1. Introduction
The demand on energy is growing with the increased population and improved lifestyle. In the meantime, the volume of oil produced under natural reservoir drives is declining [
1]. Most oil reservoirs currently apply one or more improved oil recovery (IOR) mechanisms to boost their production [
2]. IOR stimulates reservoir production by means of micro- and macroscopic sweep mechanisms [
3]. Water/brine flooding is amongst the first alternatives to sustain reservoir production, owing to its relatively low cost [
4]. Water flooding is generally used in light oil reservoirs owing to its high recovery factor, favorable mobility ratio, good sweep efficiency, suitability for long-term injection, and competitive cost [
5]. Nevertheless, water flooding is ineffective for reservoirs of high oil viscosity [
6]. The big disparity in the viscosities of the displacing phase (water) and the displaced phase (oil) induce viscous fingering, which leads to an unfavorable mobility ratio and early breakthrough of the displacing fluid [
7]. Polymer flooding includes the addition of polymers to the brine as an attempt to bridge the disparity in viscosity between the injected and the reservoir fluids [
4]. More favorable mobility ratios are therefore expected relative to brine injection [
8]. Generally, polymer flooding enhances the sweep efficiency by three major mechanisms, including increasing the viscosity of the water/brine, minimizing water/brine permeability through swept areas, and covering a larger volume of the reservoir [
3]. Polymer flooding typically does not generally increase the microscopic displacement efficiency of light oils, though recent studies strongly suggest that viscoelastic polymeric solution may be able to improve the microscopic displacement efficiency relative to waterflood [
9]. In practice, two commercial polymers, partially hydrolyzed polyacrylamides (HPAM) and xanthan gums, are commonly used in oil field enhanced oil recovery (CEOR) applications. HPAM is a water-soluble polyelectrolyte with negative charges on the polymer chains. Xanthan gums, which are polysaccharides, show excellent viscosifying ability and high tolerance to salinity but degrade with an increase in temperature and are sensitive to biodegradation [
10]. Additionally, clay (bentonite) of smectite groups is being utilized in different oil field applications as well, especially in drilling fluids technology as a rheology enhancer. Recently, Abdelhady [
11] reported that the efficiency of HPAM-based polymer flooding was enhanced with the addition of clay due to its superior rheological property. Still, the widely employed HPAM and PAM polymers suffer hydrolysis and degradation under high temperature, mechanical stress, and salinity conditions [
12,
13,
14,
15,
16]. Polymer molecular chains undergo scission, leading to a great reduction in the polymer viscosity [
17,
18]. Hence, a high concentration of polymer is utilized, which incurs huge additional cost [
19]. In addition, the reservoir heterogeneity leads to extraneous water channeling from different layers to the oil swept zone and also polymer loss to the adjacent zone [
2].
The application of nanotechnology in the petroleum industry has evolved significantly over the past decade. Nevertheless, the use of NPs in CEOR field application is very limited. The advantages of combining polymer flooding with NPs, i.e., nanohybrid, including rheology enhancement, salt tolerance, thermal stability, shear tolerance, and other rock–fluid/fluid–fluid interactions [
3]. Still, results suggest that some nanohybrids, e.g., SiO
2/HPAM, suffer from reduced viscosity with an increasing temperature and salt concentration due to thermal degradation and the accumulation of the sodium cations onto the amide group, which leads to shrinkage of the polymer chains and the minimization of crosslinking [
16,
20]. Metallic NPs have been used extensively in the development of HPAM water-based drilling fluids [
21,
22]. Nevertheless, limited studies have addressed metallic oxide NP’s performance for CEOR applications [
23]. Shah [
24] performed laboratory CEOR experiments using CuO NPs for oil with an API gravity of 15 in a Berea sandstone core. The rheological properties improved and 1 wt.% CuO NP achieved a 71% oil recovery by virtue of the enhanced hydrophilic characteristics of the nanofluid [
25]. It was suggested that if the electronegativity difference between the metal and the oxygen atoms is in between 0.5 and 1.7, the metal oxide is considered polar or hydrophilic, and if the electronegativity difference is < 0.5, the metal oxide is considered non-polar or hydrophobic [
25]. For CuO, the electronegativity difference is 1.5 [
26]; hence, cupric oxide is hydrophilic and effectively disperses in water. The addition of CuO to a polymer solution should, in principle, enhance the temperature and the salt tolerance, as confirmed by published studies [
21,
22,
24].
Another issue relating to HPAM polymer flooding is the tendency of the polymer for adsorption onto the formation. Although adsorption of the polymer may mitigate reservoir heterogeneity and act as a conformance control agent, polymer retention leads to a reduction in fluid viscosity, irreversibly reservoir damage [
27], and loss of polymer. It is noted that, depending on the size of polymer molecules, their aggregation state, and the rheology of the polymer solution, some polymer molecules are trapped, rather than adsorbed, within reservoir pores. Adsorption of polymer molecules onto NPs, or vice versa, may mitigate the risk of polymer molecules’ retention [
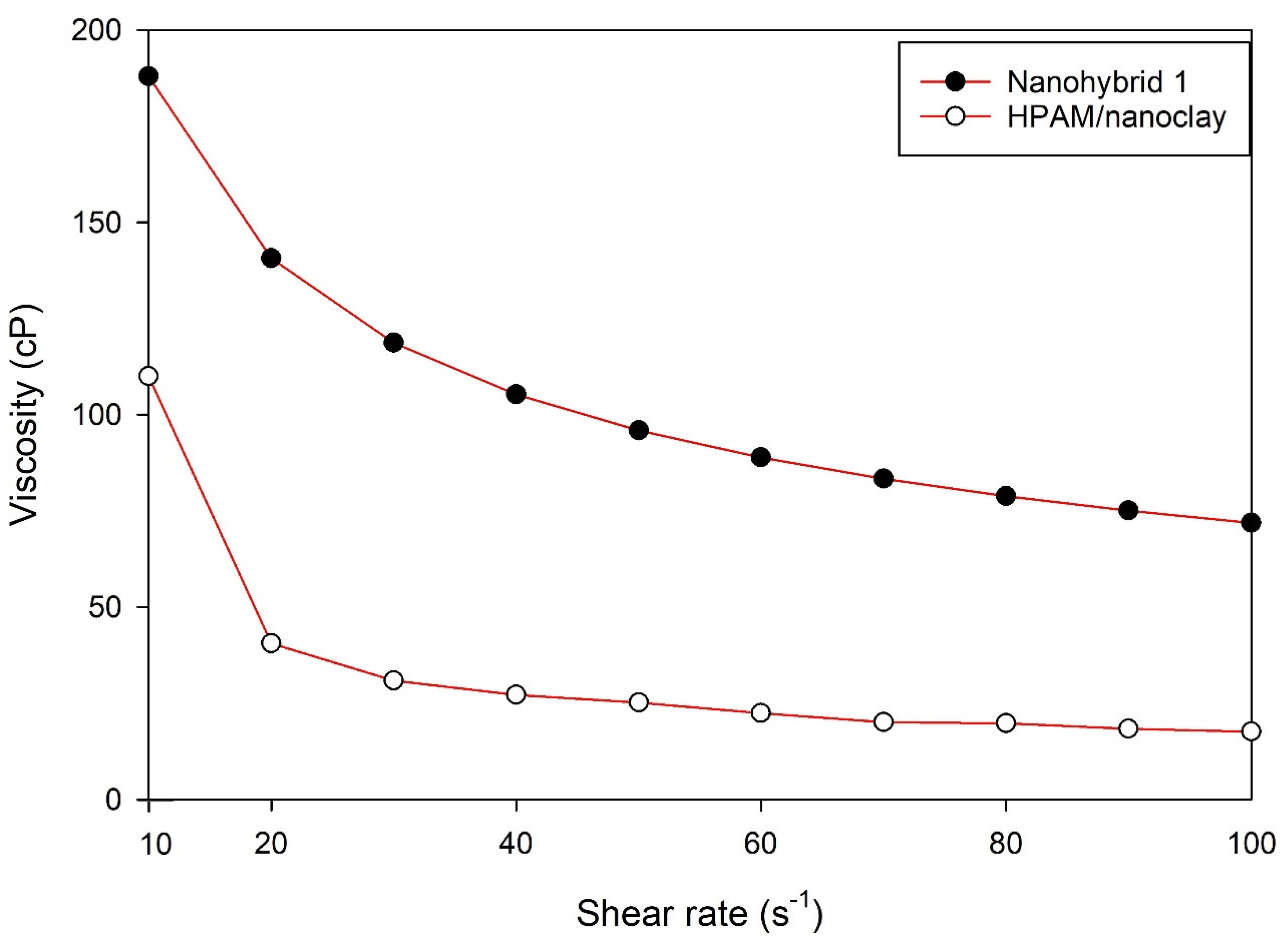
3]. Nanoclay, on the other hand, aids in proper plugging of undesired channels, i.e., profile modification, and helps to maintain proper polymer rheology owing to its effective interaction with HPAM molecules [
20,
28]. Additionally, nanoclay works as a filler in the polymer matrix to enhance its mechanical strength. Recently, Tiwari and their group [
29] reported that nanoclay can be used as a loss circulation agent for drilling fluids and can enhance the rheology without increasing the weight of the fluid. Kumar et al. [
23] developed a novel grafted copolymer onto clay/CuO and explored its application in drilling shale wells under harsh reservoir conditions. Thus, formulating a nanohybrid system consisting of nanoclay in addition to CuO and HPAM polymer can be a better alternative to overcome reservoir heterogeneity and a harsh environment.
In the current study, nanohybrid systems comprising of a blend of nanoclay and CuO NPs together with HPAM polymer were tested with the objective of improving the rheological properties of HPAM as well as its thermal and salt tolerance. The preparation steps of the nanohybrid are convenient and not too exhaustive, which promotes the field applicability of the system. Control samples consisting of HPAM were tested in the same conditions in order to isolate the role of the nano-additives. Furthermore, oil recovery tests were carried out in sandpacks at 85 °C and a salt concentration of 1 wt.% and a comparison between the nanohybrid performance and HPAM was made.
4. Conclusions
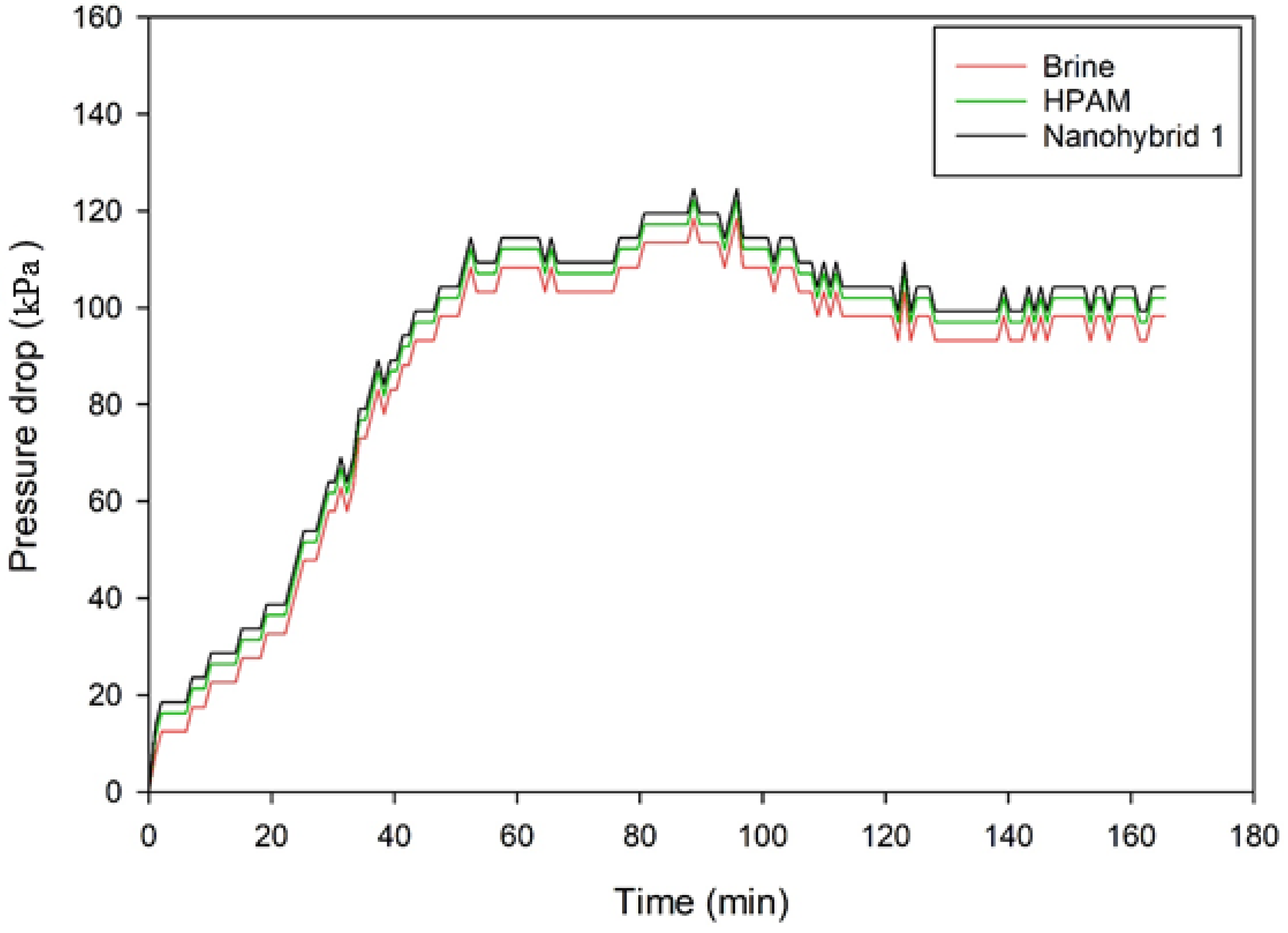
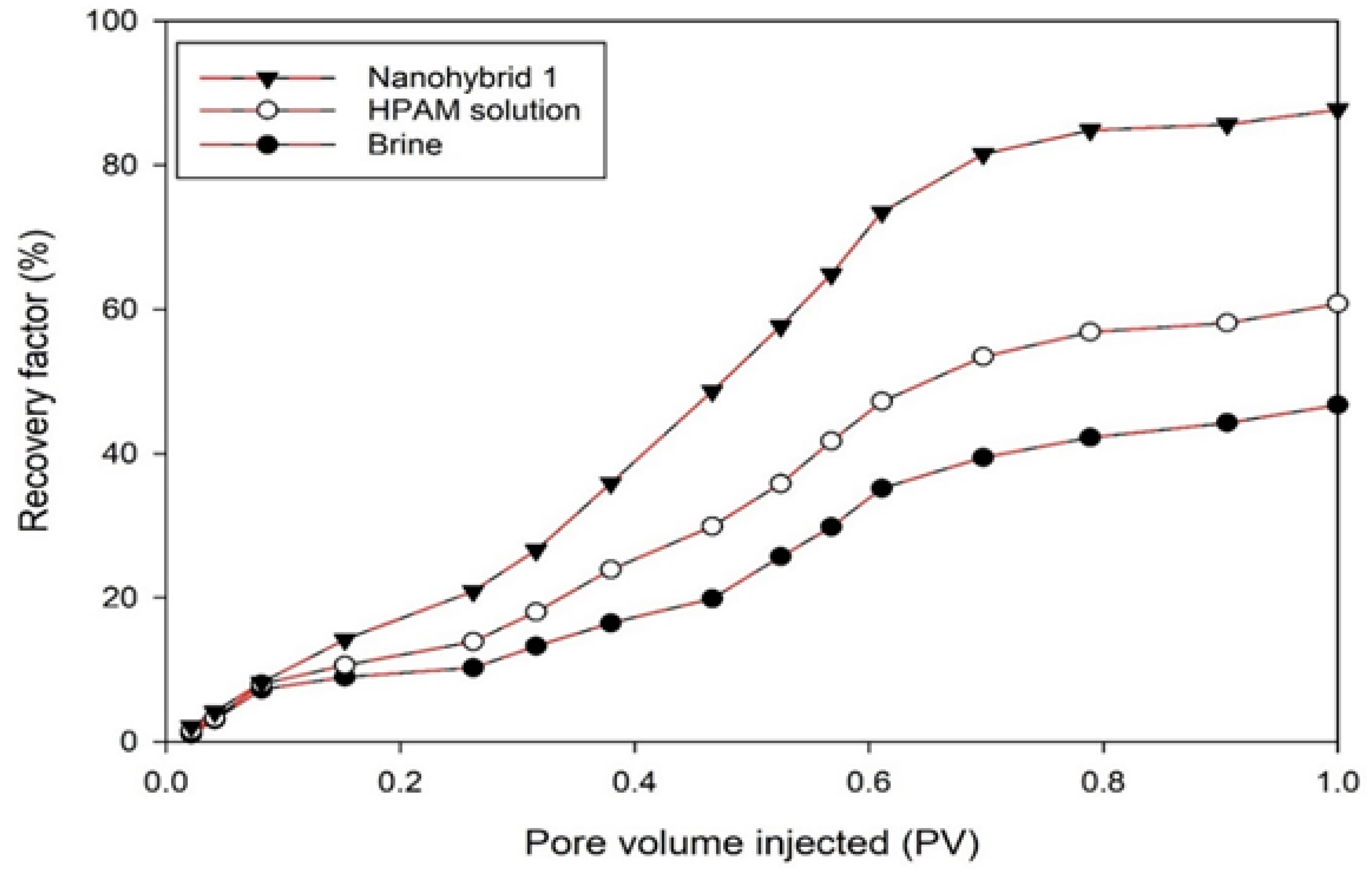
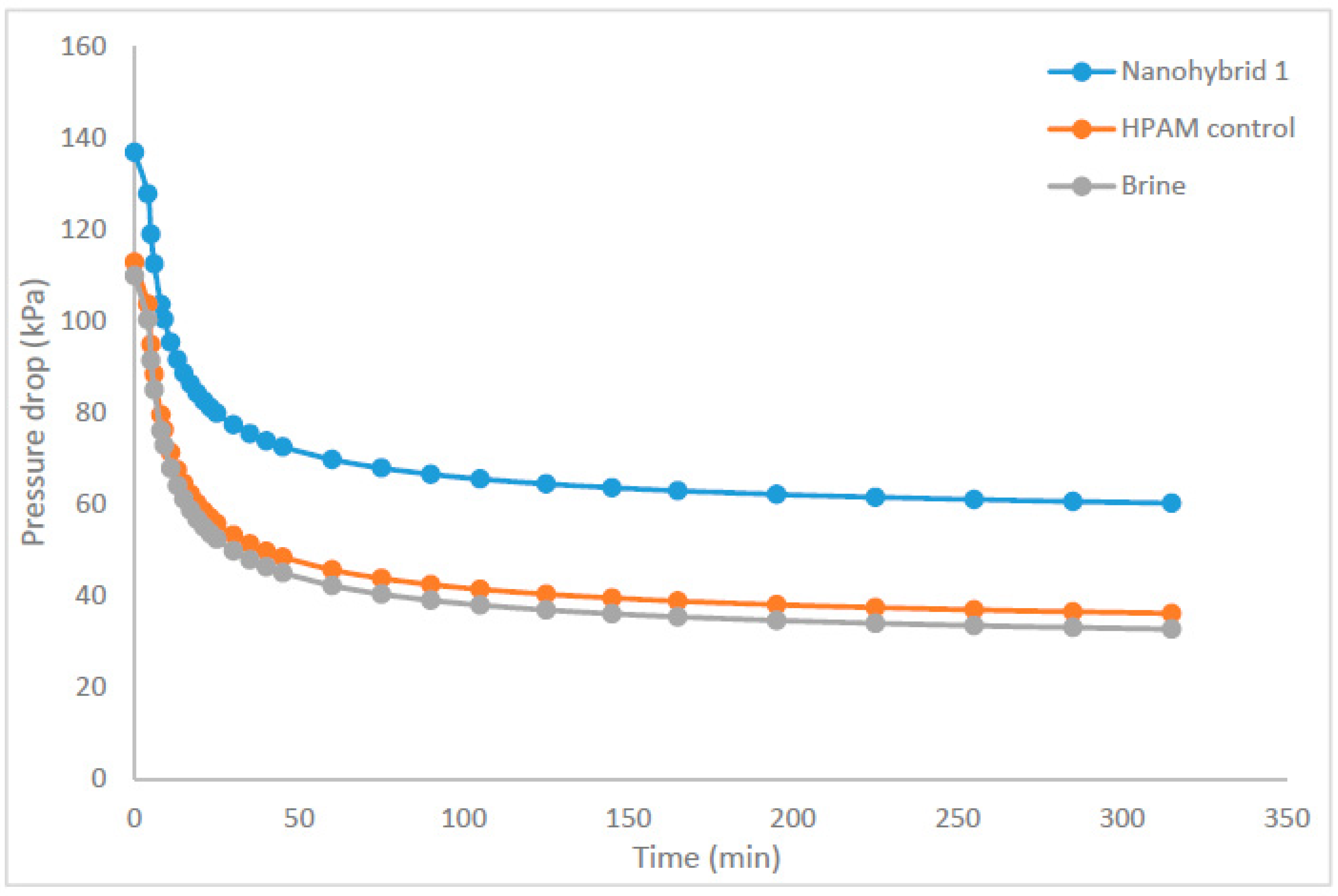
The rheological properties of a newly formulated nanohybrid composed of CuO NPs, nanoclay, and HPAM polymer were investigated under various conditions, including the additive content, salinity, temperature, and shear rate. Sandpack experiments were used to determine the recovery ratio among the nanohybrid system, HPAM polymer solution, and brine injection. The flooding tests were conducted in a linear sandpack at simulated reservoir conditions, i.e., ~2413.2 kPa of injection pressure and 85°C. Based on the analysis of the results, the following conclusions can be drawn:
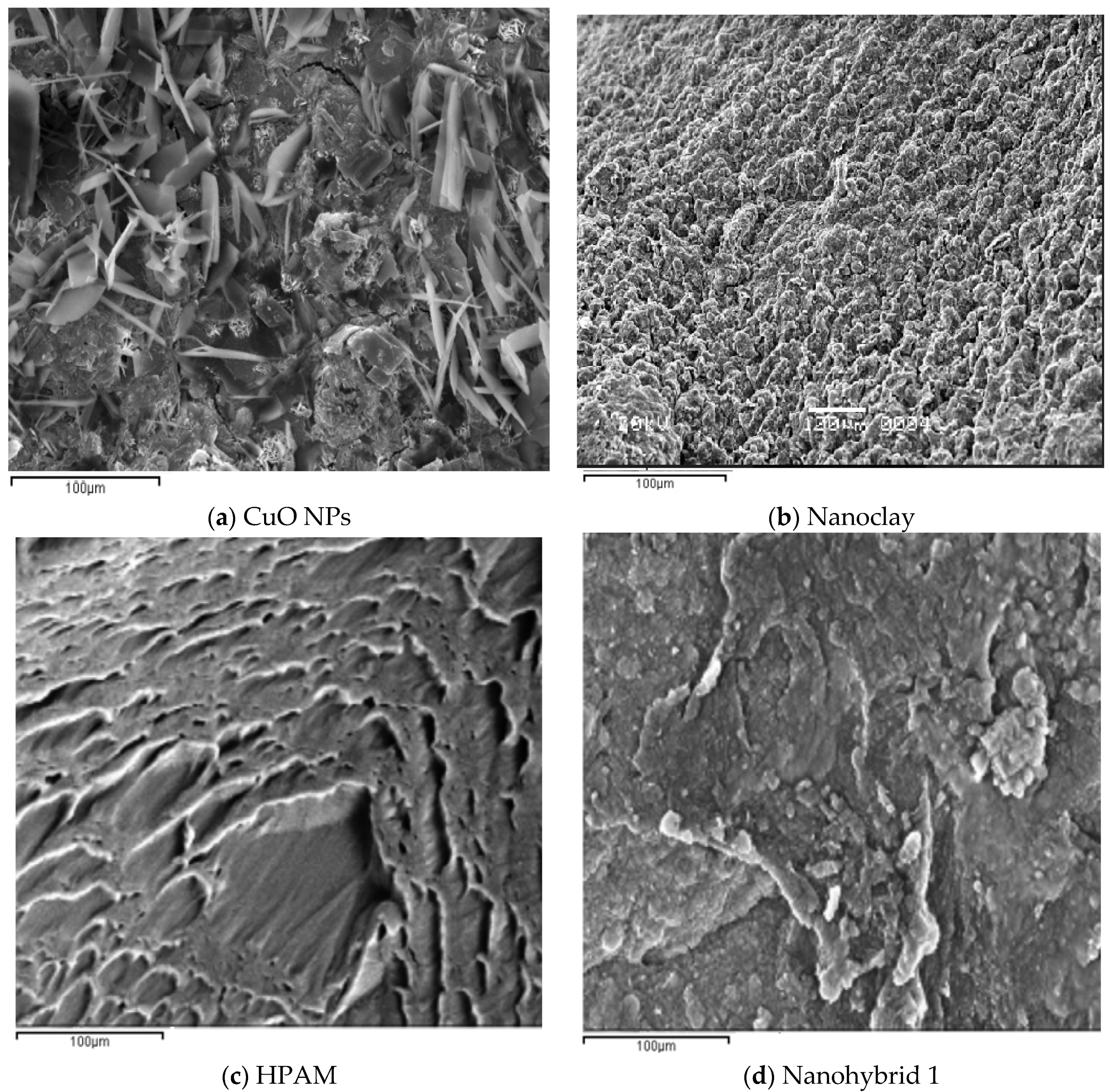
SEM images of the nanohybrid suggests that the morphology of the HPAM surface was changed due to the adsorption of CuO NPs and dispersion of nanoclay in the HPAM matrix, which promoted the entanglement of the polymer chains even at high temperatures and salt concentrations. Subsequently, the nanohybrid system maintained proper rheological properties, which in turn resulted in piston-like displacement of the oil and increased the recovery ratio/factor.
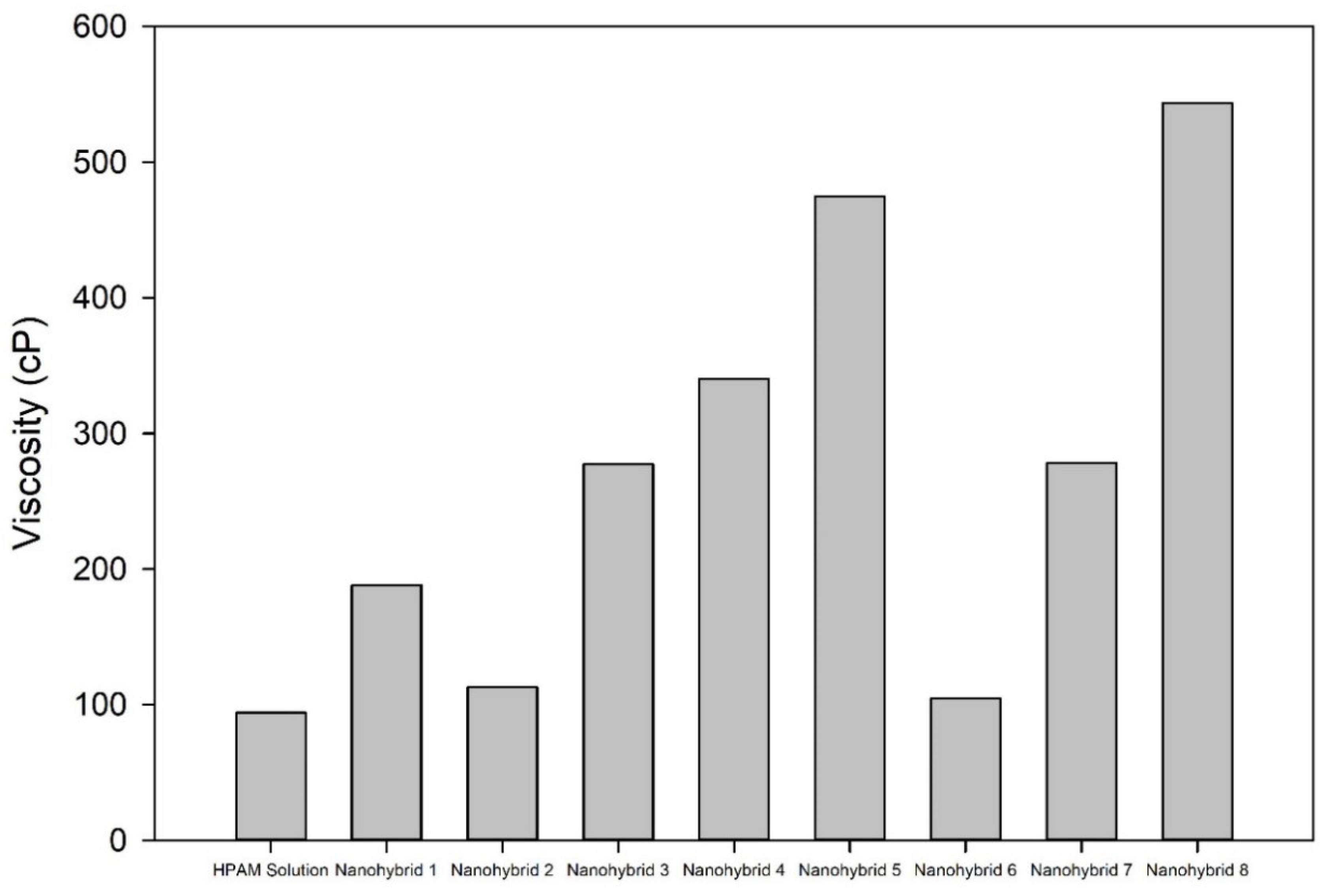
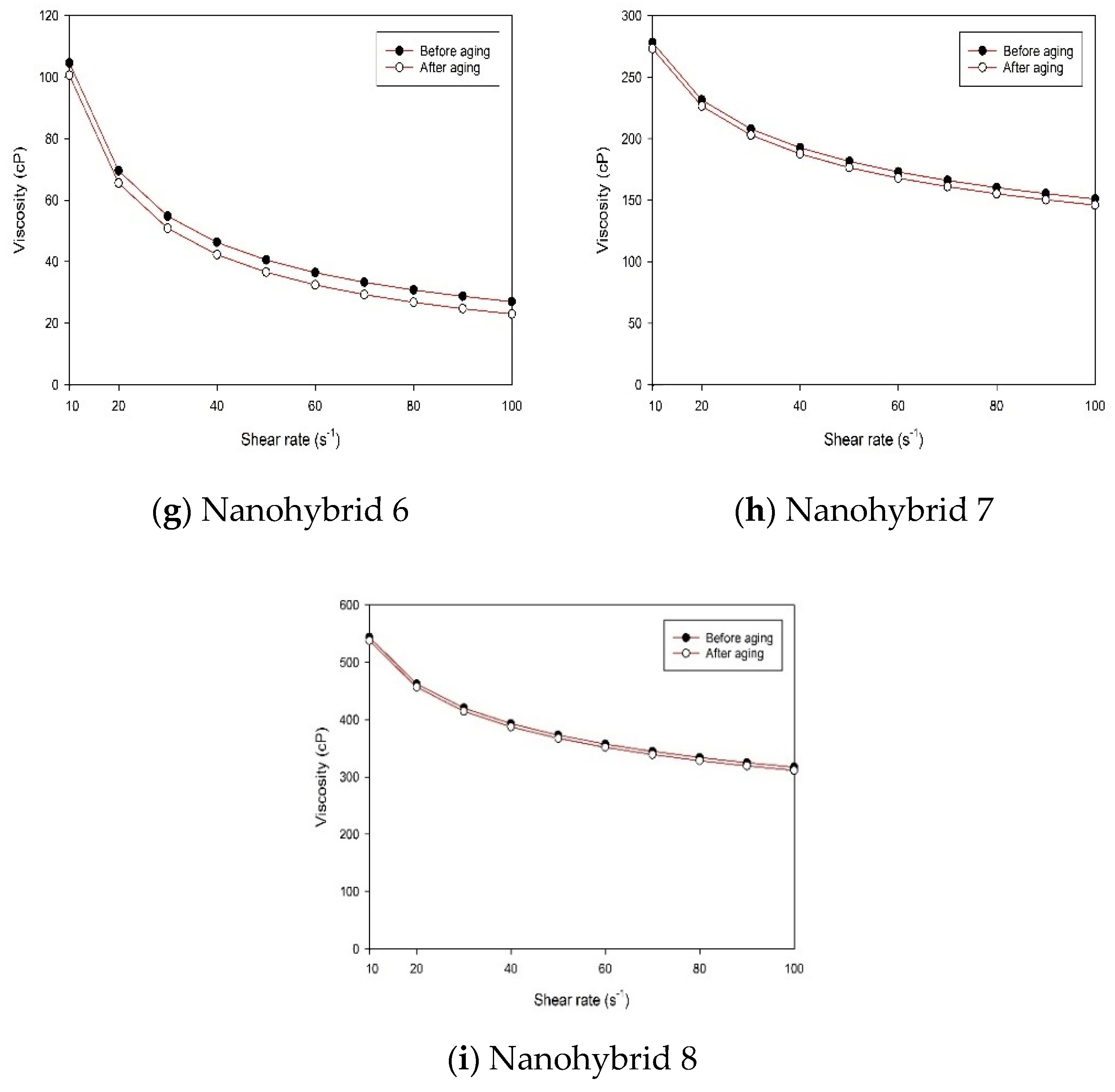
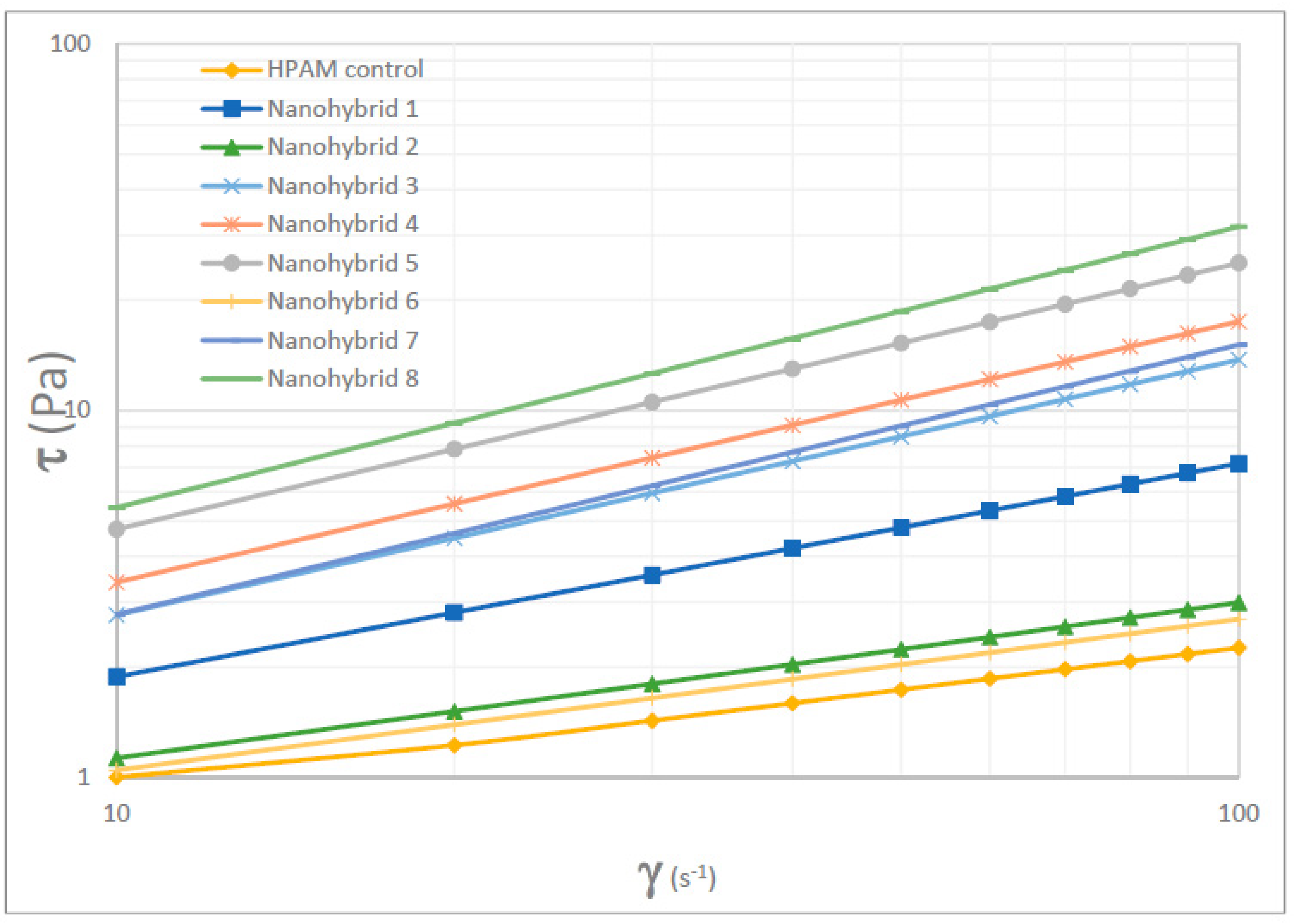
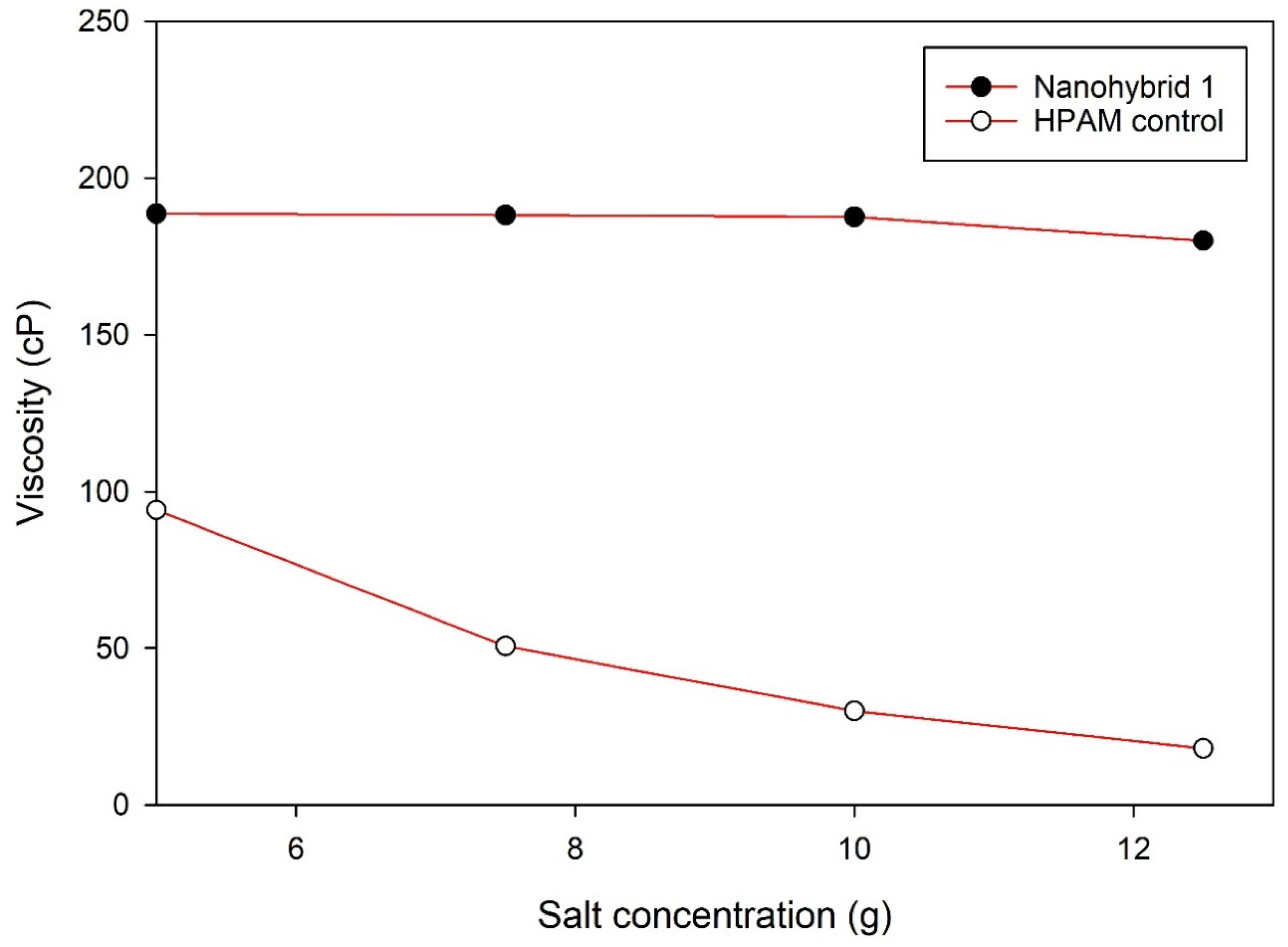
The rheological properties of the nanohybrid system depended on the content of the NPs (both CuO and nanoclay). Nanohybrid 1, consisting of 2.5 g of HPAM, 5 g of KCl, 0.5 g of nanoclay, and 0.2 g of CuO NPs in 500 mL of DI water, provided the most appropriate viscosity, which provides a suitable mobility ratio and scaling coefficient, while avoiding injectivity issues.
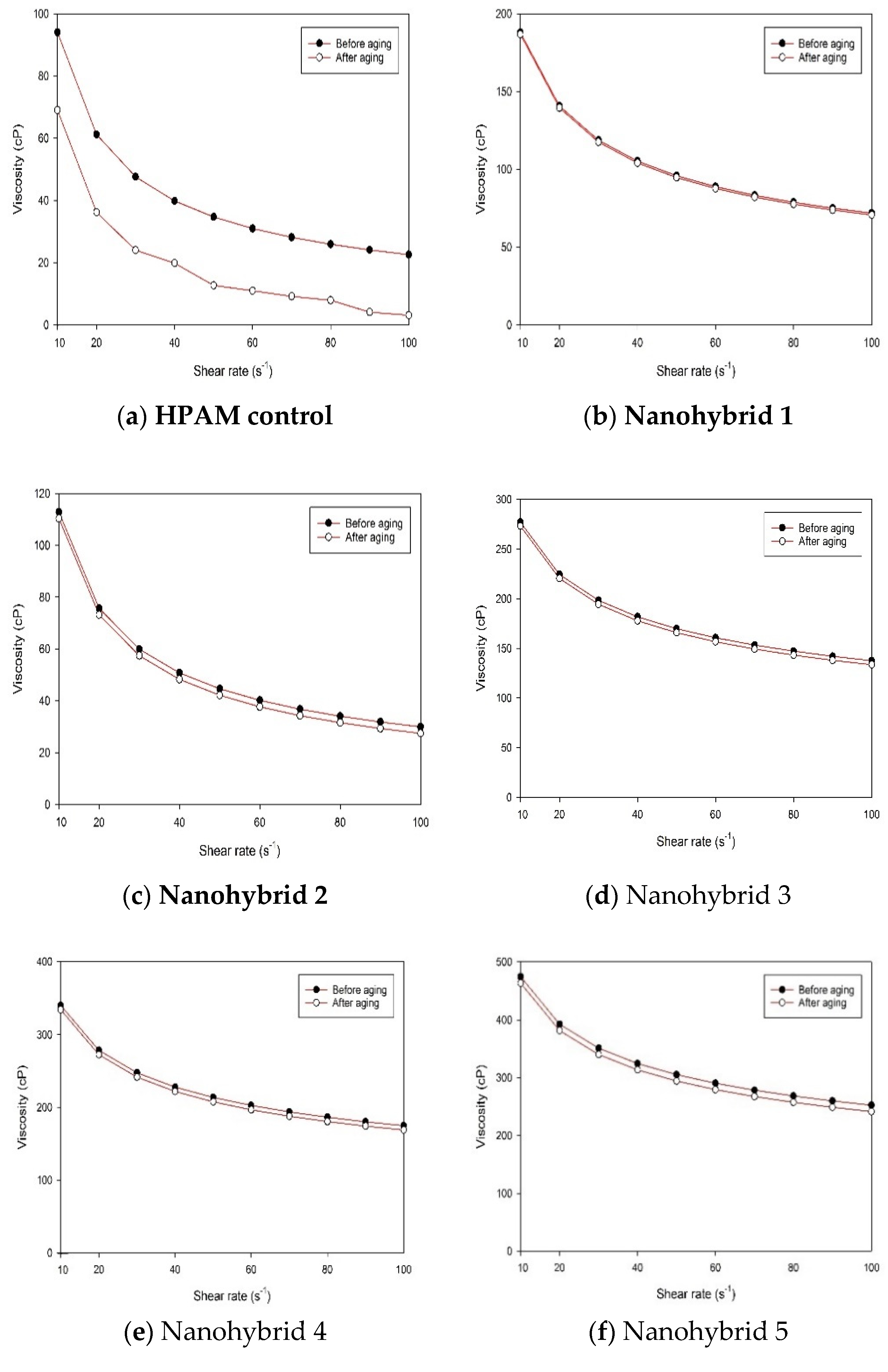
Combining CuO NPs and nanoclay helped to maintain higher viscosity at different shear rates, relative to the sole nanoclay additive or HPAM solution.
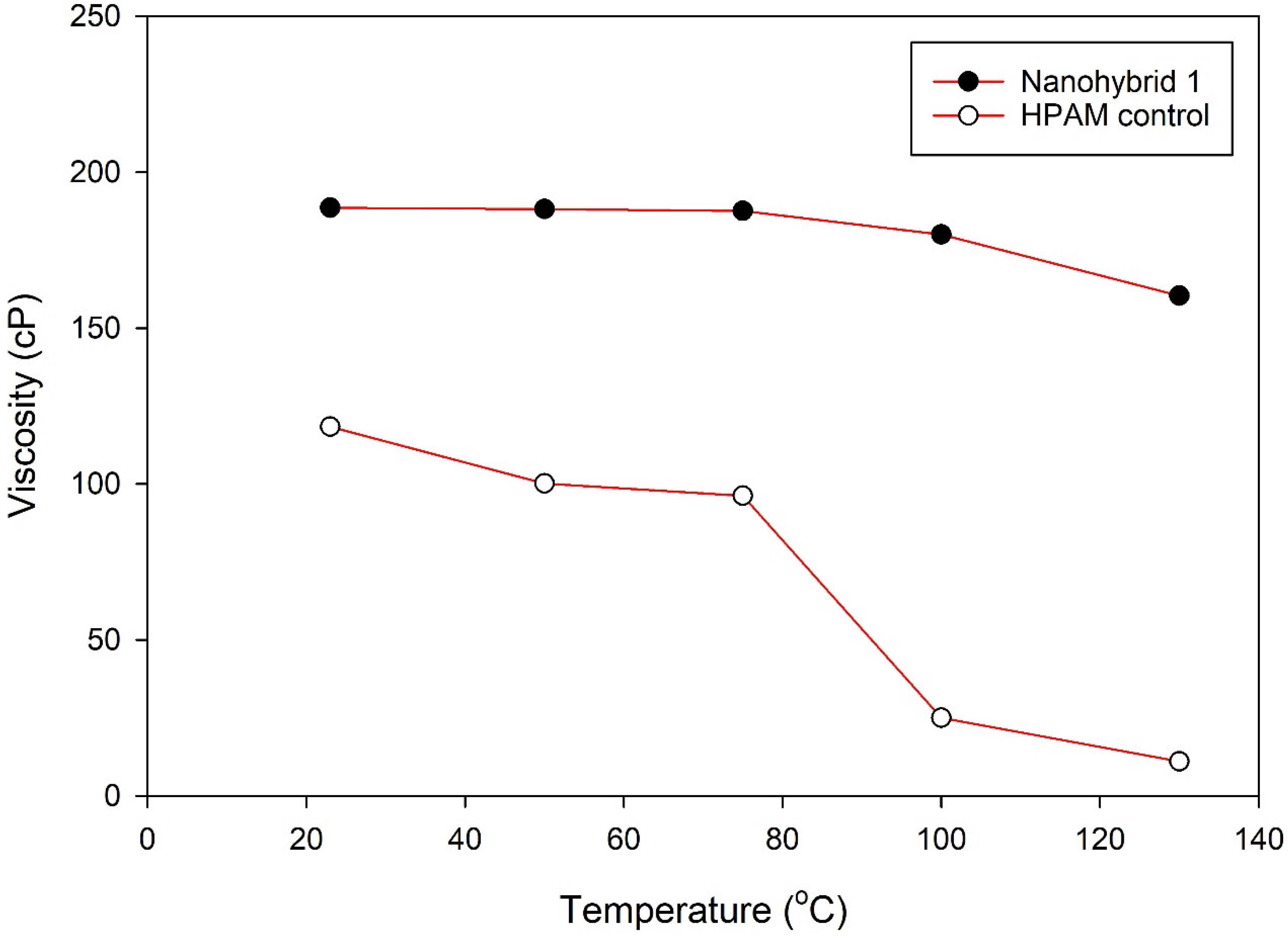
The combined effect of CuO NPs and nanoclay preserved the HPAM polymer from thermal and mechanical degradation and contributed to the superior viscoelastic property, in addition to the much desired pseudoplastic behavior.
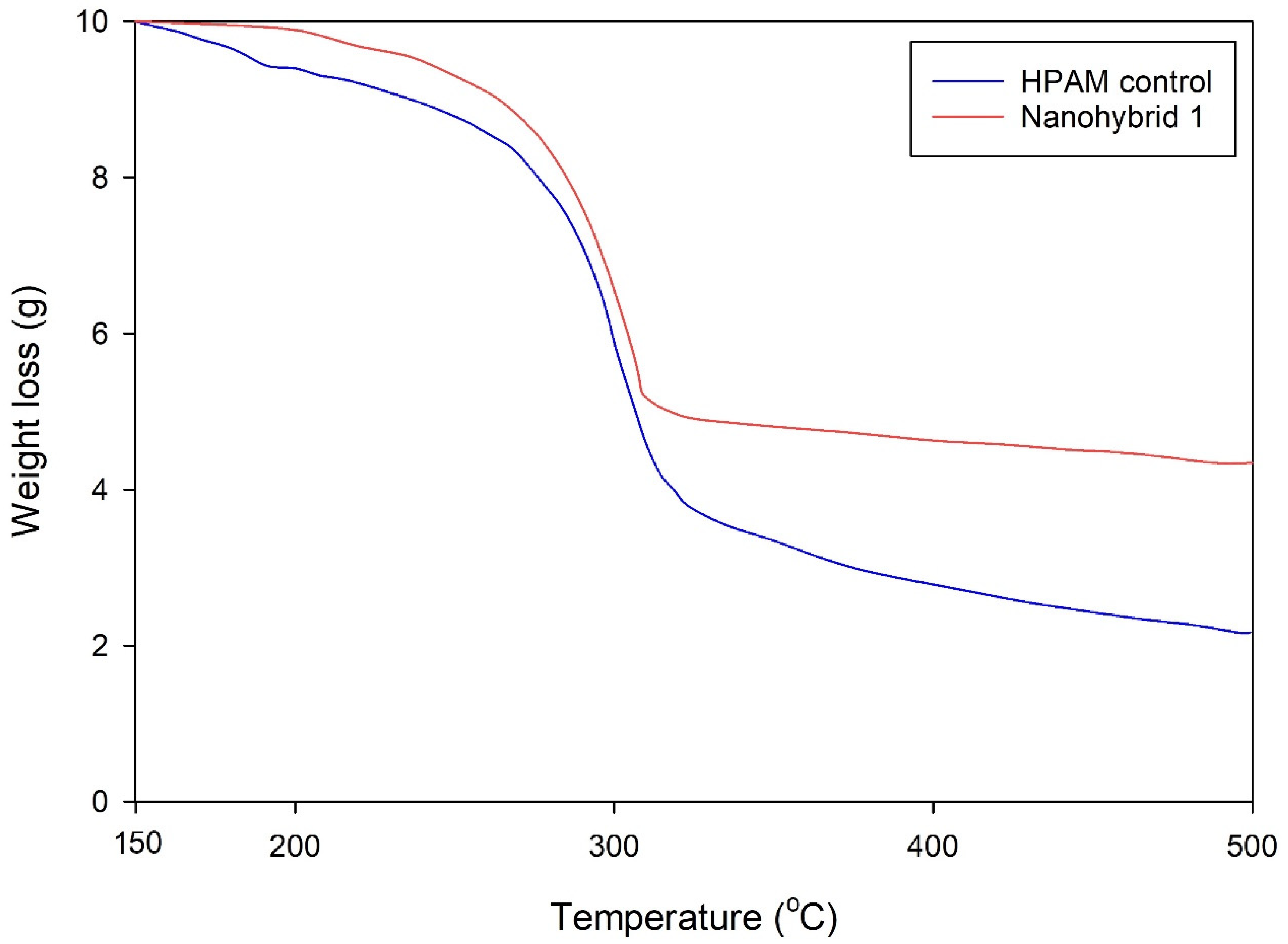
The TGA results suggested that the nanohybrid system displayed remarkable temperature stability relative to HPAM polymer and potential applicability to a high-temperature reservoir without compromising the rheological properties of the flooding agent.