Preparation of Curcumin Nanosuspension with Gum Arabic as a Natural Stabilizer: Process Optimization and Product Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Curcumin Nanosuspension
2.2. Size Measurement of Curcumin Particle in the Nanosuspension
2.3. Response Surface Methodology Optimization of the Nanosuspension Preparation Process
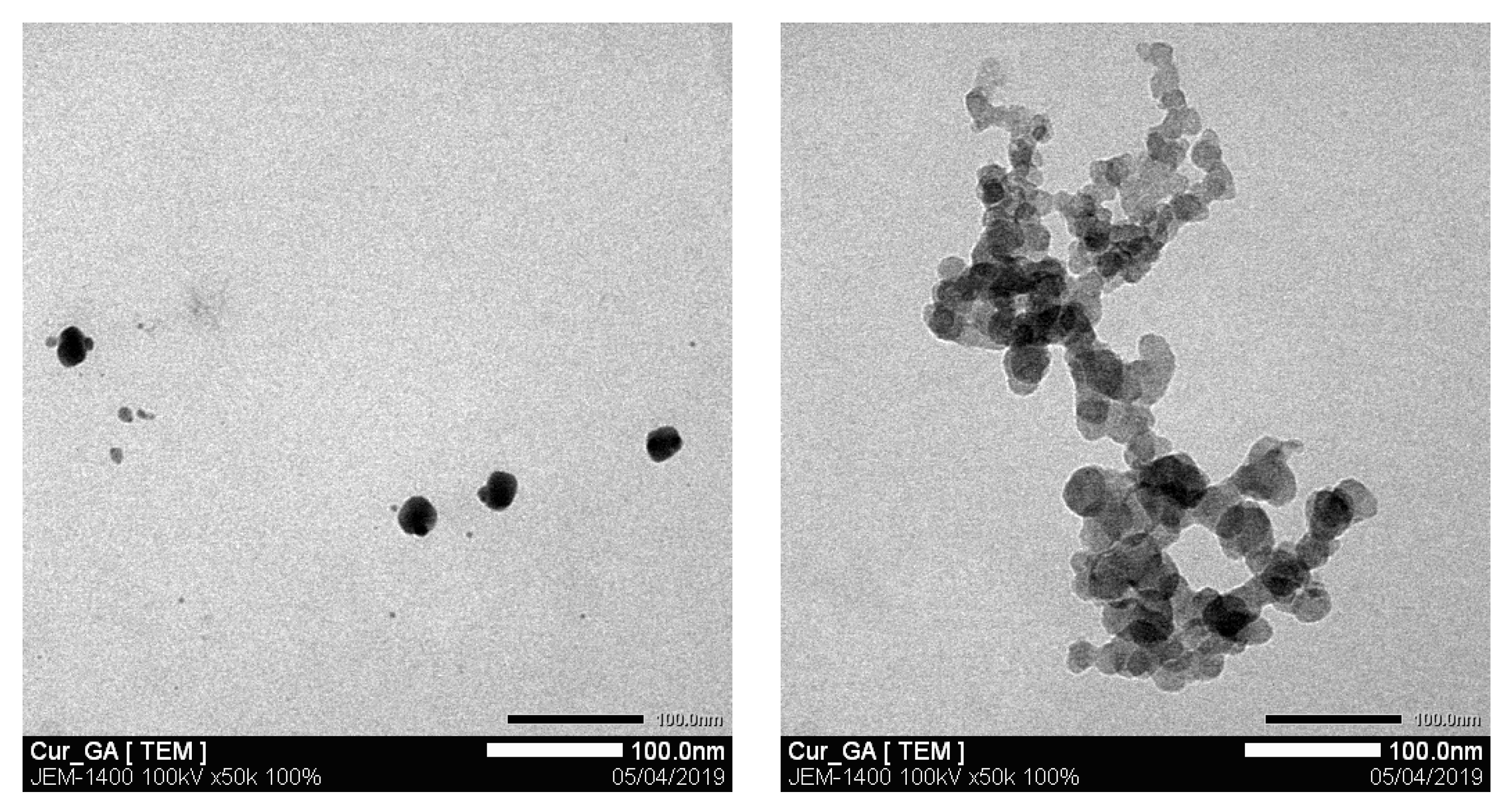
2.4. Morphological Evaluation of the Nanosuspension Product
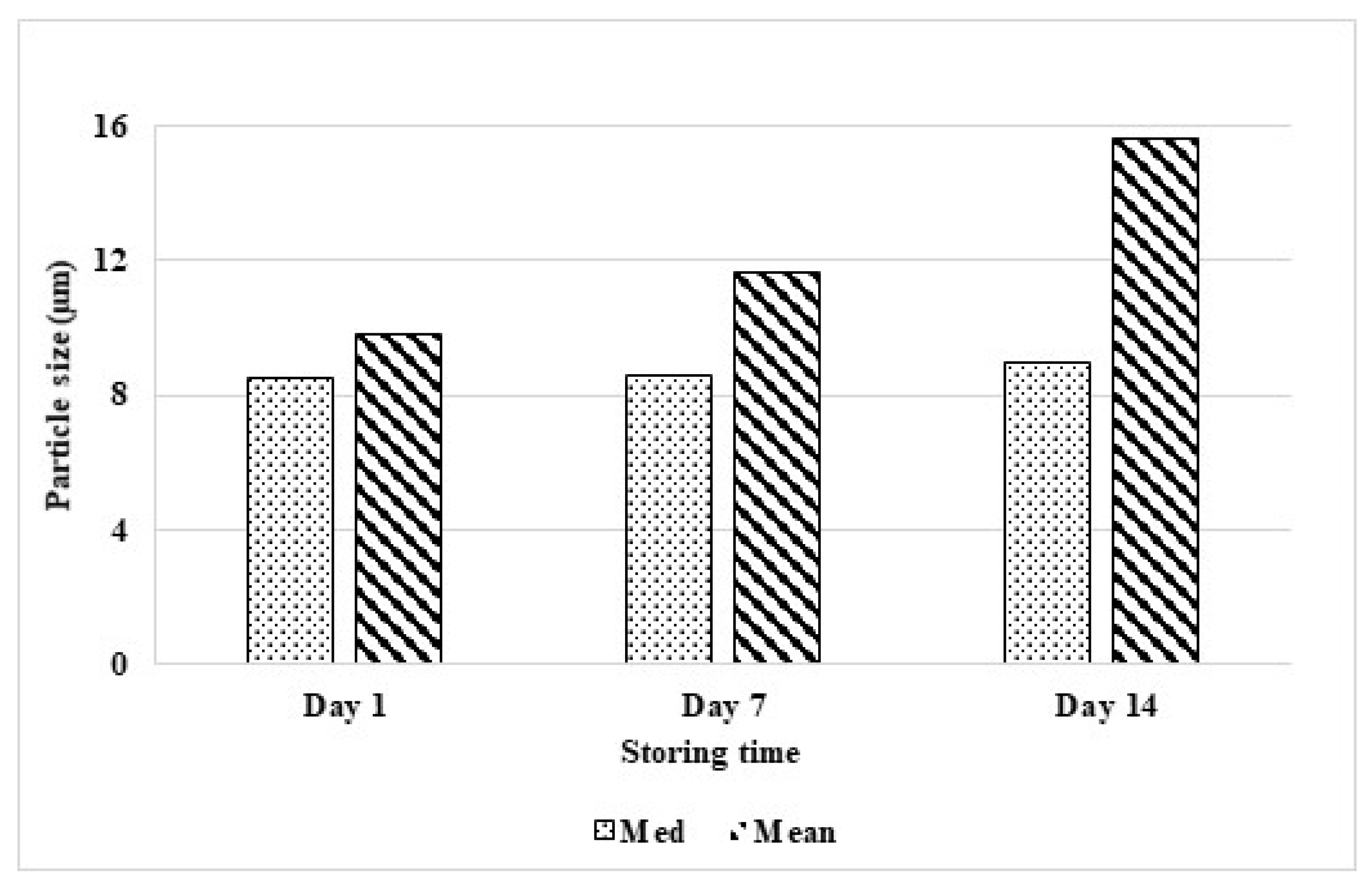
2.5. Stability Test
2.6. Scalability Test
3. Results
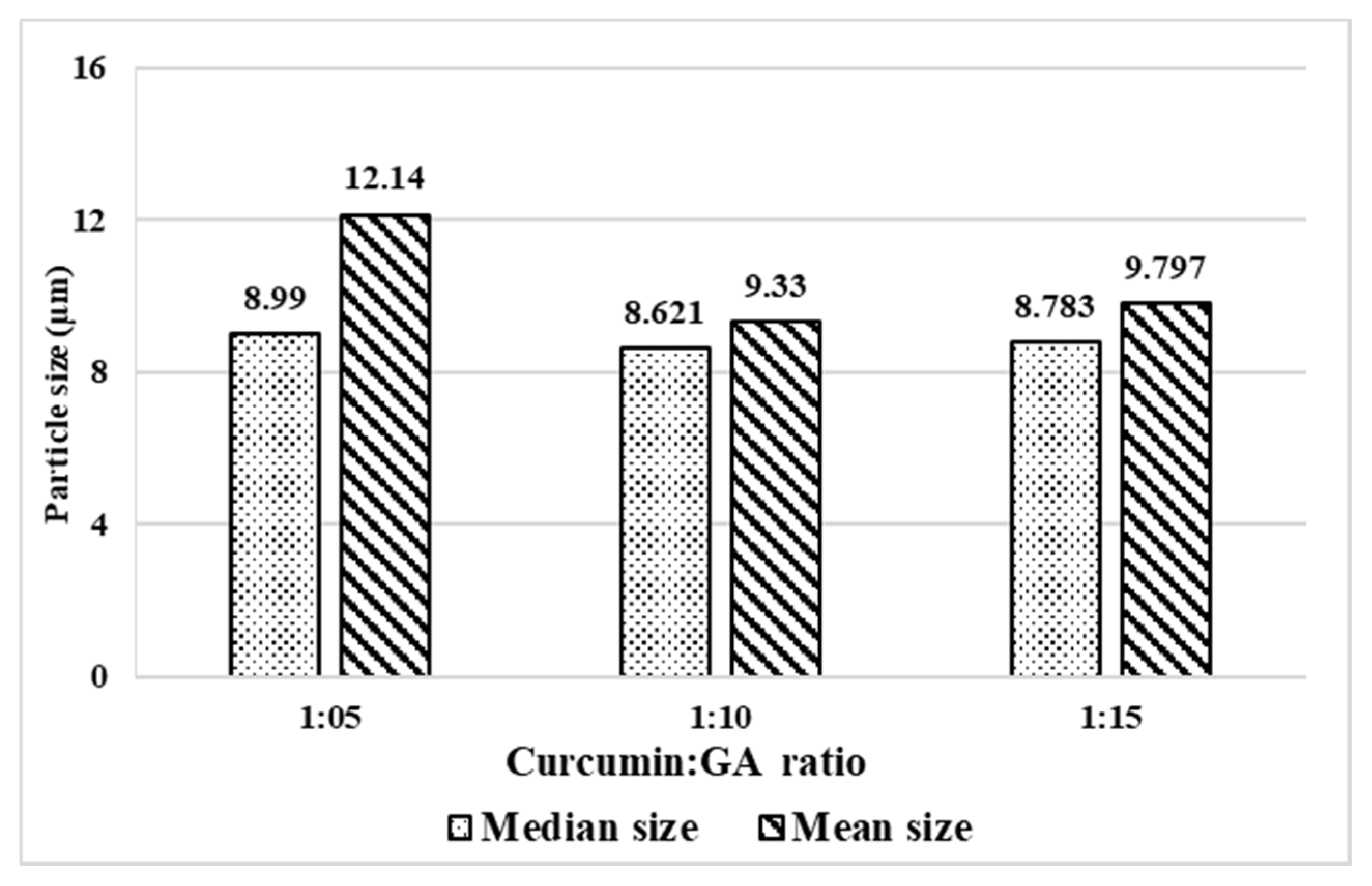
3.1. Single Factor Investigations
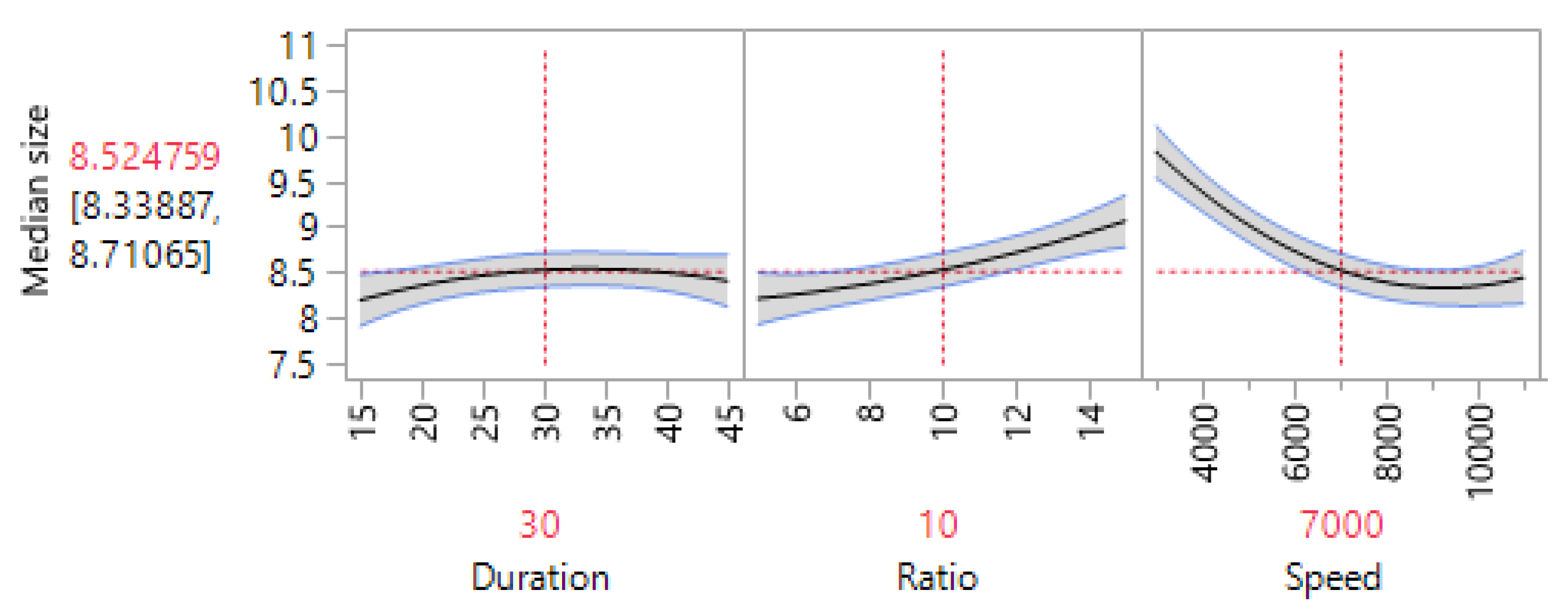
3.2. RSM Optimization Results
3.3. Scalability Test
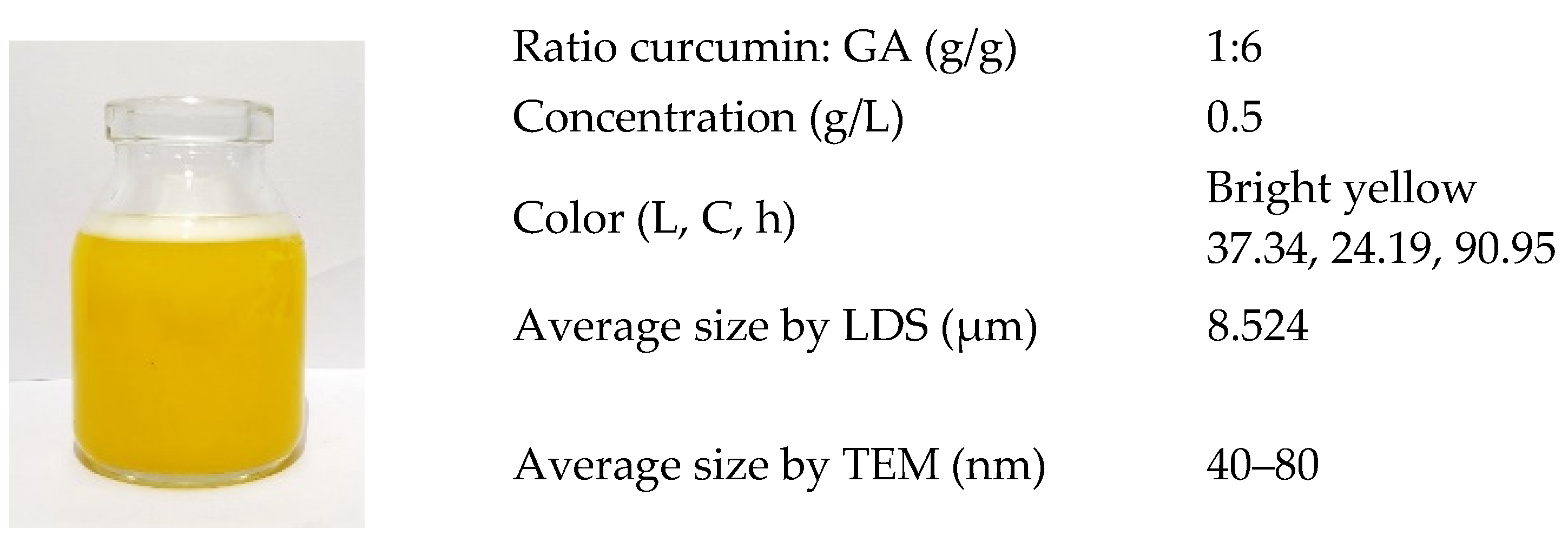
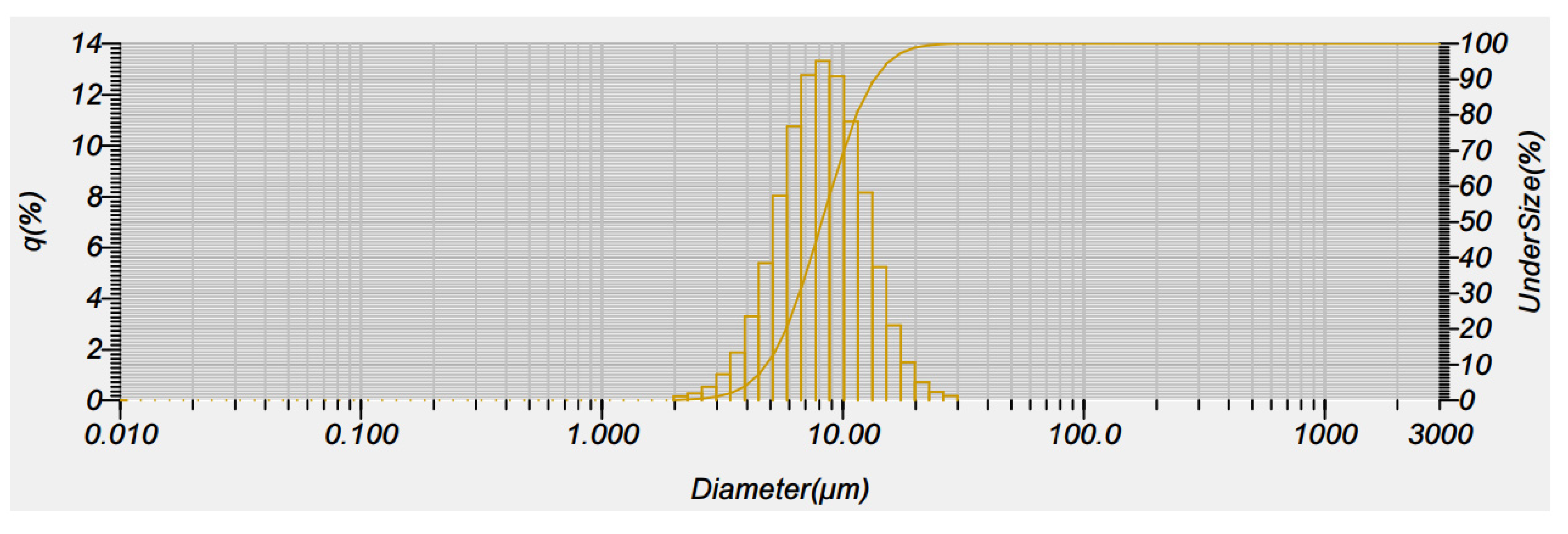
3.4. Characterization and Stability Test of the Nanosuspension Product
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- Basniwal, R.K.; Khosla, R.; Jain, N. Improving the Anticancer Activity of Curcumin Using Nanocurcumin Dispersion in Water. Nutr. Cancer 2014, 66, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Cano-Higuita, D.M.; Malacrida, C.R.; Telis, V.R.N. Stability of Curcumin Microencapsulated by Spray and Freeze Drying in Binary and Ternary Matrices of Maltodextrin, Gum Arabic and Modified Starch. J. Food Process. Preserv. 2015, 39, 2049–2060. [Google Scholar] [CrossRef]
- Jusnita, N.; Haditjaroko, L.; Yusron, M.; Noor, E. Production of Nanocurcumin from Curcuma Xanthorriza Roxb. by Homogenization. J. Biol. Agric. Healthc. 2014, 4, 79. Available online: https://www.iiste.org/Journals/index.php/JBAH/article/view/14485 (accessed on 1 June 2020).
- Gao, Y.; Li, Z.; Sun, M.; Li, H.; Guo, C.; Cui, J.; Li, A.; Cao, F.; Xi, Y.; Lou, H.; et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev. Ind. Pharm. 2010, 36, 1225–1234. [Google Scholar] [CrossRef]
- Aditya, N.P.; Yang, H.; Kim, S.; Ko, S. Fabrication of amorphous curcumin nanosuspensions using β-lactoglobulin to enhance solubility, stability, and bioavailability. Colloids Surf. B 2015, 127, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.P.; Hazarika, H.; Bharadwaj, R.; Loying, P.; Baishya, R.; Dash, S.; Das, M.K. Curcumin-docetaxel co-loaded nanosuspension for enhanced anti-breast cancer activity. Expert Opin. Drug Deliv. 2016, 13, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Motevalli, S.M.; Eltahan, A.S.; Liu, L.; Magrini, A.; Rosato, N.; Guo, W.; Bottini, M.; Liang, X.-J. Co-encapsulation of curcumin and doxorubicin in albumin nanoparticles blocks the adaptive treatment tolerance of cancer cells. Biophys. Rep. 2019, 5, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yuan, H.; Zhang, C.; Chen, W.; Cheng, W.; Chen, X.; Ye, X. Preparation and in-vitro/in-vivo evaluation of curcumin nanosuspension with solubility enhancement. J. Pharm. Pharmacol. 2016, 68, 980–988. [Google Scholar] [CrossRef]
- Moorthi, C.; Senthil Kumar, C.; Mohan, S.; Kathiresan, K. SLS/βCD-curcumin nanosuspension: Preparation, characterization and pharmacological evaluation. J. Pharm. Res. 2013, 7, 219–223. [Google Scholar] [CrossRef]
- Mai, H.; Nguyen, T.; Le, T.; Nguyen, D.; Bach, L. Evaluation of Conditions Affecting Properties of Gac (Momordica Cocochinensis Spreng) Oil-Loaded Solid Lipid Nanoparticles (SLNs) Synthesized Using High-Speed Homogenization Process. Processes 2019, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Mai, H.C.; Le, T.T.T.; Diep, T.T.; Le, T.H.N.; Nguyen, D.T.; Bach, L.G. Development of Solid Lipid Nanoparticles of Gac (Momordica cocochinensis Spreng) Oil by Nano-Emulsion Technique. Asian J. Chem. 2018, 30, 293–297. [Google Scholar] [CrossRef]
- Nhan, N.P.T.; Hien, T.T.; Nhan, L.T.H.; Anh, P.N.Q.; Huy, L.T.; Nguyen, T.C.T.; Nguyen, D.T.; Bach, L.G. Application of Response Surface Methodology to Optimize the Process of Saponification Reaction from Coconut Oil in Ben Tre-Vietnam. Solid State Phenom. 2018, 279, 235–239. [Google Scholar] [CrossRef]
- Abbas, S.; Bashari, M.; Akhtar, W.; Li, W.W.; Zhang, X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014, 21, 1265–1274. [Google Scholar] [CrossRef]
- Homayouni, A.; Amini, M.; Sohrabi, M.; Varshosaz, J.; Nokhodchi, A. Curcumin nanoparticles containing poloxamer or soluplus tailored by high pressure homogenization using antisolvent crystallization. Int. J. Pharm. 2019, 562, 124–134. [Google Scholar] [CrossRef]
- Bach, L.T.; Hue, B.T.B.; Tram, N.T.T.; Thu, D.N.A.; Dung, L.T. Chemical constituents from n-hexane and ethyl acetate extracts of Euphorbia hirta L. grown in Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022083. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.T.; Nguyen, V.T.; Minh, L.V.; Trieu, L.H.; Cang, M.H.; Bui, L.B.; Le, X.T.; Danh, V.T. Determination of the phytochemical screening, total polyphenols, flavonoids content, and antioxidant activity of soursop leaves (Annona muricata Linn.). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062011. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Minh, L.V.; Trieu, L.H.; Bui, L.M.; Lam, T.D.; Hieu, V.Q.; Khang, T.V.; Trung, L.N.Y. Evaluation of total polyphenol content, total flavonoid content, and antioxidant activity of Plectranthus amboinicus leaves. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062017. [Google Scholar] [CrossRef]
- Trinh, P.T.N.; Nguyen, T.Q.; Hau, N.V.; Hung, Q.T.; Du, C.V.; Tuan, N.T.; Thuy, N.T.L.; Dung, L.T. Chemical constituents of the stem of Coccinia grandis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022080. [Google Scholar] [CrossRef]
- Thuong Nhan, N.P.; Tan Thanh, V.; Huynh Cang, M.; Lam, T.D.; Cam Huong, N.; Hong Nhan, L.T.; Thanh Truc, T.; Tran, Q.T.; Bach, L.G. Microencapsulation of Lemongrass (Cymbopogon citratus) Essential Oil Via Spray Drying: Effects of Feed Emulsion Parameters. Processes 2020, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.N.Q.; Bach, L.G.; Nguyen, T.D.; Le, N.T.H. Efficient Method for Preparation of Rutin Nanosuspension Using Chitosan and Sodium Tripolyphosphate Crosslinker. J. Nanosci. Nanotechnol. 2019, 19, 974–978. [Google Scholar] [CrossRef]
- Minh, N.P.; Bach, L.G.; Loan, L.Y.; Tram, V.T.B.; Van Truyen, T. Production of dried tea from okra (Abelmoschus Esculentus). J. Pharm. Sci. Res. 2019, 11, 279–283. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol11issue02/jpsr11021903.pdf (accessed on 1 June 2020).





| Value | Factors | ||
|---|---|---|---|
| Homogenization Time (D) (min) | Ratio (R) (g/g) | Speed (S) (rpm) | |
| Lower limit (−1) | 15 | 5 | 3000 |
| Center point (0) | 30 | 10 | 7000 |
| Upper limit (+1) | 45 | 15 | 11,000 |
| Encoding variables (Xi) | |||
| Experiment No. | Independent Variables | Experimental Response | ||
|---|---|---|---|---|
| X1 (min) | X2 (g/g) | X3 (rpm) | Y (µm) | |
| 1 | −1 (15) | −1 (5) | −1 (3000) | 8.827 |
| 2 | −1 (15) | −1 (5) | +1 (11,000) | 7.782 |
| 3 | −1 (15) | 0 (10) | 0 (7000) | 8.272 |
| 4 | −1 (15) | +1 (15) | −1 (3000) | 10.571 |
| 5 | −1 (15) | +1 (15) | +1 (11,000) | 8.383 |
| 6 | 0 (30) | −1 (5) | 0 (7000) | 7.992 |
| 7 | 0 (30) | 0 (10) | −1 (3000) | 9.718 |
| 8 | 0 (30) | 0 (10) | 0 (7000) | 8.625 |
| 9 | 0 (30) | 0 (10) | 0 (7000) | 8.595 |
| 10 | 0 (30) | 0 (10) | +1 (11,000) | 8.457 |
| 11 | 0 (30) | +1 (15) | 0 (7000) | 9.197 |
| 12 | +1 (45) | −1 (5) | −1 (3000) | 9.346 |
| 13 | +1 (45) | −1 (5) | +1 (11,000) | 8.632 |
| 14 | +1 (45) | 0 (10) | 0 (7000) | 8.242 |
| 15 | +1 (45) | +1 (15) | −1 (3000) | 10.186 |
| 16 | +1 (45) | +1 (15) | +1 (11,000) | 8.497 |
| Independent Variable | Coeff. | Std Error | t Ratio | Prob > |t| |
|---|---|---|---|---|
| Intercept | 8.525 | 0.076 | 112.210 | <0.0001 |
| Homogenization time | 0.107 | 0.051 | 2.100 | 0.080 |
| Ratio | 0.426 | 0.051 | 8.390 | 0.000 |
| Speed | −0.690 | 0.051 | −13.590 | <0.0001 |
| Homogenization time × Ratio | −0.205 | 0.057 | −3.610 | 0.011 |
| Homogenization time × Speed | 0.104 | 0.057 | 1.830 | 0.117 |
| Ratio × Speed | −0.265 | 0.057 | −4.670 | 0.003 |
| Homogenization time2 | −0.225 | 0.099 | −2.280 | 0.063 |
| Ratio2 | 0.112 | 0.099 | 1.140 | 0.299 |
| Speed2 | 0.605 | 0.099 | 6.130 | 0.001 |
| Suspension Volume | Diameter of the Container (cm) | Median Particle Size (µm) |
|---|---|---|
| ×1 (100 mL) | 6.1 | 8.5242 |
| ×2 (200 mL) | 7.9 | 8.4407 |
| ×3 (300 mL) | 8.9 | 8.3408 |
| ×5 (500 mL) | 12.4 | 8.5701 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, B.H.; Truong, H.N.; Phan Nguyen, Q.A.; Nguyen Phu, T.N.; Hong Nhan, L.T. Preparation of Curcumin Nanosuspension with Gum Arabic as a Natural Stabilizer: Process Optimization and Product Characterization. Processes 2020, 8, 970. https://doi.org/10.3390/pr8080970
Duong BH, Truong HN, Phan Nguyen QA, Nguyen Phu TN, Hong Nhan LT. Preparation of Curcumin Nanosuspension with Gum Arabic as a Natural Stabilizer: Process Optimization and Product Characterization. Processes. 2020; 8(8):970. https://doi.org/10.3390/pr8080970
Chicago/Turabian StyleDuong, Bao Hoang, Hoai Nam Truong, Quynh Anh Phan Nguyen, Thuong Nhan Nguyen Phu, and Le Thi Hong Nhan. 2020. "Preparation of Curcumin Nanosuspension with Gum Arabic as a Natural Stabilizer: Process Optimization and Product Characterization" Processes 8, no. 8: 970. https://doi.org/10.3390/pr8080970
APA StyleDuong, B. H., Truong, H. N., Phan Nguyen, Q. A., Nguyen Phu, T. N., & Hong Nhan, L. T. (2020). Preparation of Curcumin Nanosuspension with Gum Arabic as a Natural Stabilizer: Process Optimization and Product Characterization. Processes, 8(8), 970. https://doi.org/10.3390/pr8080970





