Mechanism of Permeability and Oil Recovery during Fracturing in Tight Oil Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods and Procedures
- (1)
- Fracturing fluids with different surfactant concentrations were filtered to obtain the fracturing fluid filtrate after gel breaking;
- (2)
- After the saturated formation, water was evacuated from the core and the fracturing fluid filtrate was displaced, simulating the invasion of fracturing fluid;
- (3)
- The displacement of oil was used to simulate the oil permeation and absorption in the core, after fracturing in the deep formation. Considering the low permeability and long measurement time, the jars were sealed after each set of measurements to prevent the volatilization of crude oil and fracturing fluids;
- (4)
- During the measurements, the quality of the oil absorbent paper was first assessed and the quantity of oil absorbed was thereafter determined based on the paper’s characteristics;
- (5)
- The amounts of oil absorbed by different percolating and absorbing media at different periods were recorded and changes in the percolating and absorbing speeds as well as the recovery efficiency were calculated.
3. Results
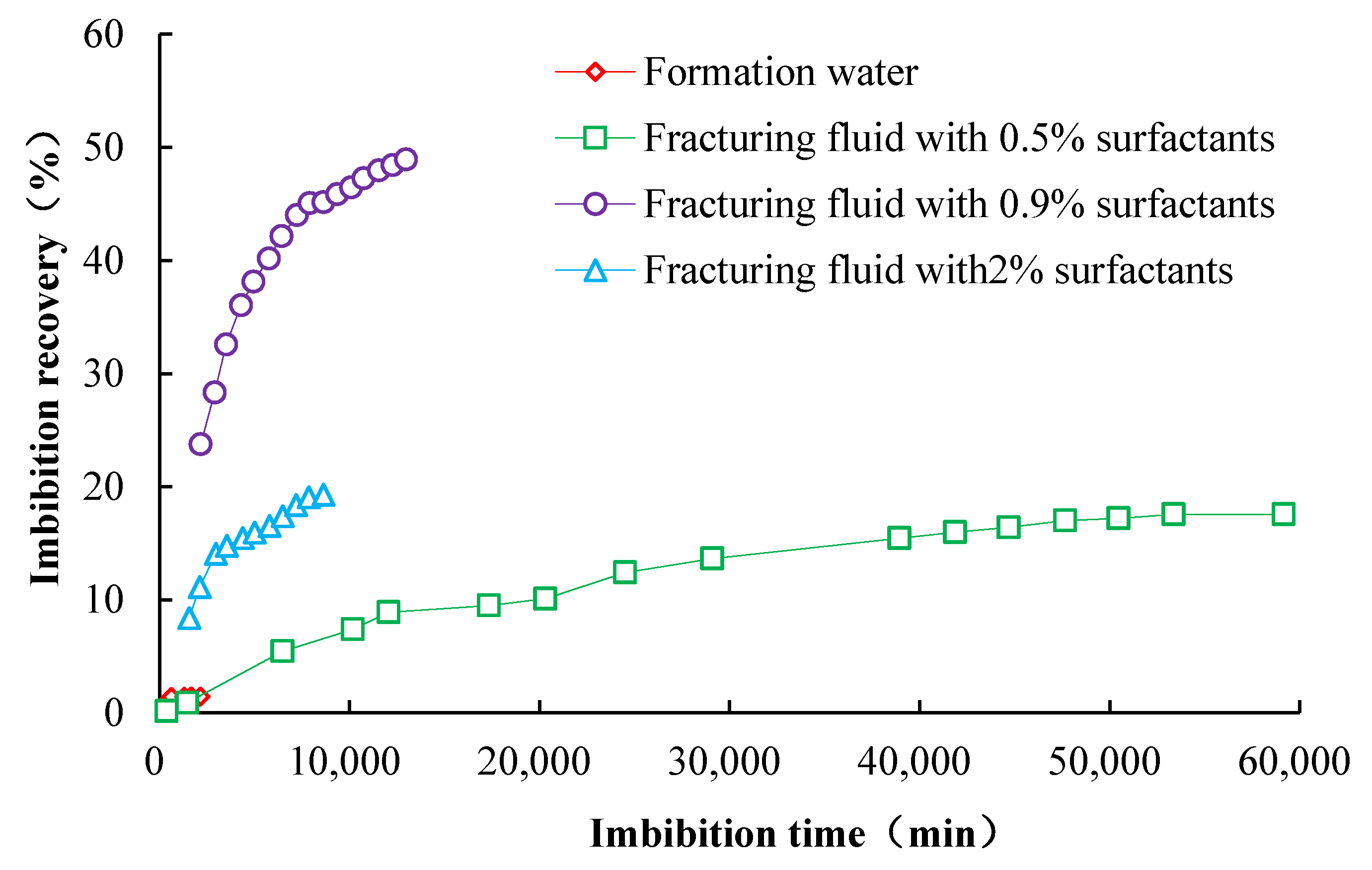
3.1. Variation in Permeability Characteristics and Absorption Rate of Fracturing Fluid System
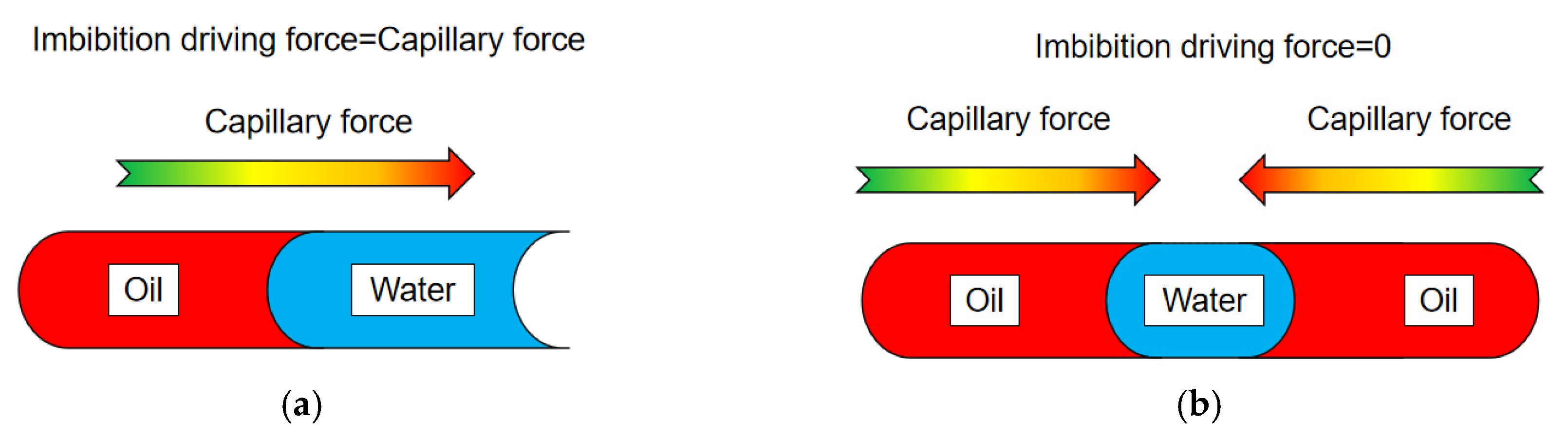
3.2. Carrying Effect of Fluid Flow with Crude Oil Particles through Pores
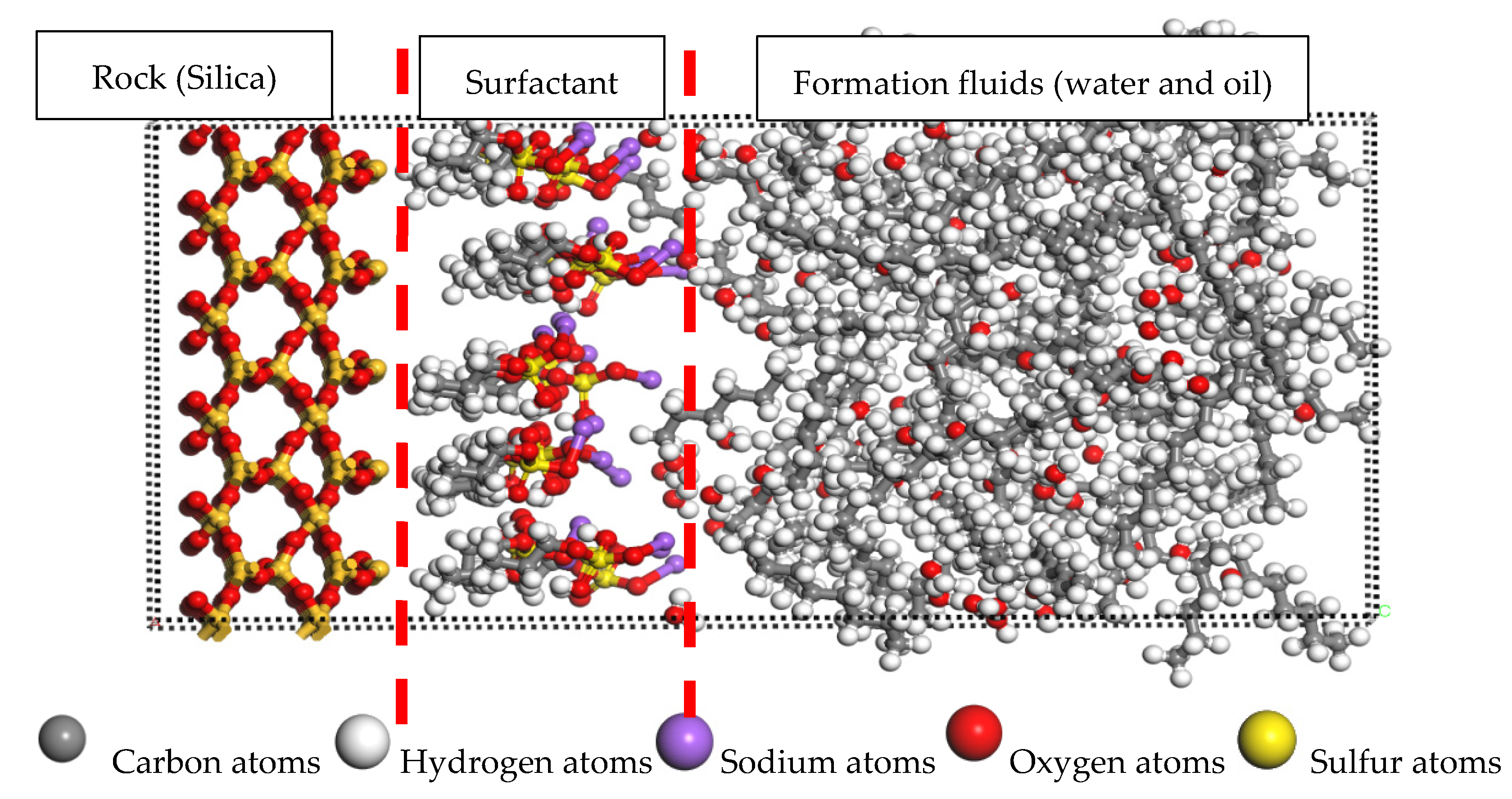
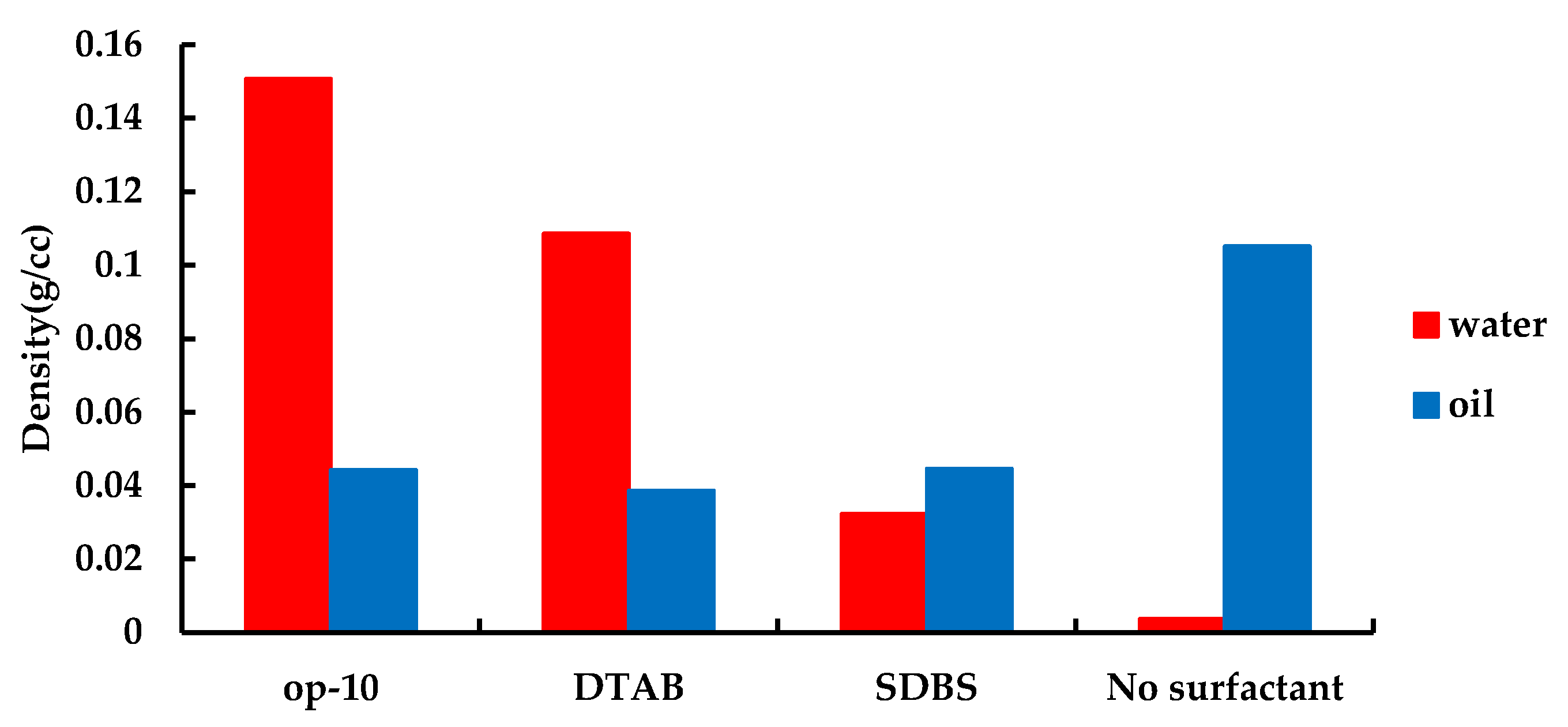
3.3. Influence of Surfactant on Rock Adsorption Characteristics during Infiltration Process
4. Conclusions
- (1)
- The main principle of fracturing fluid percolation and oil absorption in tight oil reservoirs is that the surfactant in the fracturing fluid system changes the wettability of rock and gradually disperses the crude oil particles to avoid the rapid stabilization of the percolation and absorption system. The fluid carrying through the fracture wall can enhance the imbibition velocity and prolong the imbibition time;
- (2)
- The percolation and absorption times of surfactants with different concentrations in the percolation and absorption medium vary. Therefore, a reasonable surfactant concentration should be used to maximize the degree of infiltration and recovery; the optimum OP-10 concentration in the fracturing fluid was found to be 0.9%;
- (3)
- During the fracturing and permeability development of tight oil reservoirs, the combination of fracturing permeability and stimulation can be used to increase the flow frequency and velocity of fluids in fractures. Dispersing the crude oil into small droplets makes it easier for the crude oil to seep out, avoiding the equilibrium of capillary force in the pore duct and prolonging the stability time of seeping and absorbing;
- (4)
- The wettability of rocks fundamentally changed when OP-10 surfactants and DTAB surfactants were added. From the wet surface of the oil to the wet surface of water, the crude oil adsorbed on the rock surface gradually separates from the rock surface under the action of van der Waals force and the separated oil droplets drive into the surface cracks under the action of capillary forces.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Gharbi, M.; Blunt, M.J. 2D dynamic pore-scale network model of imbibition. Dev. Water Sci. 2004, 55, 71–82. [Google Scholar]
- Mason, G.; Morrow, N.R. Developments in spontaneous imbibition and possibilities for future work. J. Pet. Sci. Eng. 2013, 110, 268–293. [Google Scholar] [CrossRef]
- Benavente, D.; Pla, C.; Cueto, N.; Galvañ, S.; Martínez-Martínez, J.; Garcia-Del-Cura, M.A.; Ordóñez, S. Predicting water permeability in sedimentary rocks from capillary imbibition and pore structure. Eng. Geol. 2015, 195, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, N.; Nikitin, V.; Legros, J.; Istasse, E.; Schramm, L.; Wassmuth, F. Microgravity investigations of capillary-driven imbibition and drainage in inhomogeneous porous media. Acta Astronaut. 2004, 54, 39–52. [Google Scholar] [CrossRef]
- Rezaveisi, M.; Ayatollahi, S.; Rostami, B. Experimental Investigation of Matrix Wettability Effects on Water Imbibition in Fractured Artificial Porous Media. J. Pet. Sci. Eng. 2012, 86, 165–171. [Google Scholar] [CrossRef]
- Li, Y.; Flores, G.; Xu, J.; Yue, W.-Z.; Wang, Y.-X.; Luan, T.-G.; Gu, Q.-B. Residual air saturation changes during consecutive drainage–imbibition cycles in an air–water fine sandy medium. J. Hydrol. 2013, 503, 77–88. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, M.; Ridgway, C.; Gane, P. Pore wall rugosity: The role of extended wetting contact line length during spontaneous liquid imbibition in porous media. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 443, 286–295. [Google Scholar] [CrossRef]
- Fernø, M.A.; Haugen, A.; Wickramathilak, S.; Howard, J.; Graue, A.; Mason, G.; Morrow, N.R. Magnetic resonance imaging of the development of fronts during spontaneous imbibition. J. Pet. Sci. Eng. 2013, 101, 1–11. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Ji, W.; Wang, X.; Chen, L. Investigation on the variation of shale permeability with spontaneous imbibition time: Sandstones and volcanic rocks as comparative study. J. Nat. Gas Sci. Eng. 2015, 27, 1546–1554. [Google Scholar] [CrossRef]
- Garing, C.; Voltolini, M.; Ajo-Franklin, J.B.; Benson, S.M. Pore-scale Evolution of Trapped CO2 at Early Stages Following Imbibition Using Micro-CT Imaging. Energy Procedia 2017, 114, 4872–4878. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Zhang, S.; Du, Z.; Zeng, F. Characteristics of oil distributions in forced and spontaneous imbibition of tight oil reservoir. Fuel 2018, 224, 280–288. [Google Scholar] [CrossRef]
- Yang, B.; Kang, Y.; Lu, X.; You, L.; Zhang, H.; Chen, Z. Experimental investigation of the pore shape factor in fluid imbibition model-taking the Longmaxi shale in Sichuan Basin as Examples. J. Pet. Sci. Eng. 2020, 193. [Google Scholar] [CrossRef]
- Gambaryan-Roisman, T. Liquids on porous layers: Wetting, imbibition and transport processes. Curr. Opin. Colloid Interface Sci. 2014, 19, 320–335. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hosseini, M.; Tangestani, E.; Mousavi, S.E.; Niazi, M. Wettability alteration and oil recovery by spontaneous imbibition of smart water and surfactants into carbonates. Pet. Sci. 2020, 17, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhao, Z.; Li, K.; Li, A.; He, B. Spontaneous Imbibition Laws and the Optimal Formulation of Fracturing Fluid during Hydraulic Fracturing in Ordos Basin. Procedia Eng. 2015, 126, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Pitois, O.; Kaddami, A.; Langlois, V. Capillary imbibition in open-cell monodisperse foams. J. Colloid Interface Sci. 2020, 571, 166–173. [Google Scholar] [CrossRef]
- Guo, J.; Li, M.; Chen, C.; Tao, L.; Liu, Z.; Zhou, D. Experimental investigation of spontaneous imbibition in tight sandstone reservoirs. J. Pet. Sci. Eng. 2020, 193, 107395. [Google Scholar] [CrossRef]
- Feldmann, F.; Strobel, G.J.; Masalmeh, S.K.; AlSumaiti, A.M. An experimental and numerical study of low salinity effects on the oil recovery of carbonate rocks combining spontaneous imbibition, centrifuge method and coreflooding experiments. J. Pet. Sci. Eng. 2020, 190, 107045. [Google Scholar] [CrossRef]
- Mehraban, M.F.; Rostami, P.; Afzali, S.; Ahmadi, Z.; Sharifi, M.; Ayatollahi, S. Brine composition effect on the oil recovery in carbonate oil reservoirs: A comprehensive experimental and CFD simulation study. J. Pet. Sci. Eng. 2020, 191, 107149. [Google Scholar] [CrossRef]
- Sheng, M.; Tian, S.; Cheng, Z.; Ge, H. Insights into the influence of fluid imbibition on dynamic mechanics of tight shale. J. Pet. Sci. Eng. 2019, 173, 1031–1036. [Google Scholar] [CrossRef]
- Ghanbari, E.; Dehghanpour, H. Impact of rock fabric on water imbibition and salt diffusion in gas shales. Int. J. Coal Geol. 2015, 138, 55–67. [Google Scholar] [CrossRef]
- Yang, L.; Ge, H.; Shi, X.; Cheng, Y.; Zhang, K.; Chen, H.; Shen, Y.; Zhang, J.; Qu, X. The effect of microstructure and rock mineralogy on water imbibition characteristics in tight reservoirs. J. Nat. Gas Sci. Eng. 2016, 34, 1461–1471. [Google Scholar] [CrossRef]
- Li, J.; McDougall, S.; Sorbie, K. Dynamic pore-scale network model (PNM) of water imbibition in porous media. Adv. Water Resour. 2017, 107, 191–211. [Google Scholar] [CrossRef]
- Chapman, E.; Yang, J.; Crawshaw, J.; Boek, E. Pore Scale Models for Imbibition of CO2 Analogue Fluids in Etched Micro-model||junctions Using Micro-fluidic Experiments and Direct Flow Calculations. Energy Procedia 2013, 37, 3680–3686. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Cheng, L.; Cao, R.; Jia, P.; Wu, J. Simulation of counter-current imbibition in single matrix and field scale using radical integral boundary element method. J. Pet. Sci. Eng. 2017, 156, 125–133. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Yao, J.; Huang, Z. Mechanistic study of cyclic water injection to enhance oil recovery in tight reservoirs with fracture deformation hysteresis. Fuel 2020, 271, 117677. [Google Scholar] [CrossRef]
- El-Amin, M.; Salama, A.; Sun, S.; El-Amin, M.F. Numerical and dimensional investigation of two-phase countercurrent imbibition in porous media. J. Comput. Appl. Math. 2013, 242, 285–296. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Perfect, E.; Donnelly, B.; Bilheux, H.Z.; Tremsin, A.S.; McKay, L.; Distefano, V.; Cai, J.; Santodonato, L. Rapid imbibition of water in fractures within unsaturated sedimentary rock. Adv. Water Resour. 2015, 77, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Haugen, A.; Fernø, M.A.; Mason, G.; Morrow, N. Capillary pressure and relative permeability estimated from a single spontaneous imbibition test. J. Pet. Sci. Eng. 2014, 115, 66–77. [Google Scholar] [CrossRef]
- Mirzaei-Paiaman, A. Analysis of counter-current spontaneous imbibition in presence of resistive gravity forces: Displacement characteristics and scaling. J. Unconv. Oil Gas Resour. 2015, 12, 68–86. [Google Scholar] [CrossRef]
- Amadu, M.; Pegg, M.J. Analytical solution to spontaneous imbibition under vertical temperature gradient based on the theory of spontaneous imbibition dynamics. J. Pet. Sci. Eng. 2019, 172, 627–635. [Google Scholar] [CrossRef]
- Roychaudhuri, B.; Tsotsis, T.; Jessen, K. An experimental investigation of spontaneous imbibition in gas shales. J. Pet. Sci. Eng. 2013, 111, 87–97. [Google Scholar] [CrossRef]






| Name | Concentration (%) | Name | Concentration (%) |
|---|---|---|---|
| Melon glue | 0.60 | Potassium persulfate | 0.50 |
| NaCl | 0.03 | OP-10 | 0.50, 0.90 |
| KCl | 0.03 | Borax (crosslinking liquid) | 0.80 |
| Experimental Schemes | Core Length (cm) | Porosity (%) | Permeability (10−3 μm2) | Interfacial Tension (mN/m) | Core Contact Angle (°) | Recovery (%) |
|---|---|---|---|---|---|---|
| Formation water | 7.8 | 11.32 | 0.1550 | 10.52 | 54.26 | 1.46 |
| Fracturing fluid1 (0.5% OP-10) | 8.5 | 9.67 | 0.3479 | 0.67 | 16.87 | 17.56 |
| Fracturing fluid2 (0.9% OP-10) | 9.7 | 10.76 | 0.1297 | 0.43 | 10.23 | 48.93 |
| Fracturing fluid3 (2% OP-10) | 9.4 | 9.54 | 0.2671 | 0.28 | 6.24 | 19.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Cao, G.; Wei, G.; Nan, X.; Cheng, Q.; Du, T.; An, H. Mechanism of Permeability and Oil Recovery during Fracturing in Tight Oil Reservoirs. Processes 2020, 8, 972. https://doi.org/10.3390/pr8080972
Bai Y, Cao G, Wei G, Nan X, Cheng Q, Du T, An H. Mechanism of Permeability and Oil Recovery during Fracturing in Tight Oil Reservoirs. Processes. 2020; 8(8):972. https://doi.org/10.3390/pr8080972
Chicago/Turabian StyleBai, Yujie, Guangsheng Cao, Guanglei Wei, Xiaohan Nan, Qingchao Cheng, Tong Du, and Hongxin An. 2020. "Mechanism of Permeability and Oil Recovery during Fracturing in Tight Oil Reservoirs" Processes 8, no. 8: 972. https://doi.org/10.3390/pr8080972
APA StyleBai, Y., Cao, G., Wei, G., Nan, X., Cheng, Q., Du, T., & An, H. (2020). Mechanism of Permeability and Oil Recovery during Fracturing in Tight Oil Reservoirs. Processes, 8(8), 972. https://doi.org/10.3390/pr8080972





