1. Introduction
Camellia sinensis is a perennial green tree that naturally grows in Asia. Nowadays, it is widely cultivated throughout the world, mainly in tropical and subtropical regions. The leaves of
Camellia sinensis have been used and named as green tea, a popular beverage in the world. In addition, green tea has been found to exhibit various medicinal activities such as anti-cancer, anti-cardiovascular diseases, anti-diabetes, and anti-aging [
1]. Polyphenols, especially catechins, are the most crucial components that contribute to the beneficial effects of green tea [
2]. Catechins (flavan-3-ols) are natural flavonols originally derived from catechu, which is the liquid extract of
Acacia catechu [
3]. Epigallocatechin, epicatechin gallate, epicatechin, and epigallocatechin gallate (EGCG) are four main catechins found in green tea [
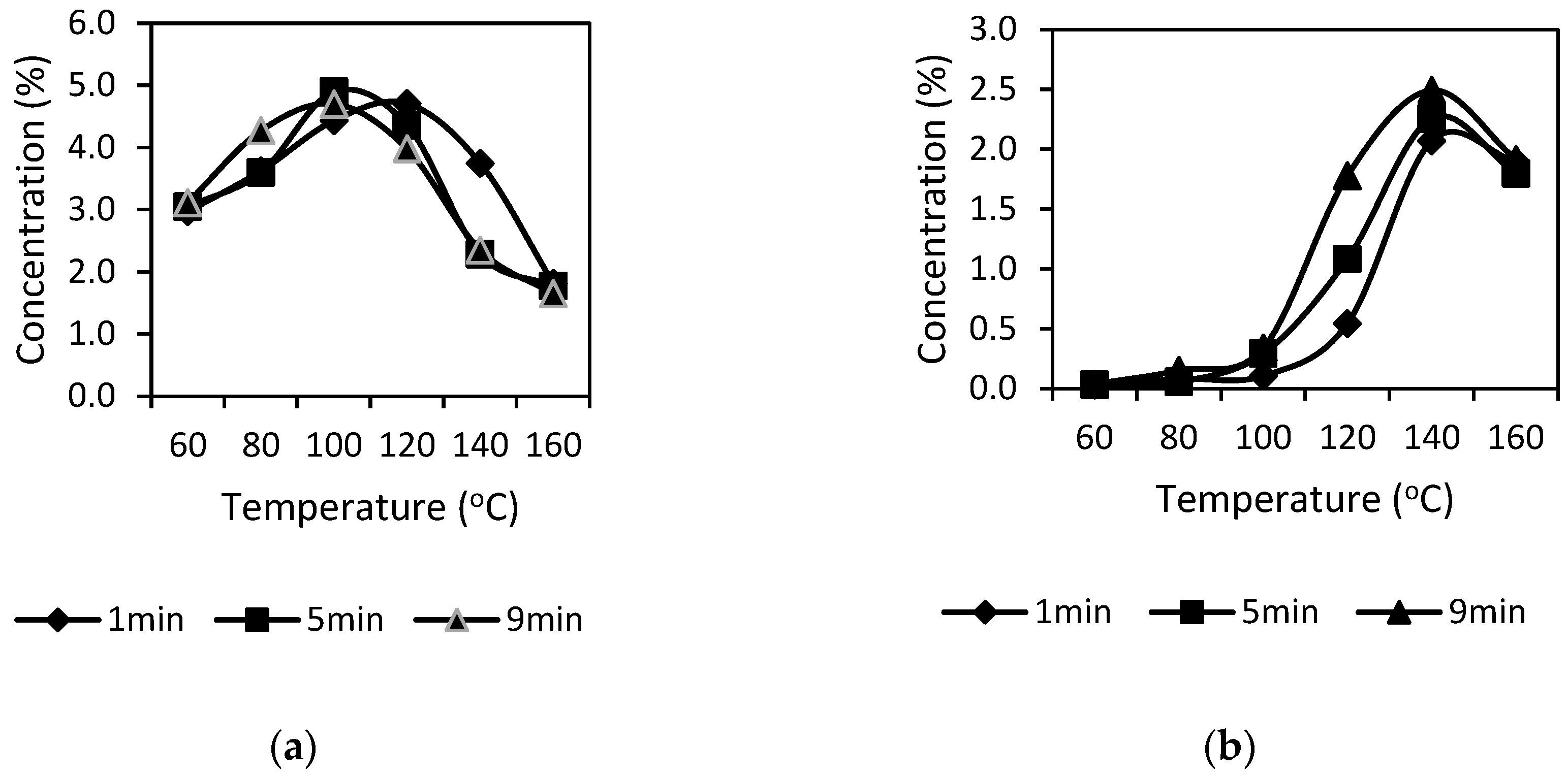
4]. Among them, EGCG generally presents with the highest content, which can make up to 15% of the total catechins content. During extraction and storage, EGCG can be degraded, and this degradation was shown to depend on many factors including temperature and duration [
4,
5]. According to Friedman, the average content of EGCG decreased by 28% after six months of storage [
5]. Previous studies have indicated that EGCG undergoes oxidation at temperatures below 44 °C, while epimerization ordinarily exists at higher temperatures [
1].
Extraction is the first step to recover the desired natural products from the plant materials. In general, heating [
6] and Soxhlet extraction [
7] are the most popular traditional techniques for extraction of green tea, which show several disadvantages like time and solvents consumption, tedium, low selectivity, and/or low efficiency [
8]. Recently, more modern extraction techniques are being developed for the extraction of bioactive components from
Camellia sinensis. Sokmen applied sequential supercritical fluid extraction to isolated catechins from green tea; the highest yield of catechin was 2.9% under the condition of pressure 25 MPa, temperature 60 °C, extraction time 3 h, and ethanol modifier flow rate at 0.5 mL/min [
9]. Vahid Ghasemzadeh-Mohammadi proposed microwave-assisted extraction and ultrasonic extraction for the optimal isolation of tea catechins. The microwave-assisted extraction was more efficient in which the best yield of catechin was 6.25% under the conditions of ultrasonic time 7.8 min, microwave power 180 W, and extraction temperature 65 °C [
10]. Pressurized liquid extraction was successfully deployed for the extraction of catechin. Four different species of green tea had been experimented with and the optimum condition was found for the extraction of catechin, which had pressure values around 150 bars with a constant temperature of around 75 °C for 60 min [
11].
While not a very new extraction technology, SWE offers numerous advantages. It was found to require less solvent volume as well as the time and cost of samples per experiment [
12]. Furthermore, the water, which is used as the solvent, has several benefits like a minor impact on the environment and worker’s health as well as easy carrying and storage. In the experiments, the use of heat can drastically change the physical and chemical properties of water. When the temperature is increased, not only do the viscosity and surface tension of water decrease, but the dielectric constant also becomes lower [
13]. In particular, the dielectric constant of water drops from 53 to 36.5 when the temperature has risen from 110 °C to 190 °C. This parameter, indicating the polarity of water, has shown to be comparable to organic solvents such as ethanol at ambient temperature [
14]. Recently, subcritical water has shown excellent potential as a substitute solvent for the extraction of natural material. They are vanillin and coumarin from vanilla beans and tonka beans [
15], caffeine from black tea leaves [
16], and asiatic acid and asiaticoside from
Centella asiatica [
17].
The regular one-factor-at-a-time method in which one factor adjusts at a time while all others are retained steady has several shortcomings, including its more laborious and time-consuming protocol. Response surface methodology (RSM), a statistical experimental design, can be the alternative method to maximize the yield of extraction. RSM consists of mathematical and statistical techniques used to explore a suitable functional interaction between a response of interest and some process variable [
18]. When applied with the subcritical water extraction method, optimal extraction procedures can be created to serve the purpose of research as well as for industrial applications [
19,
20,
21].
At present, there were few investigations on the optimization of extraction of EGCG from green tea using SWE technology combined with RSM. Therefore, we reported the potential of the SWE for the extraction of EGCG from green tea. High-Performance Liquid Chromatography (HPLC) was employed to analyze the EGCG levels. The stability of EGCG is investigated as a function that depends on temperature and extraction time. Based on the response surface methodology (RSM) experimental design, this study determines the optimum conditions for the EGCG extraction process. Moreover, the extraction process was also modeled and evaluated at a larger scale in order to apply to industrial production.
2. Materials and Methods
2.1. Plant Materials
Green tea (Camellia sinensis) leaves were obtained as a dry sample from Thai Nguyen province, Vietnam. They were grounded and sieved to a particle size of less than 2.5 mm. The sample was stored in the dry environment at ambient temperature. Moisture content (7.75%) was determined before extraction.
2.2. Subcritical Water Extraction (SWE) Procedure
The SWE was performed using an Accelerated Solvent Extractor (ASE) 350 system from Dionex Corporation (Sunnyvale, CA, USA). Two grams of sample were mixed with 4 g of diatomaceous earth and placed in a 100 mL stainless extraction cell. A stainless-steel frit and a cellulose filter (Dionex) were placed at the bottom of the extraction cell to prevent the contamination of the powder from infiltrating into the collection bottle. The extraction cells were arranged in a cell tray and the samples were extracted under certain conditions. After extraction, the obtained extracts were transferred into collection bottles and stored at 4 °C in the refrigerator for further analyses.
2.3. Conventional Water Extraction
Green tea leaves extracts were heated (2 g of grounded green tea leaves, mixed with 40 mL of water (ratio 20 mL:1 g)) at 60 °C for 2 h. The extraction mixture was continuously stirred with a magnetic stirrer. After that, the extraction mixture was cooled and filtered through a filter. The extraction solution was centrifuged at a speed of 4000 rpm for 10 min, and the supernatant was accumulated; the solvent was vaporized under vacuum and stored at 4 °C in the refrigerator for following evaluation [
22].
2.4. HPLC Analysis
Here, an HPLC method was applied for catechins (EGCG and GCG) analysis. The HPLC was performed using the Shimadzu SPD-20A system (Shimadzu Co., Ltd., Kyoto, Japan) with C18 column (250 mm × 4.6 mm, 5 µm). A mixture of methanol-0.1% phosphoric acid solution was used as the mobile phase. The elution mode was a binary, high-pressure gradient system, and the elution gradients were: 0–38 min, 25% methanol; 38–40 min, 100% methanol. Other running conditions included the detection wavelength (272 nm), the flow rate (1 mL/min), the injection volume (10 µL), and the column temperature (25 °C).
2.5. Degradation Assays
The stability of catechins (EGCG and GCG) during extraction by SWE system was investigated. Independent experiments applied a series of extraction times (1, 3, 5, 7, and 9 min) and temperatures (60 °C, 80 °C, 100 °C, 120 °C, 140 °C, and 160 °C) with purified water were tested. Therefore, one experimental configuration resulted in 30 conditions for the extraction of EGCG.
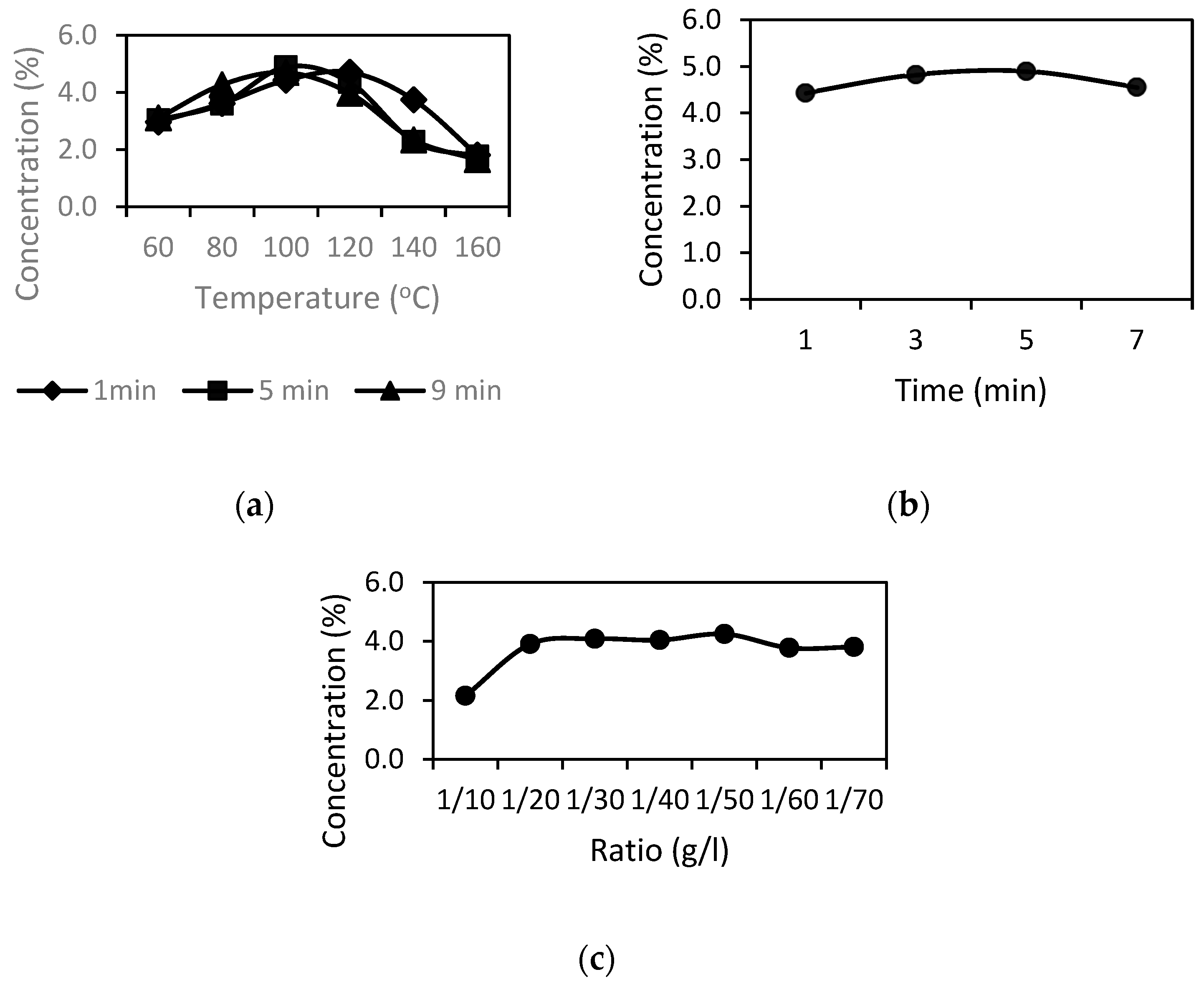
2.6. Single-Factor Analysis
Prior to the development of the RSM study, the set of tests was carried out to select the experimental ranges for the independent variables. Three factors which respond to yield include: extraction time (min), extraction temperature (°C), and sample/solvent ratio (g/mL). When optimizing experimental factors, one factor was modified, while other factors were maintained at a specified value.
2.7. RSM Procedure
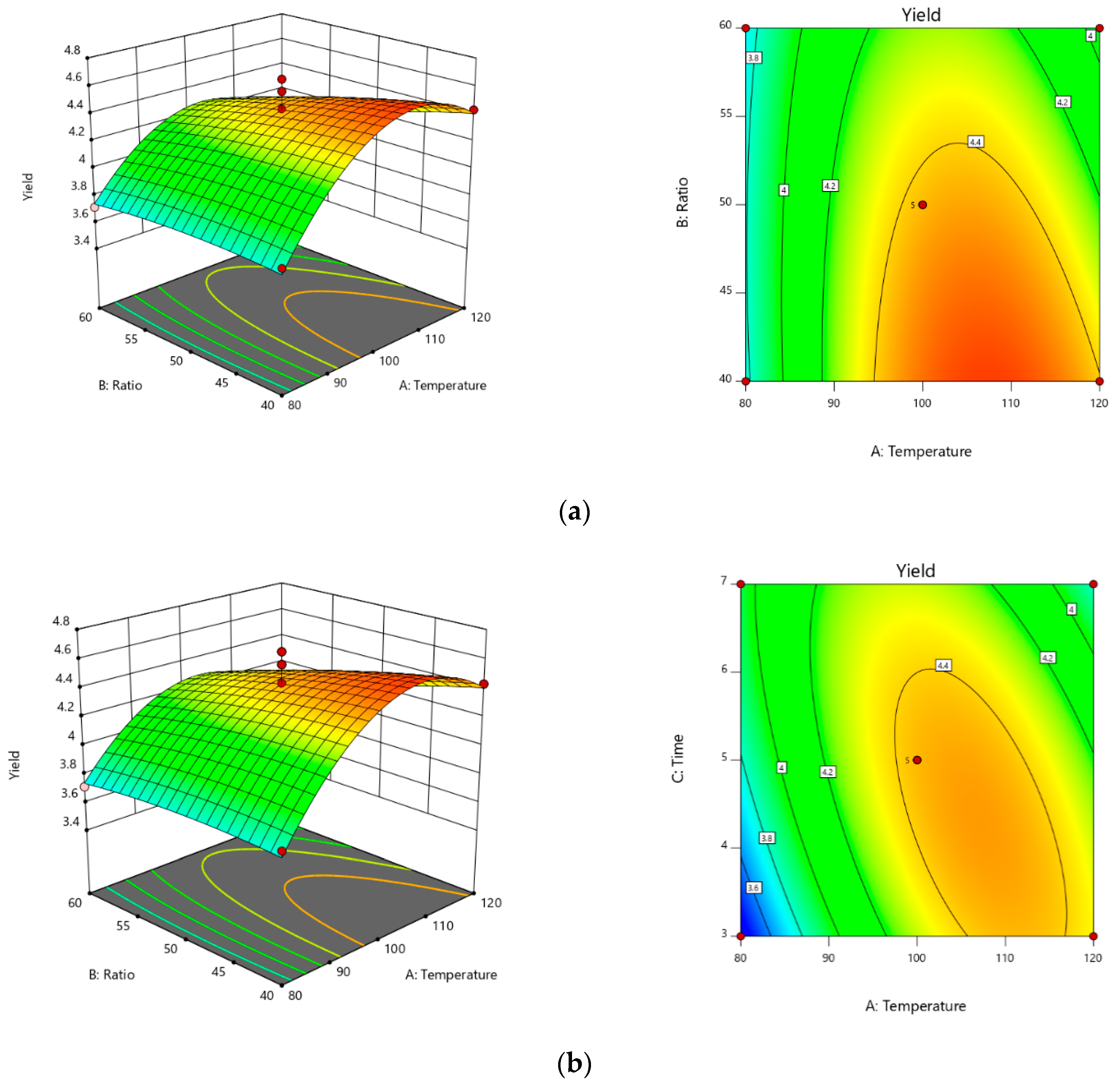
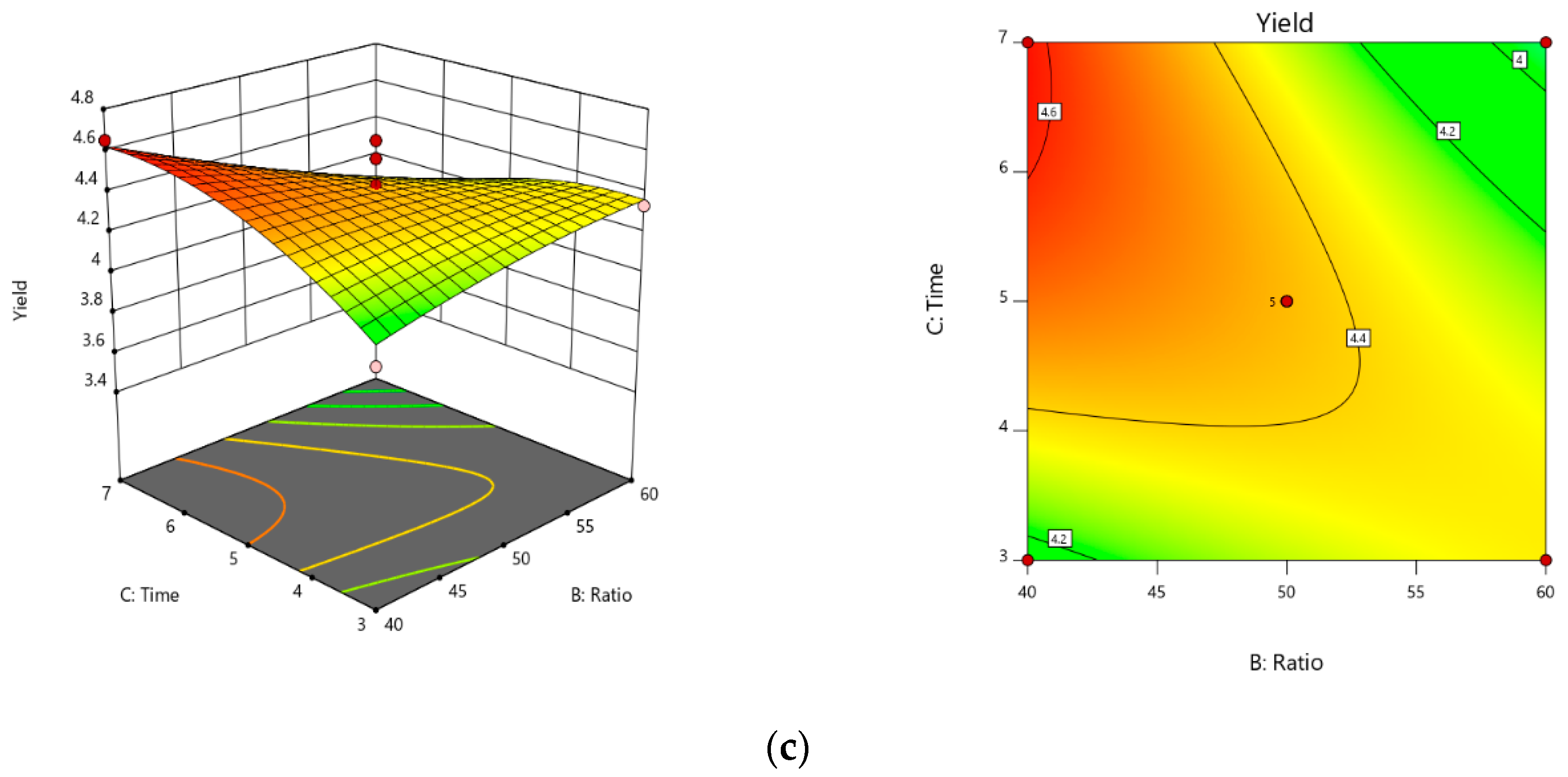
From the results of the single factor analysis, the three level-three factor Box–Behnken design was used in this study, requiring 17 experiments and five center points shown in
Table 1. Multiple regressions explained the behavior of the system to fit a second-order polynomial model as follows:
Y is the response function, is an intercept, and , , and are the coefficients of the linear, quadratic, and interactive terms, respectively. Accordingly, and represent the coded independent variables.
Design-Expert 7.1.6 (Trial Version, State-Ease Inc., Minneapolis, MN, USA) program package was run to design the experiment and handled the data. Analysis of variance (ANOVA) was conducted to check the fitness of the model and the statistical significance of the regression coefficients. Last, optimal conditions were counted from the final model and verified by an actual experiment attempt.
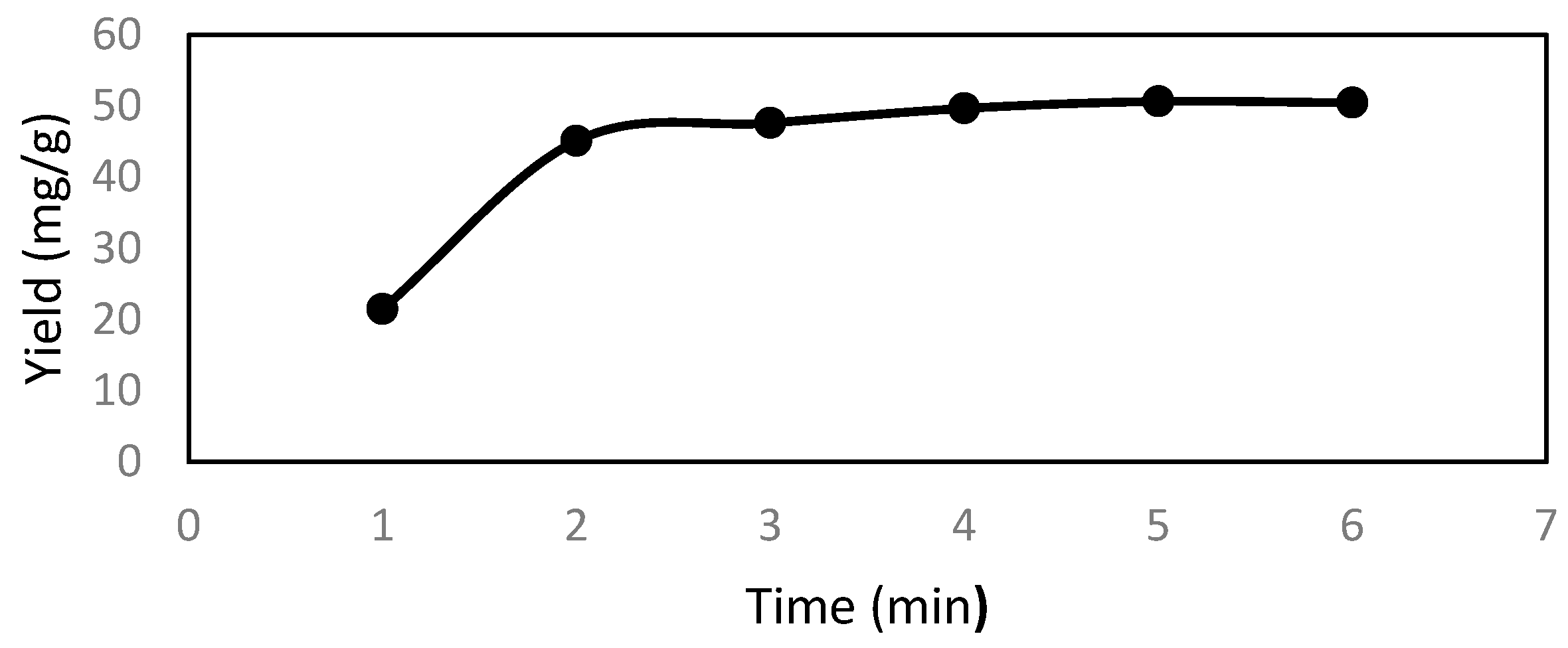
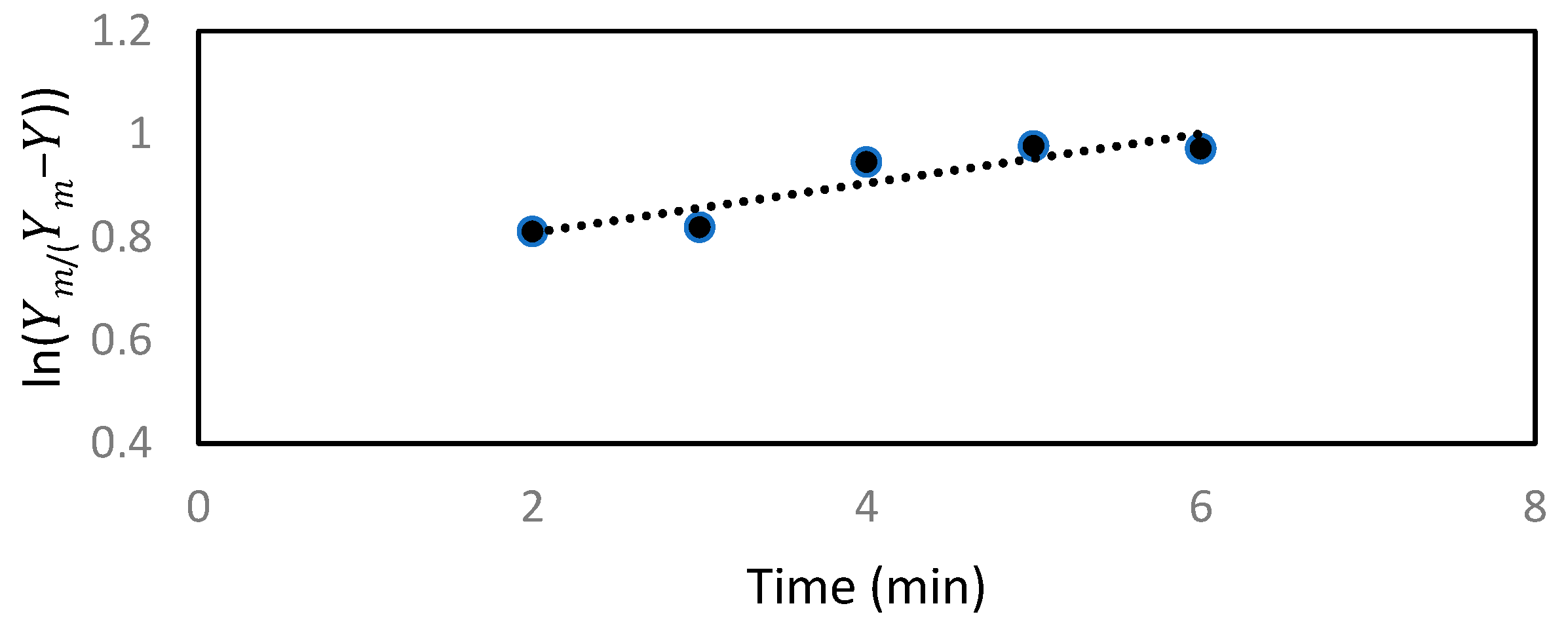
2.8. Modeling of the Extraction Process
Extracts of active ingredients from natural products are involved in mass transfer from solid to liquid. The kinetics of the active ingredients are based on two concurrent processes: the rapid part is the washing stage, where the active ingredients in the cell and surface are quickly extracted by direct washing with the solvents; and the slow one is diffusion stage, where remaining active ingredients in the cell are transferred by diffusion from the solid particles to the solvent [
23]. The pace of the extraction process depends on these slow steps, and the rate is minimal when transported through the solid matrix.
Based on previous studies [
24,
25], the steady-state kinetic model leads to a first-order rate equation as shown in Equation (2):
where
C∞ is the concentration (mg/10 g) in infinite time (
t = ∞),
C is the concentration of the extracted ingredients in the solution (mg/10 g) at time
t,
k is the overall rate constant, and
b is an intercept.
When replacing the maximum yield (
Ym) in the experiment with
C, Equation (2) becomes:
We will use Equation (3) to be suitable with the experimental data, and to obtain values for Ym and k.
4. Conclusions
The application of SWE is a reasonable replacement for the conventional extraction methods, due to the facts that quicker extraction time needs to be utilized for cost-effective extraction and the commonly available water is used as an extraction solvent in this technology, and because of the chance to directly use acquired extracts as semi-products or products for food or pharmaceutical industry without an additional process of separation or purification. Therefore, in this study, SWE was used for the recovery of EGCG from Camellia sinensis. Moreover, the response surface methodology was used and verified to be suitable for the optimization of the SWE condition extraction. RSM results validate that optimal values of extraction time, extraction temperature, and solvent/solid ratio are, respectively, 6 min, 120 °C, and 40 mL/g. Additionally, EGCG yield reached 4.665% under the above-optimized extraction conditions. The concentration of EGCG in the extracts was determined by HPLC. Moreover, our results confirmed that mathematical modeling of the extraction of EGCG from green tea is possible, yielding a useful tool for process control, even though there remains the problem of the complex interaction of the extraction conditions. This model also the presents potential for analysis of extraction processes on other active ingredients from natural products.
Overall, SWE is not only competent and environmentally friendly, but is also a highly selective and fast method. A main disadvantage of SWE is the high functional pressure, which needs expensive equipment. However, in the case of bioactive compounds such as antioxidants like EGCG, cost should not play a restricting role. Because natural antioxidants could be wanted, food components and expenses are given back by other compensations such as the high purity of extracts and the effectiveness of the technique. Therefore, SWE is a candidate to take the place of organic solvents used in the extraction of weak polar and even non-polar natural compounds. Further studies to set up a cost-efficiency pilot version of commercial equipment and scale-up of the extraction process are ongoing.