Accumulation of Airborne Toxic Elements and Photosynthetic Performance of Lolium multiflorum L. Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Trace Elements Analysis
2.3. Gas Exchange Measurements
2.4. Statistical Analysis
3. Results
3.1. Trace Elements Accumulation and Meteorological Conditions
3.2. Gas Exchange Parameters
3.3. Relations between Trace Element Accumulation and Photosynthetic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergqvist, C.; Herbert, R.; Persson, I.; Greger, M. Plants influence on arsenic availability and speciation in the rhizosphere, roots and shoots of three different vegetables. Environ. Pollut. 2014, 184, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.K.; Singh, N.K.; Rai, U.N. Chromium phytotoxicity in radish (Raphanus sativus): Effects on metabolism and nutrient uptake. Bull. Environ. Contam. Toxicol. 2013, 91, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Renaut, J.; Komatsu, S. Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteomics 2009, 9, 2602–2621. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavymetal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.; He, Z.; Stoffella, P.; Yang, X. Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J. Zhejiang Univ. Sci. B 2008, 9, 210–220. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Myśliwa-Kurdziel, B.; Strzałka, K. Influence of metals on biosynthesis of photosynthetic pigments. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Prasad, M.N.V., Strzałka, K., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 201–227. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M.; Singh, M.P.V.V.B. Adaptation strategies of plants against heavy metal toxicity: A short review. Biochem. Pharmacol. 2015, 4, 161. [Google Scholar]
- Clijsters, H.; Van Assche, F. Inhibition of photosynthesis by heavy metals. Photosynth. Res. 1985, 7, 31–40. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chao, Y.Y.; Huang, W.D.; Kao, C.H. Effect of nitrogen deficiency on antioxidant status and Cd toxicity in rice seedlings. Plant Growth Regul. 2011, 64, 263–273. [Google Scholar] [CrossRef]
- Li, F.; Qi, J.; Zhang, G.; Lin, L.; Fang, P.; Ta, A.; Xu, J. Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two Kenaf (Hibiscus cannabinus L.) plant seedlings. J. Integr. Agric. 2013, 12, 610–620. [Google Scholar] [CrossRef]
- Dagupta, N.; Datt, P.N.; Das, S. Photosynthesis and antioxidative enzyme activities in five Indian mangroves with respect to their adaptability. Acta Physiol. Plant 2010, 33, 803–810. [Google Scholar] [CrossRef]
- Vaverkova, M.; Adamcova, D. Heavy metals uptake by select plant species in the landfill area of Stepanovice, Czech Republic. Pol. J. Environ. Stud. 2014, 23, 2265–2269. [Google Scholar] [CrossRef]
- Bačeva, K.; Stafilov, T.; Šajn, R.; Tănăselia, C. Moss biomonitoring of air pollution with heavy metals in the vicinity of a ferronickel smelter plant. J. Environ. Sci. Health A 2012, 47, 645–656. [Google Scholar] [CrossRef]
- Giordano, S.; Adamo, P.; Spagnuolo, V.; Tretiach, M.; Bargagli, R. Accumulation of airborne trace elements in mosses, lichens and synthetic materials exposed a turban monitoring stations: Towards a harmonisation of the moss-bag technique. Chemosphere 2013, 90, 292–299. [Google Scholar] [CrossRef]
- Pajak, M.; Jasik, M. Heavy metal (Zn, Pb, Cd) concentration in soil and moss (Pleurozium schreberii) in the Brynica district, southern Poland. Iforest 2011, 4, 176–180. [Google Scholar] [CrossRef]
- Gerdol, R.; Marchesini, R.; Iacumin, P.; Brancaleoni, L. Monitoring temporal trends of air pollution in an urban area using mosses and lichens as biomonitors. Chemosphere 2014, 108, 388–395. [Google Scholar] [CrossRef]
- Demková, L.; Bobul’ská, L.; Árvay, J.; Jezný, T.; Ducsay, L. Biomonitoring of heavy metals contamination by mosses and lichens around Slovinky tailing pond (Slovakia). J. Environ. Sci. Health A 2016, 52, 30–36. [Google Scholar] [CrossRef]
- Will-Wolf, S.; Jovan, S.; Amacher, M.C. Lichen elemental content bioindicators for air quality in upper Midwest, USA: A model for large-scale monitoring. Ecol. Indic 2017, 78, 253–263. [Google Scholar] [CrossRef]
- Budka, A.; Kayzer, D.; Borowiak, K.; Zbierska, J.; Wolna-Maruwka, A.; Schroeter-Zakrzewska, A.; Chlebowska, A. Visible tobacco leaf injury indices as indicators of cumulative tropospheric ozone effect. Arch. Environ. Prot. 2014, 40, 53–65. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kandziora-Ciupa, M.; Ciepał, R.; Barczyk, G. Robinia pseudoacacia and Melandrium album in trace elements biomonitoring and air pollution tolerance index study. Int. J. Environ. Sci. Technol. 2016, 13, 1741–1752. [Google Scholar] [CrossRef]
- Liang, J.; Fang, H.L.; Zhang, T.L.; Wang, X.X.; Liu, Y.D. Heavy metal in leaves of twelve plant species from seven different areas in Shanghai, China. Urban For. Urban Green. 2017, 27, 390–398. [Google Scholar] [CrossRef]
- Illi, J.C.; Vancetta, T.; Alves, D.D.; Osório, D.M.M.; Bianchin, L.; De Quevedo, D.M.; Juchem, F. Integrated assessment of air pollution by metals and source apportionment using ryegrass (Lolium multiflorum Lam.) in southern Brazil. Environ. Sci. Pollut. Res. 2017, 24, 2790–2803. [Google Scholar] [CrossRef]
- Fang, Z.; Lou, L.; Tai, Z.; Wang, Y.; Yang, L.; Hu, Z.; Cai, Q. Comparative study of Cd uptake and tolerance of two Italian ryegrass (Lolium multiflorum) cultivars. PeerJ 2017, 5, e3621. [Google Scholar] [CrossRef]
- Borowiak, K.; Zbierska, J.; Barałkiewicz, D.; Hanć, A.; Budka, A.; Kayzer, D.; Kawala, A. Biomonitoring of Air Pollution by Trace Elements Using Italian Ryegrass (Lolium multiflorum L.’Lema’). Pol. J. Environ. Stud. 2014, 23, 681–688. [Google Scholar]
- VDI 3957 Part 2. German proposal to grass culture. In Biological Measuring Techniques for the Determination and Evaluation of Effects of Air Pollutants on Plants (Bioindication). Method of Standardised Grass Exposure; Beuth Verlag: Berlin, Germany, 2003. [Google Scholar]
- Klumpp, A.; Ansel, W.; Klumpp, G.; Breuer, J.; Vergne, P.; José Sanz, M.; Rasmussen, S.; Ro-Poulsen, H.; Ribas Artola, A.; Peñuelas, J.; et al. Airborne trace element pollution in 11 European cities assessed by exposure of standardised ryegrass cultures. Atmos. Environ. 2009, 43, 329–339. [Google Scholar] [CrossRef]
- Alves, D.D.; Osório, D.M.M.; Rodrigues, M.A.S.; Illi, J.C.; Bianchin, L.; Benvenuti, T. Concentrations of PM2. 5-10 and PM2. 5 and metallic elements around the Schmidt Stream area, in the Sinos River Basin, southern Brazil. Braz. J. Biol. 2015, 75, 43–52. [Google Scholar] [CrossRef]
- Jasaitis, D.; Vasiliauskienė, V.; Chadyšienė, R.; Pečiulienė, M. Surface ozone concentration and its relationship with UV radiation, meteorological parameters and radon on the eastern coast of the Baltic Sea. Atmosphere 2016, 7, 2. [Google Scholar] [CrossRef]
- Singh, A.A.; Agrawal, S.B. Tropospheric ozone pollution in India: Effects on crop yield and product quality. Environ. Sci. Pollut. Res. 2017, 24, 4367–4382. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, T.H.; Choi, B.C.; Lee, B.S.; Lee, J.H. Effect of CO, NOx and SO2 on ROS production, photosynthesis and ascorbate–glutathione pathway to induce Fragaria×annasa as a hyperaccumulator. Redox Biol. 2014, 2, 91–98. [Google Scholar] [CrossRef]
- Seyyednejad, S.M.; Niknejad, M.; Koochak, H. A review of some different effects of air pollution on plants. Res. J. Environ. Sci. 2011, 5, 302–309. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ding, H. Variation in air pollution tolerance index of plants near a steel factory: Implications for landscape-plant species selection for industrial areas. WSEAS Trans. Environ. Dev. 2008, 4, 24–32. [Google Scholar]
- Pavlík, M.; Pavlíková, D.; Zemanová, V.; Hnilička, F.; Urbanová, V.; Száková, J. Trace elements present in airborne particulate matter--stressors of plant metabolism. Ecotoxicol. Environ. Saf. 2012, 79, 101–107. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Redondo-Gómez, S.; Mateos-Naranjo, E. Growth and photosynthetic limitation analysis of the Cd-accumulator Salicornia ramosissima under excessive cadmium concentrations and optimum salinity conditions. Plant Physiol. Biochem. 2016, 109, 103–113. [Google Scholar] [CrossRef]
- Pereira, M.P.; de Almeida Rodriguez, L.C.; Corréa, F.F.; de Castro, E.M.; Ribeiro, V.E.; Pereira, F.J. Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees 2015, 30, 807–814. [Google Scholar] [CrossRef]
- Shanying, H.E.; Yang, X.; He, Z.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Tóth, T.; Zsiros, O.; Kis, M.; Garab, G.; Kovács, L. Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC 6803. Plant Cell Environ. 2016, 35, 2075–2086. [Google Scholar] [CrossRef]
- Tran, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar] [CrossRef]
- Solís-Domínguez, F.A.; González-Chávez, M.Z.; Carrillo-González, R.; Rodriguez-Vázquez, R. Accumulation and localization of cadmium in Echinochloa polystachya grown within a hydroponic system. J. Hazard. Mater. 2007, 141, 630–636. [Google Scholar] [CrossRef]
- Chen, L.; Long, X.H.; Zhang, Z.H.; Zheng, X.T.; Rengel, Z.; Liu, Z.P. Cadmium accumulation and translocation in two Jerusalem artichoke (Helianthus tuberosus L.) cultivars. Pedosphere 2001, 21, 573–580. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestiers, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef]
- Shi, G.R.; Cai, Q.S. Photosynthetic and anatomic responses of peanut leaves to cadmium stress. Photosynthetica 2008, 46, 627–630. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total Environ. 2017, 581, 209–220. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Rahman, M.M.; Islam, M.N.; Miah, M.A.M.; Tasmen, A. Effect of arsenic on photosynthesis, growth and yield of five widely cultivated rice (Oryza sativa L.) varieties in Bangladesh. Chemosphere 2007, 67, 1072–1079. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Gajewska, E.; Niewiadomska, E.; Tokarz, K.; Słaba, M.; Skłodowska, M. Nickel-induced changes in carbon metabolism in wheat shoots. J. Plant Physiol. 2013, 170, 369–377. [Google Scholar] [CrossRef]
- Velikova, V.; Tsonev, T.; Loreto, F.; Centritto, M. Changes in photosynthesis, mesophyll conductance to CO2, and isoprenoid emissions in Poplar nigra plants exposed to excess nickel. Environ. Pollut. 2011, 159, 1058–1066. [Google Scholar] [CrossRef]
- Papazoglou, E.G.; Karantounias, G.A.; Vemmos, S.N.; Bouranis, D.L. Photosynthesis and growth responses of giant reed (Arundo donax L.) to the heavy metals Cd an Ni. Environ. Int. 2005, 31, 243–249. [Google Scholar] [CrossRef]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis n developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef]
- Rodriguez, E.; Santos, C.; Azevedo, R.; Moutnho-Pereira, J.; Correia, C. Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol. Biochem. 2012, 53, 94–100. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Photosynthetic performance of Salvinia natans exposed to chromium and zinc rich wastewater. Braz. J. Plant Physiol. 2008, 20, 61–70. [Google Scholar] [CrossRef]
- Vernay, P.; Gauthier-Moussard, C.; Jean, L.; Bordas, F.; Faure, O.; Ledoigt, G.; Hitmi, A. Effect of chromium species on phytochemical and physiological parameters in Datura innoxia. Chemosphere 2008, 72, 763–771. [Google Scholar] [CrossRef]
- Leal-Alvarado, D.A.; Espadas-Gil, F.; Sáenz-Carbonell, L.; Talavera-May, C.; Santamaria, J.M. Lead accumulation reduces photosynthesis in the lead hyper-accumulator Salvinia minima Baker by affecting the cell membrane and inducing stomatal closure. Aquat. Toxicol. 2016, 171, 37–47. [Google Scholar] [CrossRef]
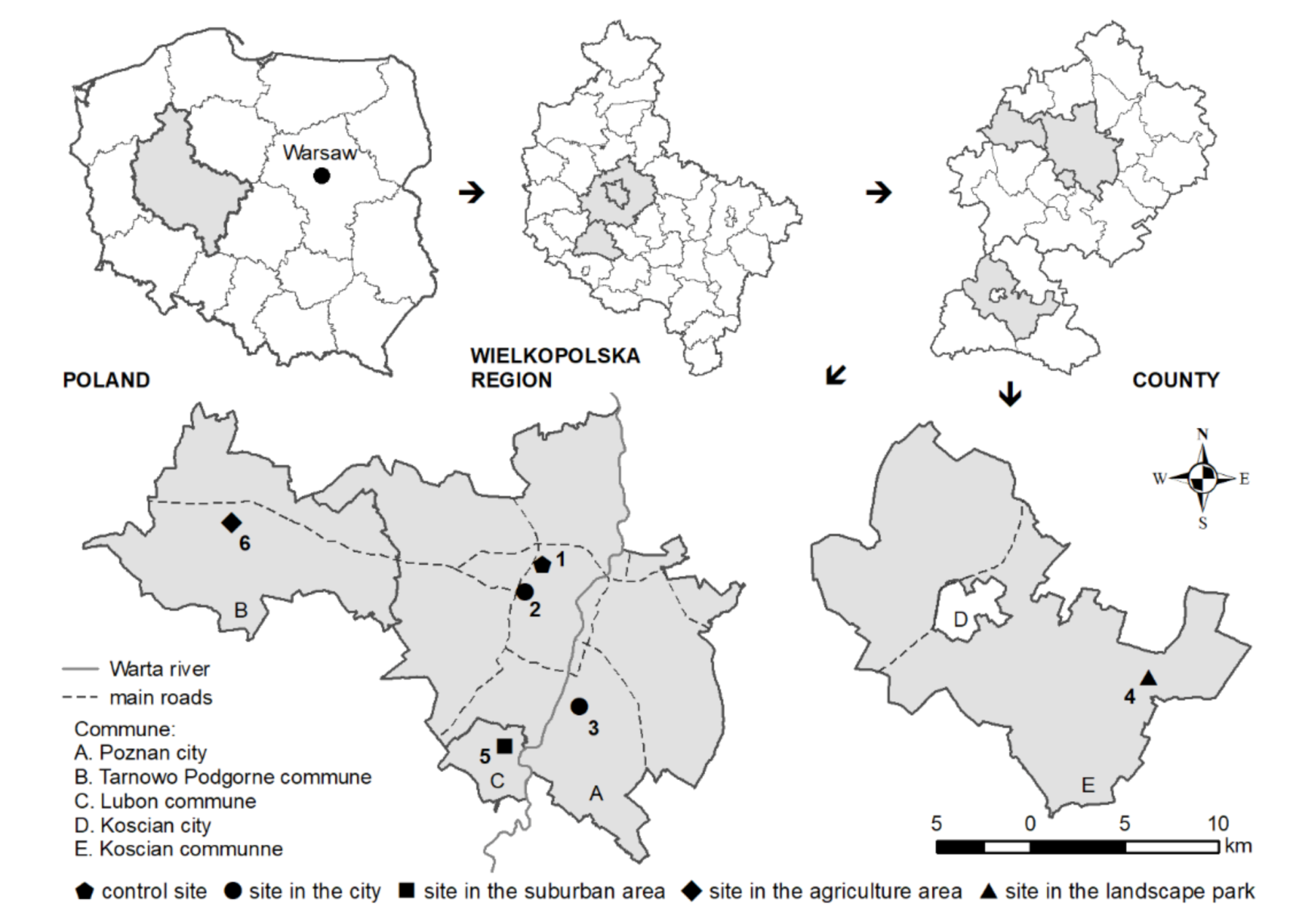
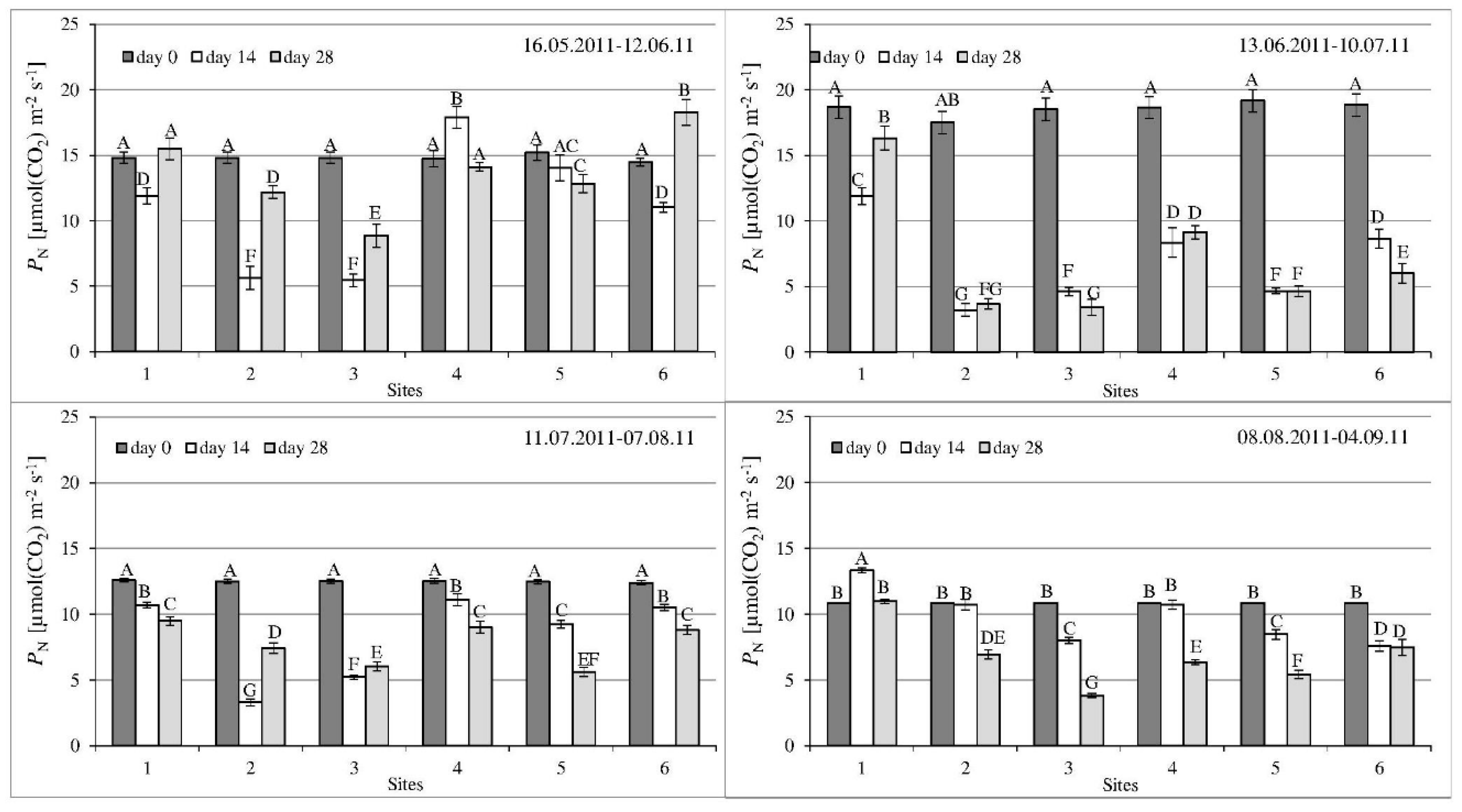
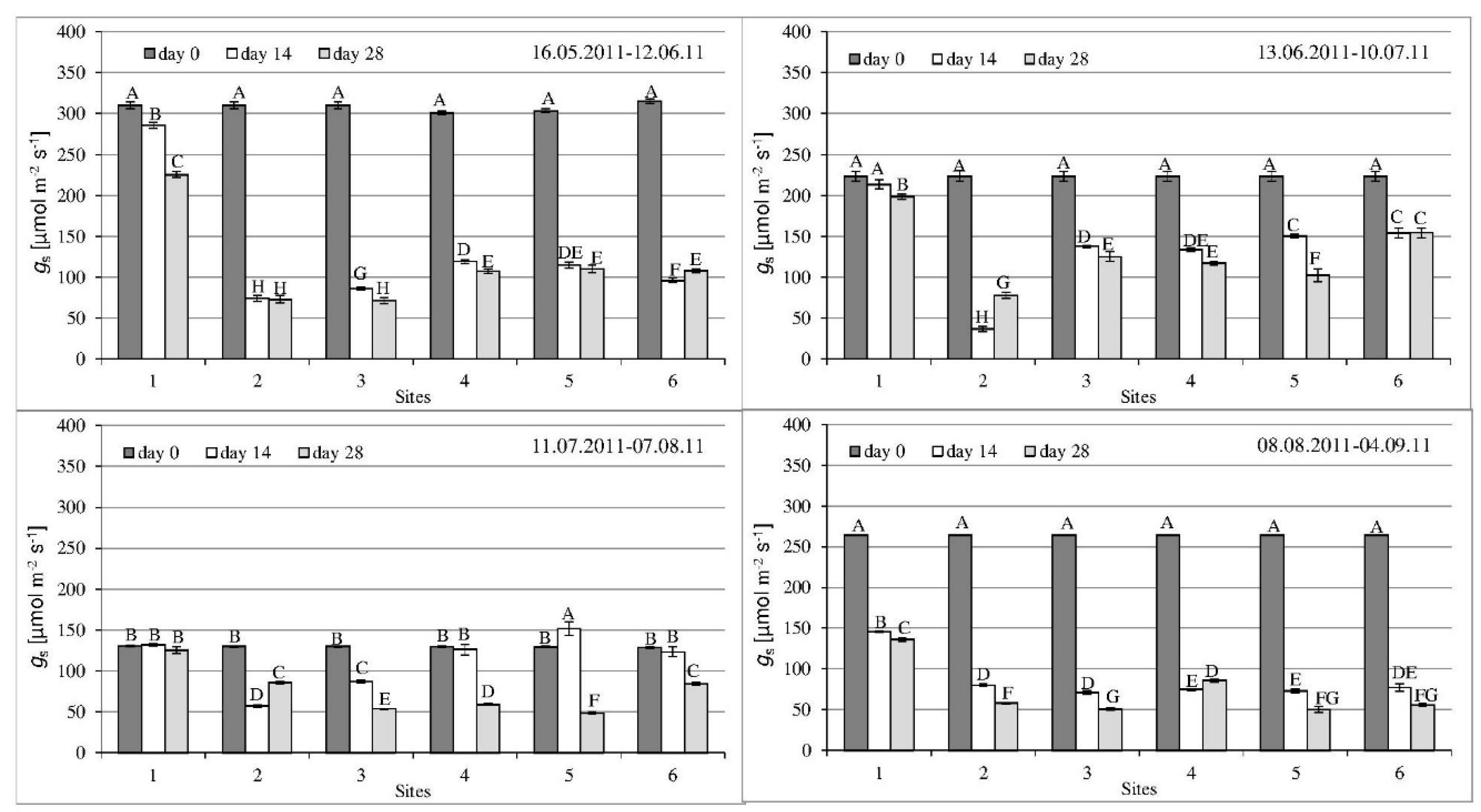
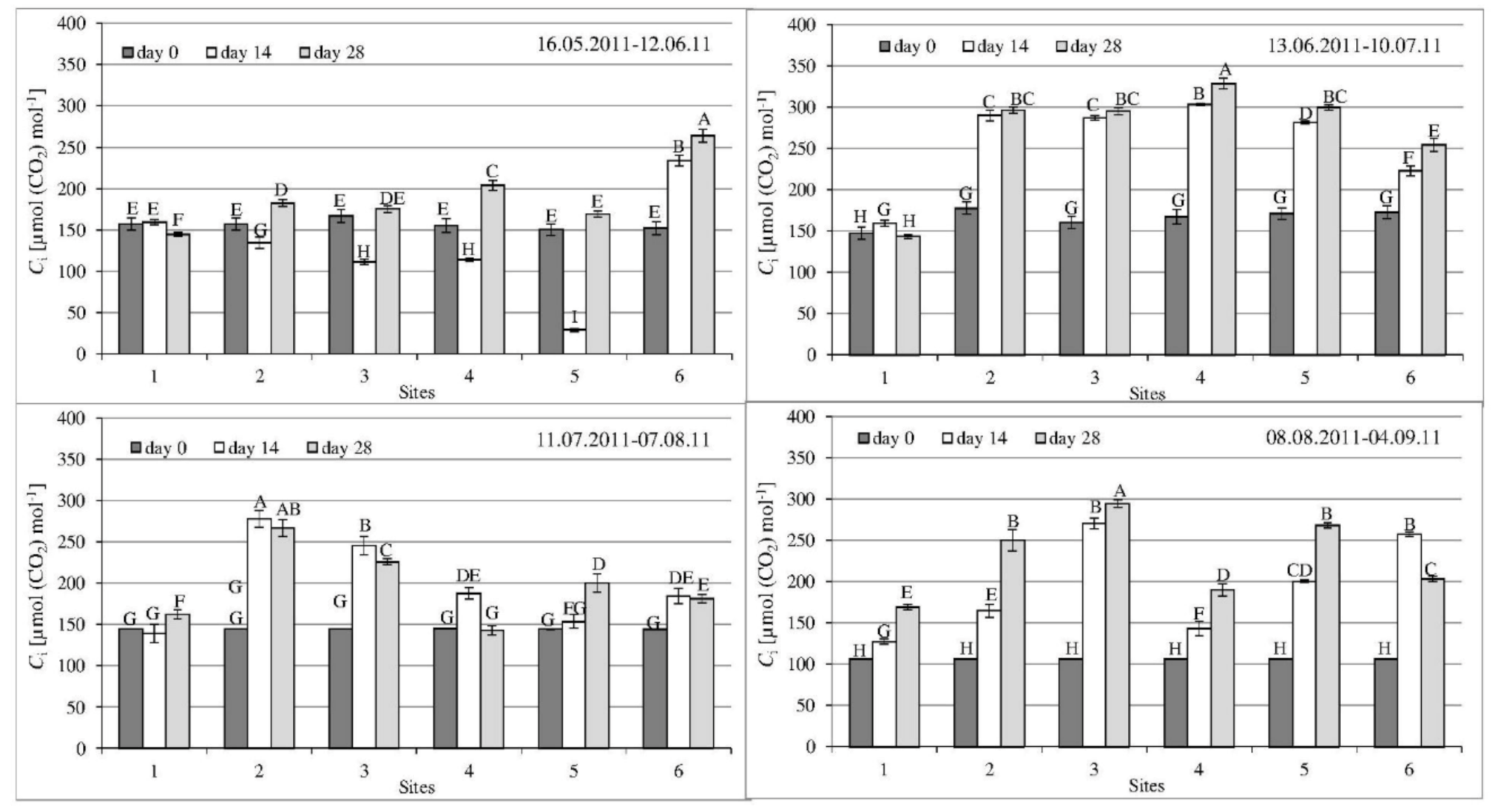
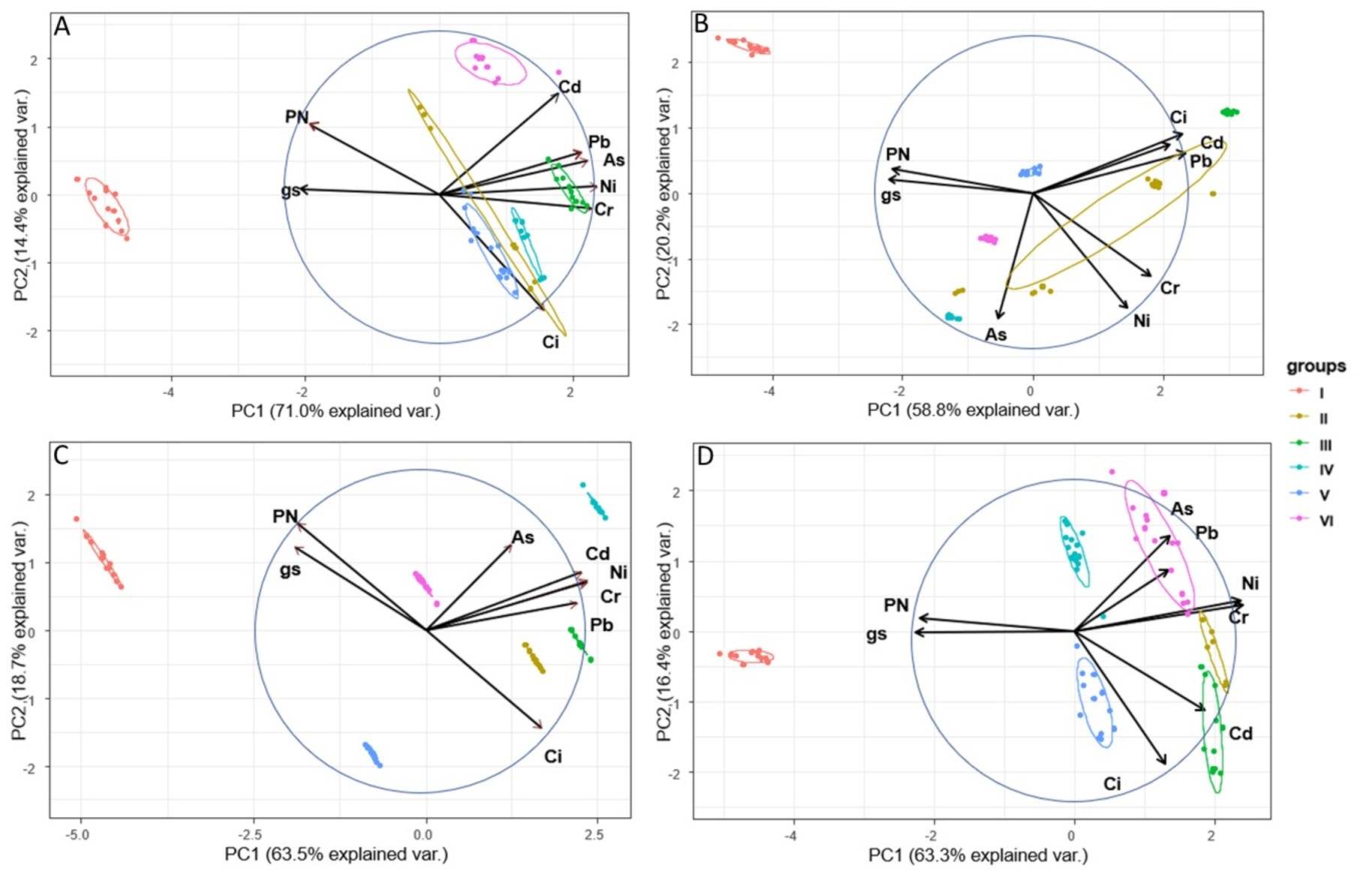
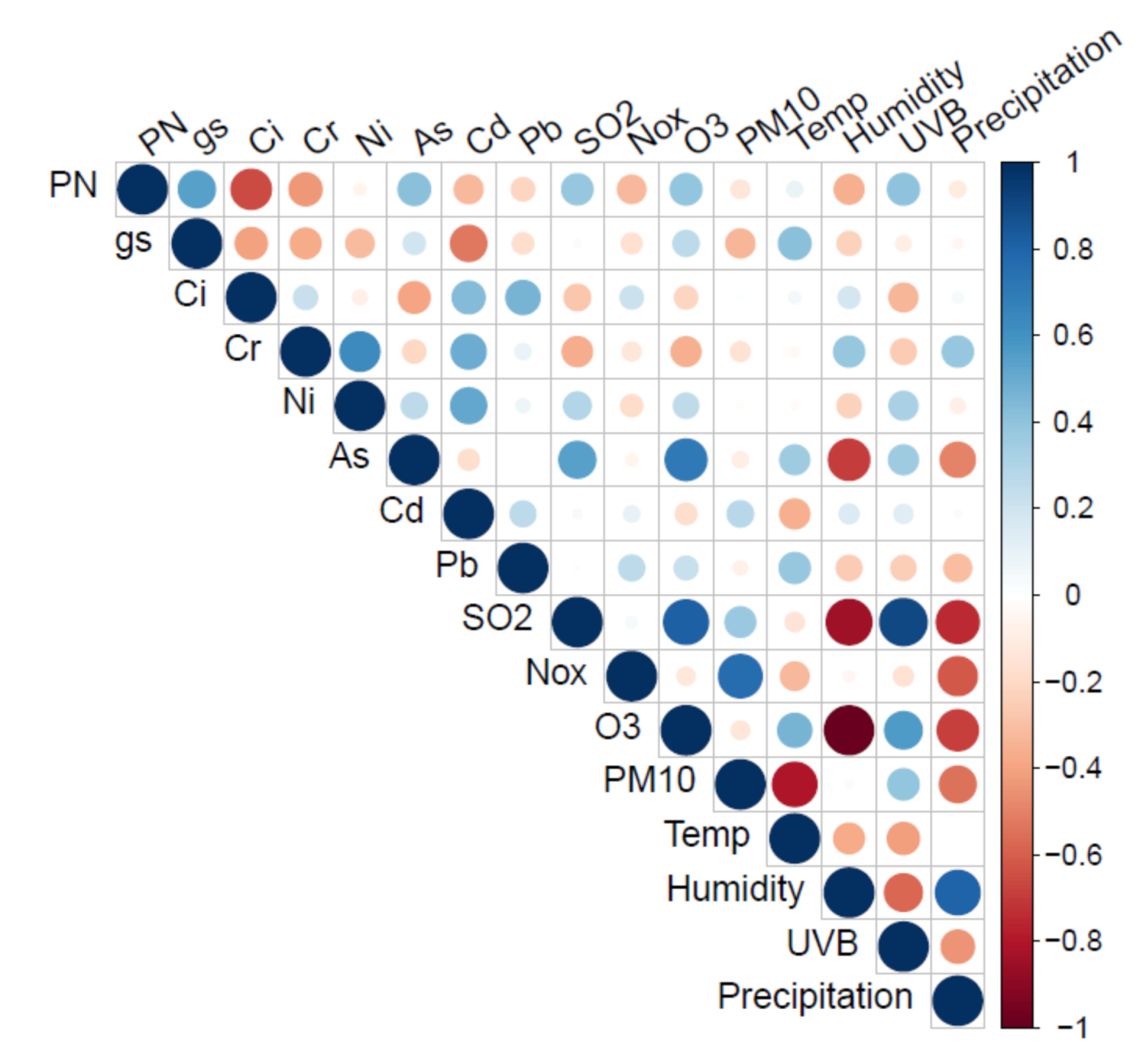
| Parameter | 1st Series | 2nd Series | 3rd Series | 4th Series |
|---|---|---|---|---|
| Cr | 75.80 *** | 123.90 *** | 60.49 *** | 98.96 *** |
| Ni | 15.87 *** | 15.23 *** | 31.26 *** | 117.97 *** |
| As | 6.80 *** | 428.58 *** | 68.45 *** | 12.62 *** |
| Cd | 16.68 *** | 22.74 *** | 14.70 *** | 348.14 *** |
| Pb | 15.16 *** | 325.14 *** | 76.00 *** | 35.88 *** |
| Series | Site | Cr (µg g−1) | Ni (µg g−1) | As (µg g−1) | Cd (µg g−1) | Pb (µg g−1) |
|---|---|---|---|---|---|---|
| 1–4 1 | 1 (control) | 0.233 ± 0.025 | 0.690 ± 0.035 | 0.018 ± 0.006 | 0.093 ± 0.001 | 0.000 ± 0.000 |
| 2 | 1.581 ± 0.072 | 1.932 ± 0.067 | 0.295 ± 0.051 | 0.156 ± 0.021 | 0.342 ± 0.029 | |
| 3 | 1.570 ± 0.083 | 1.881 ± 0.074 | 0.519 ± 0.011 | 0.224 ± 0.040 | 0.304 ± 0.014 | |
| 4 | 1.723 ± 0.067 | 2.035 ± 0.071 | 0.425 ± 0.011 | 0.149 ± 0.016 | 0.388 ± 0.028 | |
| 5 | 1.879 ± 0.350 | 1.742 ± 0.246 | 0.268 ± 0.013 | 0.162 ± 0.016 | 0.396 ± 0.013 | |
| 6 | 1.520 ± 0.086 | 1.796 ± 0.321 | 0.376 ± 0.007 | 0.248 ± 0.026 | 0.481 ± 0.017 | |
| 2 | 2 | 2.876 ± 0.262 | 1.493 ± 0.235 | 0.069 ± 0.006 | 0.190 ± 0.004 | 0.883 ± 0.066 |
| 3 | 2.114 ± 0.101 | 1.468 ± 0.081 | 0.031 ± 0.007 | 0.312 ± 0.012 | 1.086 ± 0.176 | |
| 4 | 2.204 ± 0.219 | 1.437 ± 0.101 | 0.533 ± 0.006 | 0.128 ± 0.009 | 0.275 ± 0.020 | |
| 5 | 2.049 ± 0.068 | 1.254 ± 0.040 | 0.088 ± 0.006 | 0.111 ± 0.011 | 0.322 ± 0.020 | |
| 6 | 2.508 ± 0.227 | 1.594 ± 0.206 | 0.049 ± 0.001 | 0.119 ± 0.017 | 0.164 ± 0.022 | |
| 3 | 2 | 2.872 ± 0.218 | 1.923 ± 0.083 | 0.044 ± 0.004 | 0.285 ± 0.021 | 0.234 ± 0.027 |
| 3 | 3.384 ± 0.159 | 2.255 ± 0.100 | 0.036 ± 0.005 | 0.347 ± 0.006 | 0.210 ± 0.031 | |
| 4 | 4.469 ± 0.858 | 2.222 ± 0.603 | 0.225 ± 0.016 | 0.322 ± 0.016 | 0.167 ± 0.020 | |
| 5 | 1.829 ± 0.133 | 1.205 ± 0.050 | 0.041 ± 0.004 | 0.122 ± 0.006 | 0.038 ± 0.018 | |
| 6 | 3.168 ± 0.382 | 1.955 ± 0.116 | 0.046 ± 0.007 | 0.225 ± 0.023 | 0.106 ± 0.002 | |
| 4 | 2 | 2.512 ± 0.117 | 1.762 ± 0.057 | 0.053 ± 0.009 | 0.381 ± 0.044 | 0.632 ± 0.082 |
| 3 | 2.368 ± 0.159 | 1.587 ± 0.063 | 0.061 ± 0.003 | 0.413 ± 0.086 | 0.181 ± 0.022 | |
| 4 | 1.921 ± 0.223 | 1.397 ± 0.105 | 0.100 ± 0.011 | 0.173 ± 0.010 | 0.028 ± 0.002 | |
| 5 | 1.768 ± 0.145 | 1.416 ± 0.152 | 0.041 ± 0.013 | 0.215 ± 0.024 | 0.069 ± 0.009 | |
| 6 | 2.373 ± 0.102 | 1.718 ± 0.061 | 0.066 ± 0.011 | 0.195 ± 0.014 | 0.673 ± 0.064 |
| Series | Temperature (°C) | Air Humidity (%) | UVB Radiation (W·m2) | PrecipitationSum (mm) |
|---|---|---|---|---|
| 16.05–12.06 | 17.67 | 64.97 | 246.19 | 10 |
| 13.06–10.07 | 18.20 | 76.34 | 103.89 | 86 |
| 11.07–07.08 | 17.00 | 81.00 | 171.72 | 155 |
| 08.08–04.09 | 16.00 | 78.25 | 181.36 | 43 |
| Means | 17.00 | 76.62 | 173.92 | 294 (sum) |
| Parameter | 1st Series | 2nd Series | 3rd Series | 4th Series |
|---|---|---|---|---|
| PN | 8.331 *** | 7.393 *** | 8.208 *** | 16.633 *** |
| gs | 1.225 ns | 66.845 *** | 6.083 *** | 1.387 ns |
| Ci | 1.542 ns | 3.327 ** | 10.649 *** | 12.788 ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiak, K.; Budka, A.; Lisiak-Zielińska, M.; Hanć, A.; Zbierska, J.; Barałkiewicz, D.; Kayzer, D.; Gaj, R.; Szymczak-Graczyk, A.; Kanclerz, J. Accumulation of Airborne Toxic Elements and Photosynthetic Performance of Lolium multiflorum L. Leaves. Processes 2020, 8, 1013. https://doi.org/10.3390/pr8091013
Borowiak K, Budka A, Lisiak-Zielińska M, Hanć A, Zbierska J, Barałkiewicz D, Kayzer D, Gaj R, Szymczak-Graczyk A, Kanclerz J. Accumulation of Airborne Toxic Elements and Photosynthetic Performance of Lolium multiflorum L. Leaves. Processes. 2020; 8(9):1013. https://doi.org/10.3390/pr8091013
Chicago/Turabian StyleBorowiak, Klaudia, Anna Budka, Marta Lisiak-Zielińska, Anetta Hanć, Janina Zbierska, Danuta Barałkiewicz, Dariusz Kayzer, Renata Gaj, Anna Szymczak-Graczyk, and Jolanta Kanclerz. 2020. "Accumulation of Airborne Toxic Elements and Photosynthetic Performance of Lolium multiflorum L. Leaves" Processes 8, no. 9: 1013. https://doi.org/10.3390/pr8091013
APA StyleBorowiak, K., Budka, A., Lisiak-Zielińska, M., Hanć, A., Zbierska, J., Barałkiewicz, D., Kayzer, D., Gaj, R., Szymczak-Graczyk, A., & Kanclerz, J. (2020). Accumulation of Airborne Toxic Elements and Photosynthetic Performance of Lolium multiflorum L. Leaves. Processes, 8(9), 1013. https://doi.org/10.3390/pr8091013









