Application of Detrended Fluctuation Analysis and Yield Stability Index to Evaluate Near Infrared Spectra of Green and Roasted Coffee Samples
Abstract
1. Introduction
- −
- Introduce new tools for the analysis of near infrared spectra;
- −
- Differentiate coffee samples based on their roasting levels using detrended fluctuation analysis.
2. Materials and Methods
2.1. Samples
2.2. Roasting Experiment
2.3. Near-Infrared Spectroscopy (NIRS)
2.4. Principal Component Analysis (PCA)
- Estimation of the correction coefficients (additive and multiplicative contributions),
- Correcting the recorded spectrum,where xorg is one original sample spectra measured by the NIR instrument, xref is a reference spectrum used for pre-processing of the entire dataset, e is the un-modeled part of xorg, xcorr is the corrected spectra, and b0 and bref,1 are scalar parameters, which differ for each sample. In most applications, the average spectrum of the calibration set is used as the reference spectrum [38]. PCA and MSC were performed using Unscrambler X 10.4 (ver. 10.4, CAMO Software AS, Oslo, Viken, Norway, 2016) software.
2.5. Agglomerative Hierarchical Clustering (AHC)
2.6. Multiple Correspondence Analysis (MCA)
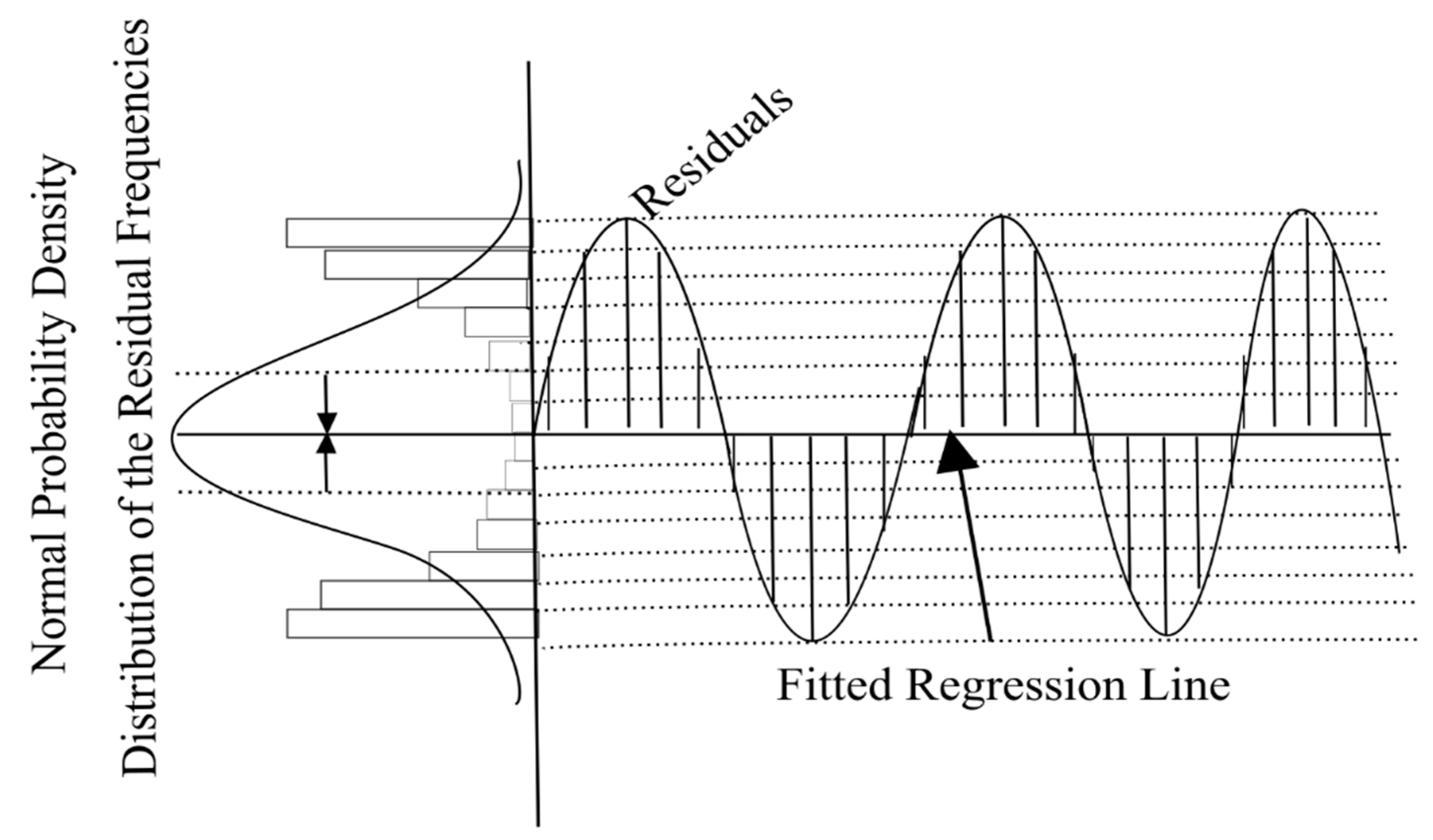
2.7. Detrended Fluctuation Analysis (DFA)
2.8. Yield Stability Index
3. Results
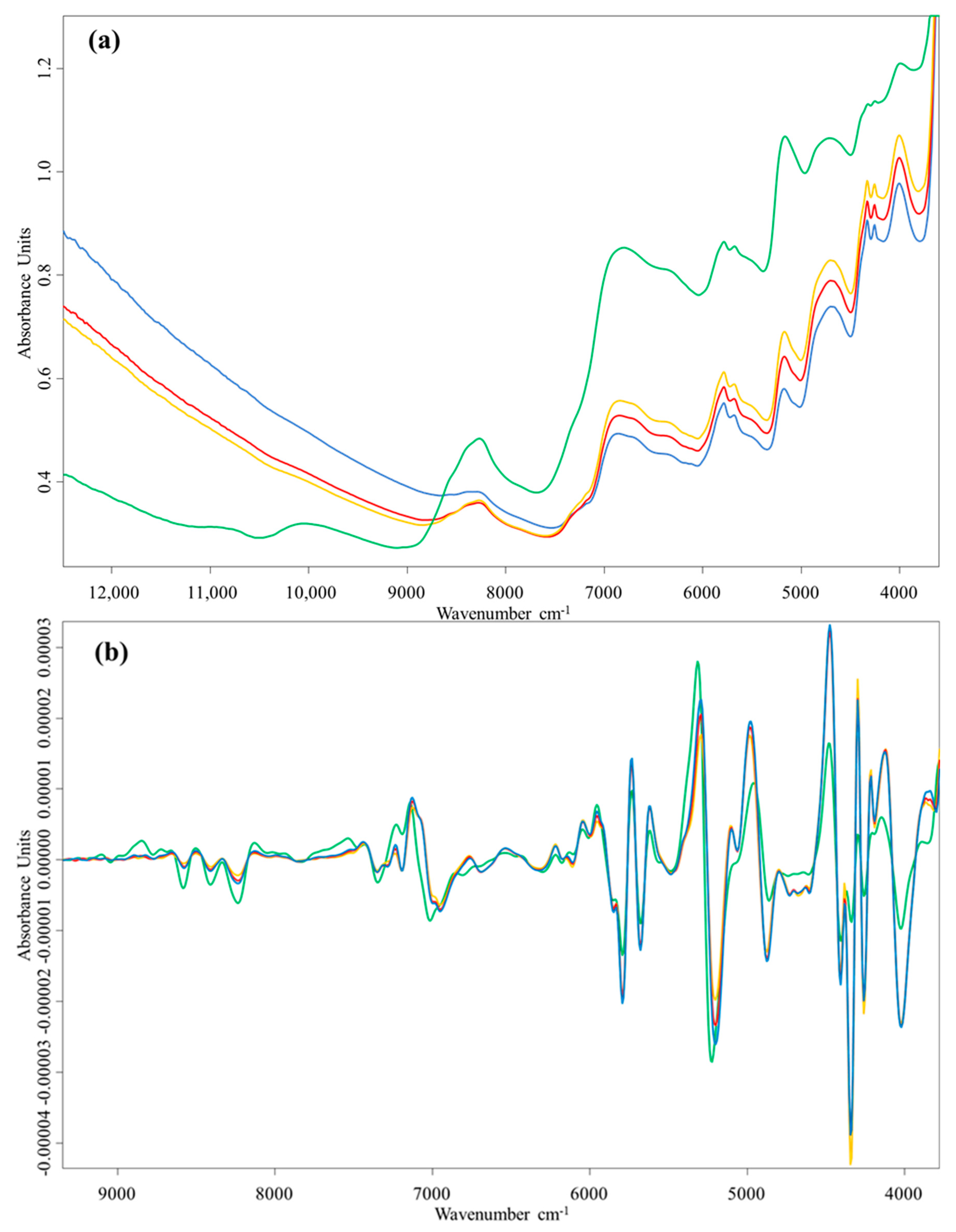
3.1. Spectral Characteristics
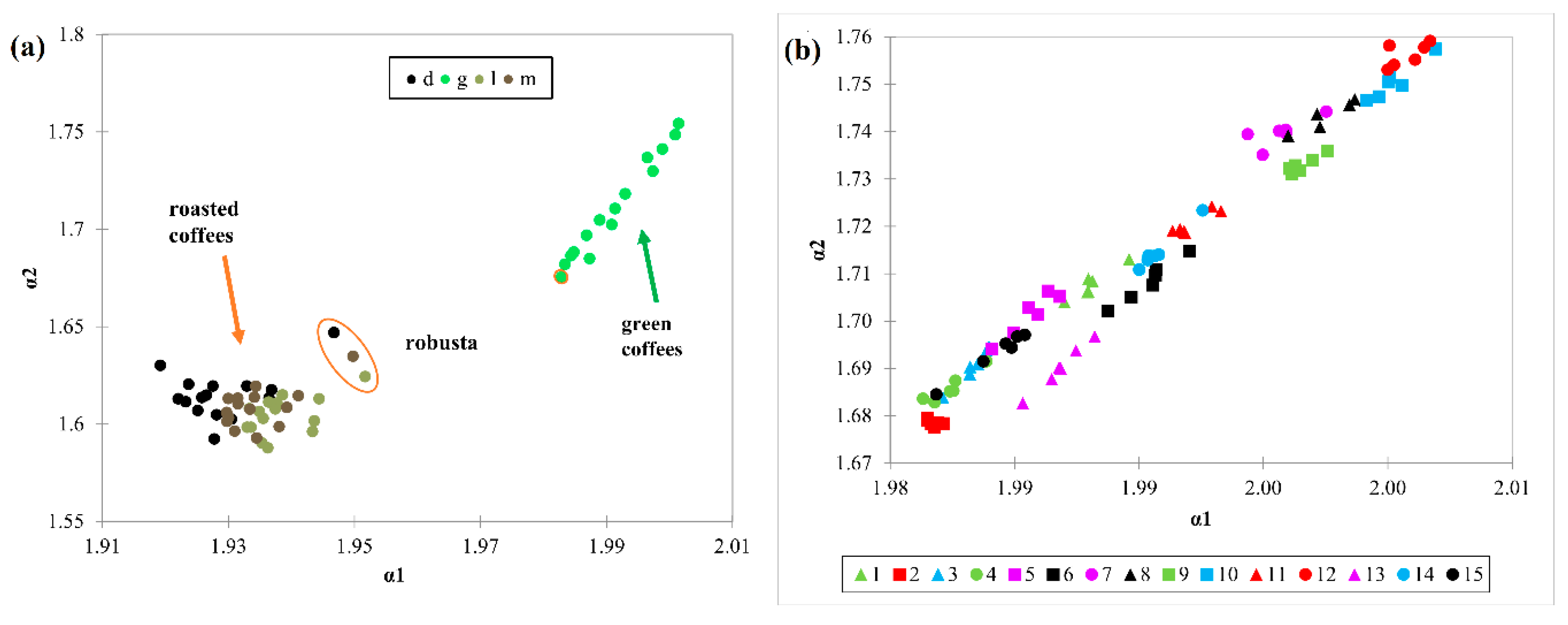
3.2. Principal Component Analysis (PCA)
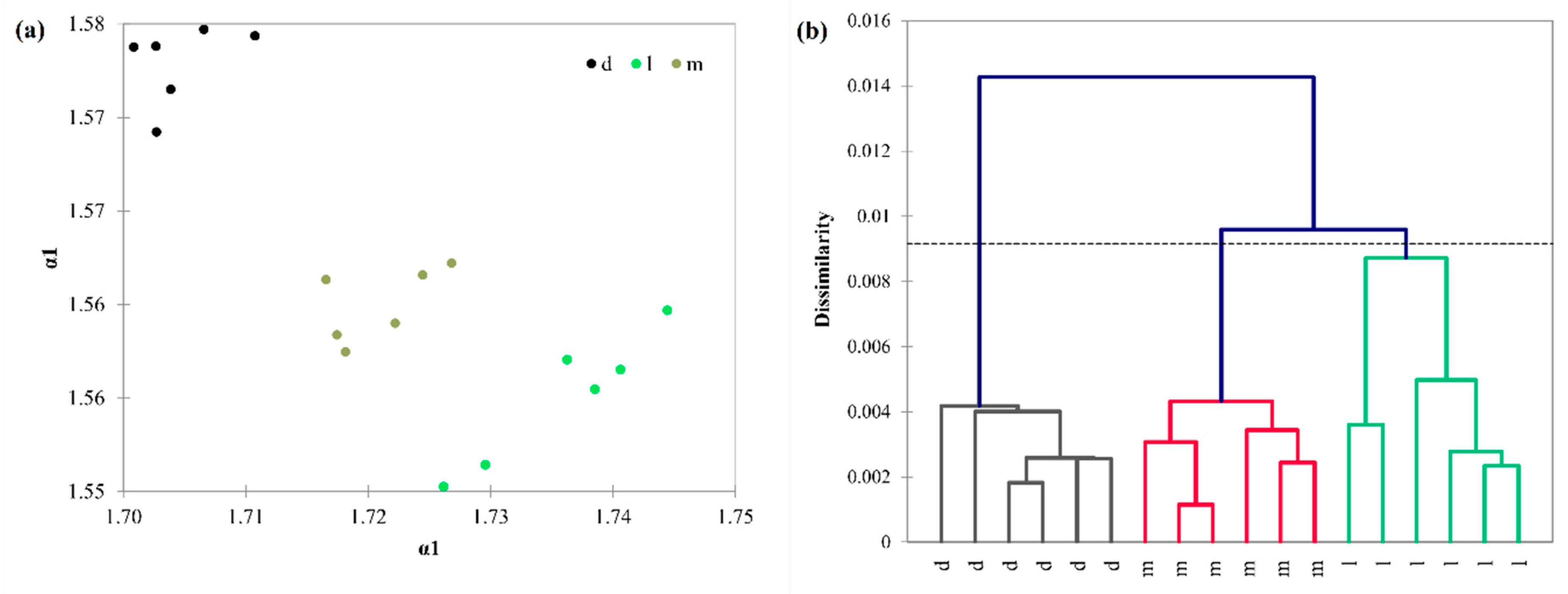
3.3. Detrended Fluctuation Analysis
3.4. Yield Stability Index
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farah, A. Coffee: Production, Quality and Chemistry; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; ISBN 9781782620044. [Google Scholar]
- Malta, M.R.; de Fassio, L.O.; Liska, G.R.; Carvalho, G.R.; Pereira, A.A.; Botelho, C.E.; Ferraz, V.P.; Silva, A.D.; Pedrosa, A.W.; Alvaro, L.N.; et al. Discrimination of genotypes coffee by chemical composition of the beans: Potential markers in natural coffees. Food Res. Int. 2020, 134, 109219. [Google Scholar] [CrossRef]
- Preedy, V.R. Coffee in Health and Disease Prevention; Academic Press: London, UK, 2014; ISBN 9780124167162. [Google Scholar]
- International Coffee Organization. ICO Indicator Prices—May 2020; International Coffee Organization: London, UK, 2020; p. 1. Available online: http://www.ico.org/prices/p1-May2020.pdf (accessed on 24 June 2020).
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Barbin, D.F.; Felicio, A.L.; Sun, D.W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- Benes, E.; Gere, A.; Fodor, M. Predicting macronutrients and energy content of snack products using FT-NIR analysis and chemometric techniques. J. Food Eng. 2020, 280, 109954. [Google Scholar] [CrossRef]
- Fodor, M.; Woller, A.; Turza, S.; Szigedi, T. Development of a rapid, non-destructive method for egg content determination in dry pasta using FT-NIR technique. J. Food Eng. 2011, 107, 195–199. [Google Scholar] [CrossRef]
- Mikola, E.; Geösel, A.; Stefanovits-Bányai, É.; Fodor, M. Quantitative determination of macro components and classification of some cultivated mushrooms using near-infrared spectroscopy. J. Food Process. Preserv. 2020, 44, e14540. [Google Scholar] [CrossRef]
- Shan, J.; Suzuki, T.; Suhandy, D.; Ogawa, Y.; Kondo, N. Chlorogenic acid (CGA) determination in roasted coffee beans by Near Infrared (NIR) spectroscopy. Eng. Agric. Environ. Food 2014, 7, 139–142. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Machado, S.; Segundo, M.A.; Lopes, J.A.; Páscoa, R.N.M.J. Rapid assessment of bioactive phenolics and methylxanthines in spent coffee grounds by FT-NIR spectroscopy. Talanta 2016, 147, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Botelho, B.G.; Oliveira, L.S.; Franca, A.S. Mid infrared spectroscopy and chemometrics as tools for the classification of roasted coffees by cup quality. Food Chem. 2018, 245, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- De Luca, S.; De Filippis, M.; Bucci, R.; Magrì, A.D.; Magrì, A.L.; Marini, F. Characterization of the effects of different roasting conditions on coffee samples of different geographical origins by HPLC-DAD, NIR and chemometrics. Microchem. J. 2016, 129, 348–361. [Google Scholar] [CrossRef]
- Alessandrini, L.; Romani, S.; Pinnavaia, G.; Rosa, M.D. Near infrared spectroscopy: An analytical tool to predict coffee roasting degree. Anal. Chim. Acta 2008, 625, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Díez, I.; González-Sáiz, J.M.; Pizarro, C. An evaluation of orthogonal signal correction methods for the characterisation of arabica and robusta coffee varieties by NIRS. Anal. Chim. Acta 2004, 514, 57–67. [Google Scholar] [CrossRef]
- Bertone, E.; Venturello, A.; Giraudo, A.; Pellegrino, G.; Geobaldo, F. Simultaneous determination by NIR spectroscopy of the roasting degree and Arabica/Robusta ratio in roasted and ground coffee. Food Control 2016, 59, 683–689. [Google Scholar] [CrossRef]
- Giraudo, A.; Grassi, S.; Savorani, F.; Gavoci, G.; Casiraghi, E.; Geobaldo, F. Determination of the geographical origin of green coffee beans using NIR spectroscopy and multivariate data analysis. Food Control 2019, 99, 137–145. [Google Scholar] [CrossRef]
- Jesztl, B.; Benes, E.; Fodor, M. FT-NIR origin identification of coffee samples. J. Food Investig. 2019, 65, 2360–2377. [Google Scholar]
- Kárpáti, Z.; Benes, E.; Fodor, M. Nutritional analysis of coffee dregs for utilization purposes using classical, ICP-OES and FT-NIR techniques. J. Food Investig. 2018, 64, 2178–2183. [Google Scholar]
- Tolessa, K.; Rademaker, M.; De Baets, B.; Boeckx, P. Prediction of specialty coffee cup quality based on near infrared spectra of green coffee beans. Talanta 2016, 150, 367–374. [Google Scholar] [CrossRef]
- Esteban-Díez, I.; González-Sáiz, J.M.; Pizarro, C. Prediction of sensory properties of espresso from roasted coffee samples by near-infrared spectroscopy. Anal. Chim. Acta 2004, 525, 171–182. [Google Scholar] [CrossRef]
- Barbosa, M.D.; dos Santos Scholz, M.B.; Kitzberger, C.S.; de Toledo Benassi, M. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Ferreira, M.M.C.; Salva, T.J.G. Chemometric models for the quantitative descriptive sensory analysis of Arabica coffee beverages using near infrared spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef]
- Márquez, C.; López, M.I.; Ruisánchez, I.; Callao, M.P. FT-Raman and NIR spectroscopy data fusion strategy for multivariate qualitative analysis of food fraud. Talanta 2016, 161, 80–86. [Google Scholar] [CrossRef]
- Grassi, S.; Amigo, J.M.; Lyndgaard, C.B.; Foschino, R.; Casiraghi, E. Beer fermentation: Monitoring of process parameters by FT-NIR and multivariate data analysis. Food Chem. 2014, 155, 279–286. [Google Scholar] [CrossRef] [PubMed]
- del Río, V.; Callao, M.P.; Larrechi, M.S.; de Espinosa, L.M.; Ronda, J.C.; Cádiz, V. Chemometric resolution of NIR spectra data of a model aza-Michael reaction with a combination of local rank exploratory analysis and multivariate curve resolution-alternating least squares (MCR-ALS) method. Anal. Chim. Acta 2009, 642, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Wang, Y.; Li, L.; Shen, S.; Deng, W.-W.; Zhang, Z.; Ning, J. Intelligent evaluation of black tea fermentation degree by FT-NIR and computer vision based on data fusion strategy. LWT 2020, 125, 109216. [Google Scholar] [CrossRef]
- Radványi, D.; Gere, A.; Sipos, L.; Kovács, S.; Jókai, Z.; Fodor, P. Discrimination of mushroom disease-related mould species based solely on unprocessed chromatograms. J. Chemom. 2016, 30, 197–202. [Google Scholar] [CrossRef]
- Hernández, J.; Galaviz, D.F.; Torres, L.; Palacio-Pérez, A.; Rodríguez-Valdés, A.; Guzmán, J.E.V. Evolution of high-viscosity gas-liquid flows as viewed through a detrended fluctuation characterization. Processes 2019, 7, 822. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, K.; Lin, Y.; Yin, H.; Hou, D.; Yu, J.; Huang, P.; Zhang, G. An Online Contaminant Classification Method Based on MF-DCCA Using Conventional Water Quality Indicators. Processes 2020, 8, 178. [Google Scholar] [CrossRef]
- Pavlov, A.N.; Runnova, A.E.; Maksimenko, V.A.; Pavlova, O.N.; Grishina, D.S.; Hramov, A.E. Detrended fluctuation analysis of EEG patterns associated with real and imaginary arm movements. Phys. A Stat. Mech. Its Appl. 2018, 509, 777–782. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Zhao, Y.; Zhang, X. Multifractal detrended fluctuation analysis on air traffic flow time series: A single airport case. Phys. A Stat. Mech. Its Appl. 2019, 531, 121790. [Google Scholar] [CrossRef]
- Ferreira, P. What detrended fluctuation analysis can tell us about NBA results. Phys. A Stat. Mech. Its Appl. 2018, 500, 92–96. [Google Scholar] [CrossRef]
- Vizvári, B.; Bacsi, Z. Technological development and the stability of technology in crop production. J. Cent. Eur. Agric. 2002, 3, 63–72. [Google Scholar]
- Bacsi, Z.; Hollósy, Z. A yield stability index and its application for crop production. Analecta Tech. Szeged. 2019, 13, 11–20. [Google Scholar] [CrossRef]
- Chau, F.-T.; Liang, Y.-Z.; Gao, J.; Shao, X.-G. Chemometrics: From Basics to Wavelet Transform; Winefordner, J.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 978-0-471-45473-1. [Google Scholar]
- Gemperline, P. Practical Guide to Chemometrics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 1574447831. [Google Scholar]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Greenacre, M.; Blasius, J. Multiple Correspondence Analysis and Related Methods, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2006; ISBN 9780429141966. [Google Scholar]
- Lan, T.H.; Gao, Z.Y.; Abdalla, A.N.; Cheng, B.; Wang, S. Detrended fluctuation analysis as a statistical method to study ion single channel signal. Cell Biol. Int. 2008, 32, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef] [PubMed]
- Papp, F.; Hajdu, P.; Tajti, G.; Toth, A.; Nagy, E.; Fazekas, Z.; Kovacs, S.; Vámosi, G.; Varga, Z.; Panyi, G. Periodic membrane potential and Ca2+ oscillations in t cells forming an immune synapse. Int. J. Mol. Sci. 2020, 21, 1568. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Fearn, T. Back to basics: Spectral pre-treatments—Derivatives. Spectrosc. Eur. 2007, 19, 32–33. [Google Scholar]



| Sample Number | Geographical Origin |
|---|---|
| 1 | Brazil, South America |
| 2 | India, Asia |
| 3 | Uganda, Africa |
| 4 | Colombia, South America |
| 5 | Uganda, Africa |
| 6 | Colombia, South America |
| 7 | Sumatra, Asia |
| 8 | Papua New Guinea, Asia |
| 9 | Guatemala, Central America |
| 10 | Kenya, Africa |
| 11 | Ethiopia, Africa |
| 12 | Panama, Central America |
| 13 | Mexico, Central America |
| 14 | India, Asia |
| 15 | Uganda, Africa |
| YSIn | Green | Light Roast | Medium Roast | Dark Roast |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 2 | 0 | 1 |
| 3 | 1 | 1 | 4 | 0 |
| 4 | 1 | 4 | 3 | 3 |
| 5 | 5 | 5 | 3 | 0 |
| 6 | 2 | 2 | 1 | 0 |
| 7 | 1 | 1 | 4 | 0 |
| 8 | 1 | 0 | 0 | 0 |
| 9 | 2 | 0 | 0 | 6 |
| 10 | 2 | 0 | 0 | 5 |
| N | 15 | 15 | 15 | 15 |
| Cut value for YSI | 0 | 0.2 | 0.1 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benes, E.; Fodor, M.; Kovács, S.; Gere, A. Application of Detrended Fluctuation Analysis and Yield Stability Index to Evaluate Near Infrared Spectra of Green and Roasted Coffee Samples. Processes 2020, 8, 913. https://doi.org/10.3390/pr8080913
Benes E, Fodor M, Kovács S, Gere A. Application of Detrended Fluctuation Analysis and Yield Stability Index to Evaluate Near Infrared Spectra of Green and Roasted Coffee Samples. Processes. 2020; 8(8):913. https://doi.org/10.3390/pr8080913
Chicago/Turabian StyleBenes, Eszter, Marietta Fodor, Sándor Kovács, and Attila Gere. 2020. "Application of Detrended Fluctuation Analysis and Yield Stability Index to Evaluate Near Infrared Spectra of Green and Roasted Coffee Samples" Processes 8, no. 8: 913. https://doi.org/10.3390/pr8080913
APA StyleBenes, E., Fodor, M., Kovács, S., & Gere, A. (2020). Application of Detrended Fluctuation Analysis and Yield Stability Index to Evaluate Near Infrared Spectra of Green and Roasted Coffee Samples. Processes, 8(8), 913. https://doi.org/10.3390/pr8080913








