.-W. Freeze-Drying, 3rd ed.; John Wiley & Sons Incorporated: Newark, NJ, USA, 2017; ISBN 978-3-527-34306-5. [Google Scholar]
- Schuchmann, H.P.; Schuchmann, H. Lebensmittelverfahrenstechnik; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 3527312307. [Google Scholar]
- Pikal, M.J. Freeze Drying. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 1. [Google Scholar]
- Gehrmann, D.; Esper, G.J.; Schuchmann, H. Trocknungstechnik in der Lebensmittelindustrie; Behr: Hamburg, Germany, 2009; ISBN 3899475178. [Google Scholar]
- Hua, T.-C.; Liu, B.-L.; Zhang, H. Freeze-Drying of Pharmaceutical and Food Products; Elsevier Science: Burlington, VT, USA, 2010; ISBN 9781845697464. [Google Scholar]
- Kharaghani, A.; Tsotsas, E.; Wolf, C.; Beutler, T.; Guttzeit, M.; Oetjen, G.-W. Freeze-Drying. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 1–47. ISBN 9783527306732. [Google Scholar]
- Meister, E.; Gieseler, H. Freeze-dry microscopy of protein/sugar mixtures: Drying behavior, interpretation of collapse temperatures and a comparison to corresponding glass transition data. J. Pharm. Sci. 2009, 98, 3072–3087. [Google Scholar] [CrossRef] [PubMed]
- Meister, E.; Sasić, S.; Gieseler, H. Freeze-dry microscopy: Impact of nucleation temperature and excipient concentration on collapse temperature data. AAPS PharmSciTech 2009, 10, 582–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohori, R.; Yamashita, C. Effects of temperature ramp rate during the primary drying process on the properties of amorphous-based lyophilized cake, Part 1: Cake characterization, collapse temperature and drying behavior. J. Drug Deliv. Sci. Technol. 2017, 39, 131–139. [Google Scholar] [CrossRef]
- Nail, S.L.; Gatlin, L.A. Freeze-Drying: Principles and Practice. In Parenteral Medications; Nema, S., Ludwig, J.D., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 907–927. ISBN 9780429201400. [Google Scholar]
- Schneid, S.C. Investigation of Novel Process Analytical Technology (PAT) Tools for Use in Freeze-Drying Processes; Friedrich-Alexander-University: Erlangen-Nürnberg, Germany, 2009. [Google Scholar]
- Andrieu, J.; Vessot, S. Characterization and Control of Physical Quality Factors during Freeze-Drying of Pharmaceuticals in Vials. In Modern Drying Technology; Tsotsas, E., Mujumdar, A.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 51–90. ISBN 9783527631667. [Google Scholar]
- Nail, S.; Tchessalov, S.; Shalaev, E.; Ganguly, A.; Renzi, E.; Dimarco, F.; Wegiel, L.; Ferris, S.; Kessler, W.; Pikal, M.; et al. Recommended Best Practices for Process Monitoring Instrumentation in Pharmaceutical Freeze Drying-2017. AAPS PharmSciTech 2017, 18, 2379–2393. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Pikal, M.J. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Rasetto, V.; Marchisio, D.L.; Fissore, D.; Barresi, A.A. On the use of a dual-scale model to improve understanding of a pharmaceutical freeze-drying process. J. Pharm. Sci. 2010, 99, 4337–4350. [Google Scholar] [CrossRef]
- Zhai, S.; Taylor, R.; Sanches, R.; Slater, N.K.H. Measurement of lyophilisation primary drying rates by freeze-drying microscopy. Chem. Eng. Sci. 2003, 58, 2313–2323. [Google Scholar] [CrossRef]
- Li, X.; Nail, S.L. Nuclear magnetic resonance imaging of freeze-drying. J. Pharm. Sci. 2006, 95, 2516–2525. [Google Scholar] [CrossRef]
- Voda, A.; Homan, N.; Witek, M.; Duijster, A.; van Dalen, G.; van der Sman, R.; Nijsse, J.; van Vliet, L.; van As, H.; van Duynhoven, J. The impact of freeze-drying on microstructure and rehydration properties of carrot. Food Res. Int. 2012, 49, 687–693. [Google Scholar] [CrossRef]
- Raman, P.; Rielly, C.D.; Stapley, A.G.F. Freeze-Drying Microscopy as a Tool to Study the Sublimation Kinetics of a Freeze-Drying Process. In Proceedings of the 19th International Drying Symposium, Lyon, France, 24–27 August 2014. [Google Scholar]
- Siebert, T.; Zuber, M.; Hamann, E.; Baumbach, T.; Karbstein, H.P.; Gaukel, V. Micro-CT visualization of structure development during freeze-drying processes. Drying Technol. 2020, 38, 376–384. [Google Scholar] [CrossRef]
- Wang, W.; Hu, D.; Pan, Y.; Zhao, Y.; Chen, G. Freeze-drying of aqueous solution frozen with prebuilt pores. AIChE J. 2015, 61, 2048–2057. [Google Scholar] [CrossRef]
- Foerst, P.; Melo de Carvalho, T.; Lechner, M.; Kovacevic, T.; Kim, S.; Kirse, C.; Briesen, H. Estimation of mass transfer rate and primary drying times during freeze-drying of frozen maltodextrin solutions based on X-ray μ-computed tomography measurements of pore size distributions. J. Food Eng. 2019, 260, 50–57. [Google Scholar] [CrossRef]
- Handbook of Industrial Drying, 4th ed.; Mujumdar, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466596658. [Google Scholar]
- Nakagawa, K.; Tamiya, S.; Sakamoto, S.; Do, G.; Kono, S. Observation of Microstructure Formation During Freeze-Drying of Dextrin Solution by in-situ X-ray Computed Tomography. Front. Chem. 2018, 6, 418. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J. Quantitative Time Resolved Neutron Imaging Methods at the High Flux Neutron Source FRM-II; TU Munich: Munich, Germany, 2006. [Google Scholar]
- Defraeye, T.; Nicolaï, B.; Mannes, D.; Aregawi, W.; Verboven, P.; Derome, D. Probing inside fruit slices during convective drying by quantitative neutron imaging. J. Food Eng. 2016, 178, 198–202. [Google Scholar] [CrossRef]
- Mannes, D.; Schmid, F.; Wehmann, T.; Lehmann, E. Design and Applications of a Climatic Chamber for in-situ Neutron Imaging Experiments. Phys. Procedia 2017, 88, 200–207. [Google Scholar] [CrossRef]
- Defraeye, T.; Aregawi, W.; Saneinejad, S.; Vontobel, P.; Lehmann, E.; Carmeliet, J.; Verboven, P.; Derome, D.; Nicolaï, B. Novel Application of Neutron Radiography to Forced Convective Drying of Fruit Tissue. Food Bioprocess Technol 2013, 6, 3353–3367. [Google Scholar] [CrossRef]
- Goff, J.A.; Gratch, S. Low-pressure properties of water from −160° to 212 °F. Trans. Amer. Soc. Heat Vent. Eng. 1946, 52, 95–121. [Google Scholar]
- Kaestner, A.P.; Hartmann, S.; Kühne, G.; Frei, G.; Grünzweig, C.; Josic, L.; Schmid, F.; Lehmann, E.H. The ICON beamline—A facility for cold neutron imaging at SINQ. Nuclear Instrum. Methods Phys. Res. Section A Accel. Spectrometers Detect. Assoc. Equip. 2011, 659, 387–393. [Google Scholar] [CrossRef]
- Siegwart, M.; Harti, R.P.; Manzi-Orezzoli, V.; Valsecchi, J.; Strobl, M.; Grünzweig, C.; Schmidt, T.J.; Boillat, P. Selective Visualization of Water in Fuel Cell Gas Diffusion Layers with Neutron Dark-Field Imaging. J. Electrochem. Soc. 2019, 166, F149–F157. [Google Scholar] [CrossRef]
- Blau, B.; Clausen, K.N.; Gvasaliya, S.; Janoschek, M.; Janssen, S.; Keller, L.; Roessli, B.; Schefer, J.; Tregenna-Piggott, P.; Wagner, W.; et al. The Swiss Spallation Neutron Source SINQ at Paul Scherrer Institut. Neutron News 2009. [Google Scholar] [CrossRef]
- Carminati, C.; Strobl, M.; Kaestner, A. KipTool, a general purpose processing tool for neutron imaging data. SoftwareX 2019, 10, 100279. [Google Scholar] [CrossRef]
- Toei, R.; Okazaki, M.; Asaeda, M. The stability of plane sublimation and a model of zone sublimation in freeze-drying of porous bodies. J. Chem. Eng. Japan JCEJ 1975, 8, 282–288. [Google Scholar] [CrossRef]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Hottot, A.; Vessot, S.; Andrieu, J. A Direct Characterization Method of the Ice Morphology. Relationship Between Mean Crystals Size and Primary Drying Times of Freeze-Drying Processes. Drying Technol. 2004, 22, 2009–2021. [Google Scholar] [CrossRef]
- Kasper, J.C.; Friess, W. The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 78, 248–263. [Google Scholar] [CrossRef]
- Pisano, R.; Arsiccio, A.; Nakagawa, K.; Barresi, A.A. Tuning, measurement and prediction of the impact of freezing on product morphology: A step toward improved design of freeze-drying cycles. Drying Technol. 2019, 579–599. [Google Scholar] [CrossRef]
- Hottot, A.; Andrieu, J.; Vessot, S.; Shalaev, E.; Gatlin, L.A.; Ricketts, S. Experimental Study and Modeling of Freeze-Drying in Syringe Configuration. Part I: Freezing Step. Drying Technol. 2009, 27, 40–48. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Charoenrein, S.; Roos, Y.H. Microstructure formation of maltodextrin and sugar matrices in freeze-dried systems. Carbohydr. Polym. 2012, 88, 734–742. [Google Scholar] [CrossRef]
- Gruber, S.; Vorhauer, N.; Schulz, M.; Hilmer, M.; Peters, J.; Tsotsas, E.; Foerst, P. Estimation of the local sublimation front velocities from neutron radiography and tomography of particulate matter. Chem. Eng. Sci. 2020, 211, 115268. [Google Scholar] [CrossRef]
- Foerst, P.; Gruber, S.; Schulz, M.; Vorhauer, N.; Tsotsas, E. Characterization of Lyophilization of Frozen Bulky Solids. Chem. Eng. Technol. 2020, 43, 789–796. [Google Scholar] [CrossRef]
- Vorhauer, N.; Först, P.; Schuchmann, H.; Tsotsas, E. Pore Network Model of Primary Freeze-Drying. In Proceedings of the International Drying Symposium, Valencia, Spain, 11–14 September 2018. [Google Scholar]

| Solid Content w/w | Total Sample Weight (g) | Freezing Temperature (°C) |
|---|---|---|
| 20 | 4.41 | −78 °C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorhauer-Huget, N.; Mannes, D.; Hilmer, M.; Gruber, S.; Strobl, M.; Tsotsas, E.; Foerst, P. Freeze-Drying with Structured Sublimation Fronts—Visualization with Neutron Imaging. Processes 2020, 8, 1091. https://doi.org/10.3390/pr8091091
Vorhauer-Huget N, Mannes D, Hilmer M, Gruber S, Strobl M, Tsotsas E, Foerst P. Freeze-Drying with Structured Sublimation Fronts—Visualization with Neutron Imaging. Processes. 2020; 8(9):1091. https://doi.org/10.3390/pr8091091
Chicago/Turabian StyleVorhauer-Huget, Nicole, David Mannes, Mathias Hilmer, Sebastian Gruber, Markus Strobl, Evangelos Tsotsas, and Petra Foerst. 2020. "Freeze-Drying with Structured Sublimation Fronts—Visualization with Neutron Imaging" Processes 8, no. 9: 1091. https://doi.org/10.3390/pr8091091
APA StyleVorhauer-Huget, N., Mannes, D., Hilmer, M., Gruber, S., Strobl, M., Tsotsas, E., & Foerst, P. (2020). Freeze-Drying with Structured Sublimation Fronts—Visualization with Neutron Imaging. Processes, 8(9), 1091. https://doi.org/10.3390/pr8091091








