The Influence of the Porous Structure of Activated Coke for the Treatment of Gases from Coal Combustion on Its Mechanical Strength
Abstract
:Introduction
1. Test Materials and Test Methods
1.1. Test Materials
1.2. Characterization of Activated Coke Samples
2. Results and Discussions
2.1. Influences of Porosity on Strength of Activated Coke in the Recycling Process
2.2. Effects of Pore Structure on Compression Strength
2.2.1. Effects of Open/Close State of Pores on Compression Strength
2.2.2. Effects of Pore Diameter Distribution on Compression Strength
2.3. Effects of Pore Structure on Abrasive Resistance
2.3.1. Effects of Open/Close State of Pores on Abrasive Resistance
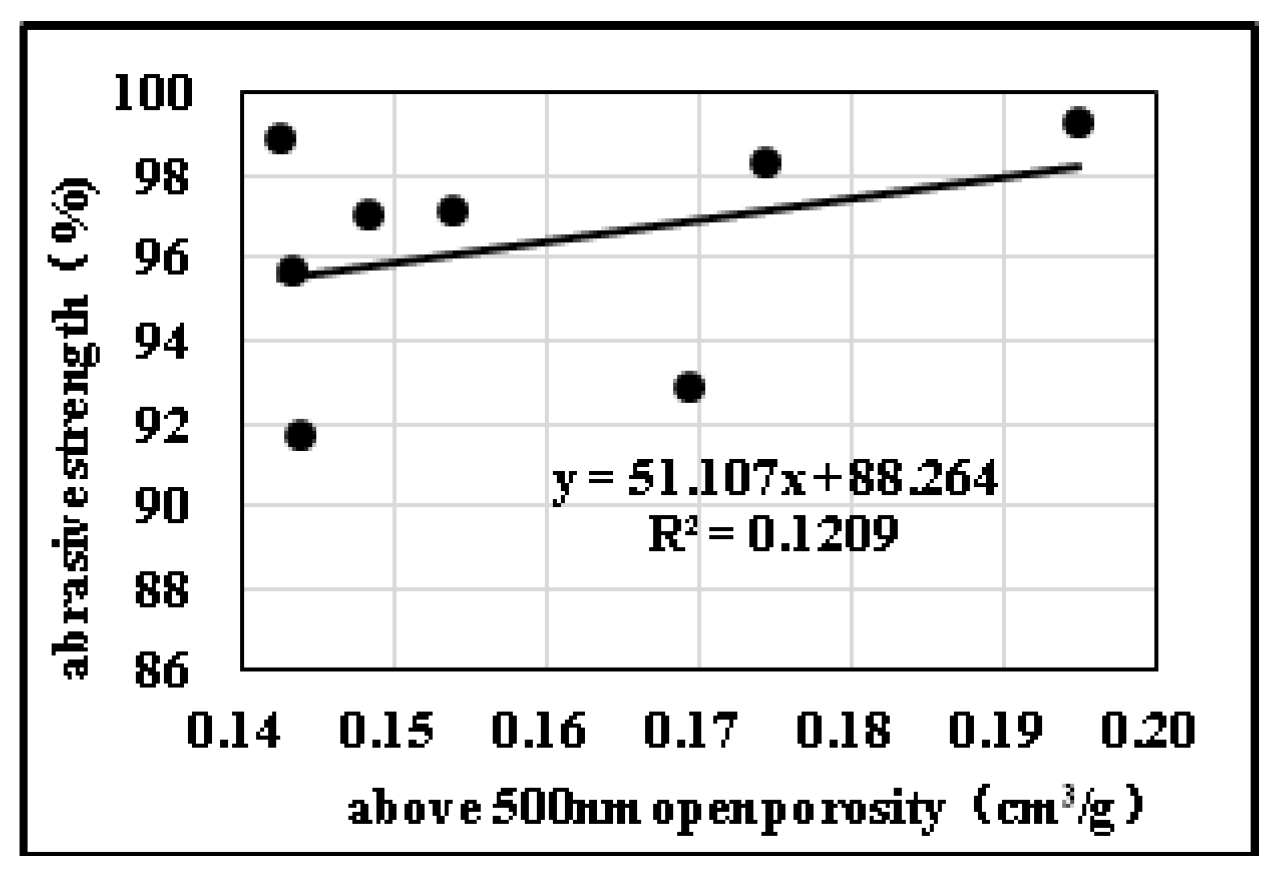
2.3.2. Influences of Pore Diameter Distribution on Abrasive Resistance
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruan, Z.Y. Desulfurization and denitration of sintering flue gas based on ammonia and activated carbon process. Iron Steel 2017, 52, 91–97. [Google Scholar]
- Liu, Z.G.; Cang, D.Q.; Dan, Z.G. Experimental study on the factors of affecting sintering flue gas desulphurization of activated carbon. Energy Metal. Ind. 2008, 27, 56–63. [Google Scholar]
- Ogriseck, S.; Vanegas, G.P.G. Experiment investigation of ammonia adsorption and nitric oxide reduction on activated coke. Chem. Eng. J. 2010, 160, 641–650. [Google Scholar] [CrossRef]
- Shao, J.G.; Zhang, J.J.; Zhang, X.; Feng, Y.; Zhang, H.; Zhang, S.; Chen, H. Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation. Fuel 2018, 224, 138–146. [Google Scholar] [CrossRef]
- Sadraei, R.; Paganini, M.C.; Calza, P.; Magnacca, G. As easy synthesis for preparing bio-based hybrid adsorbent useful for fast adsorption of polar pollutants. Nanomaterials 2019, 9, 731. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Jüntgen, H.; Richter, E.; Knoblauch, K. Catalytic NOx reduction by ammonia on carbon catalysts. Chem. Eng. Sci. 1988, 43, 419–428. [Google Scholar] [CrossRef]
- Knoblauch, K.; Richter, E.; Jüntgen, H. Application of active coke in process of SO2-and NOx-removal from flue gases. Fuel 1981, 60, 832–838. [Google Scholar] [CrossRef]
- Richter, E.; Knoblauch, K.; Jüntgen, H. Mechanisms and kinetics of SO2 adsorption and NOx reduction on active coke. Gas Sep. Purif. 1987, 1, 35–43. [Google Scholar] [CrossRef]
- Olson, D.G.; Tsuji, K.; Shiraishi, I. The reduction of gas phase air toxics from combustion and incineration sources using the MET–Mitsui–BF activated coke process. Fuel Process. Technol. 2000, 65, 393–405. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Zhu, T.; Ding, S. Adsorption and desorption of SO2, NO and chlorobenzene on activated carbon. J. Environ. Sci. 2016, 43, 128–135. [Google Scholar] [CrossRef]
- Wang, W.W.; Xu, S.P.; Wang, K.C.; Liang, J.; Zhang, W. De-intercalation of the intercalated potassium in the preparation of activated carbons by KOH activation. Fuel Process. Technol. 2019, 189, 74–79. [Google Scholar] [CrossRef]
- Mochida, I.; Korai, Y.; Shirahama, M. Removal of Sox and NOx over activated carbon fibers. Carbon 2000, 38, 227–239. [Google Scholar] [CrossRef]
- Jastrzab, K. Changes of activated coke properties in cyclic adsorption treatment of flue gases. Fuel Process. Technol. 2012, 104, 371–377. [Google Scholar] [CrossRef]
- Wu, H.; Wall, T.; Liu, G. Ash liberation from included minerals during combustion of pulverized coal: The relationship with char structure and burnout. Energy Fuels 1999, 13, 1197–1202. [Google Scholar] [CrossRef]
- Wu, H.; Bryant, G.; Wall, T. The effect of pressure on ash formation during pulverized coal combustion. Energy Fuels 2000, 14, 745–750. [Google Scholar] [CrossRef]
- Wang, P.; Xie, W.; Li, L.; Xiong, Y. Evolution of pore structure and surface characteristics of activated coke during circulations of desulfurization and regeneration. J. China Coal Soc. 2016, 41, 751–759. (In Chinese) [Google Scholar]
- GB/T9966.3-2001, Test Methods for Natural Facing Stone. Part 3: Test Methods for Bulk Density, True Density, True Porosity, and Absorption Rate. Available online: http://www.bzxz.net/bzxz/39564.html (accessed on 20 July 2020).
- Komatsubara, Y.; Shiraishi, I.; Yano, M. Preparation of active coke for the simultaneous removal of SOx and NOx in the flue gas. J. Fuel Soc. Jpn. 1985, 64, 255–263. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Chang, L.; Wu, D.; Fang, Z.; Shi, Y. Understandings on the scattering property of the mechanical strength data of solid catalysts: A statistical analysis of iron-based high-temperature water-gas shift catalysts. Catal. Today 1999, 51, 73–84. [Google Scholar] [CrossRef]
- Xing, X. A statistical theory of brittle fracture. Acta Phys. Sin. 1980, 29, 718–731. [Google Scholar]
- Chen, L.; Fang, K.; Fu, S. Effects of Pore and Crack on Stress Concentration in Welded Joint. Hot Work. Technol. 2014, 43, 213–215. [Google Scholar]
- Tang, Z.; Huang, R.; Jiao, Y.; Tan, F.; Zhu, X. Theoretical closure model for rock joints considering interaction of deformations of substrate deformation and asperity. Chin. J. Geotech. Eng. 2017, 39, 1800–1806. [Google Scholar]

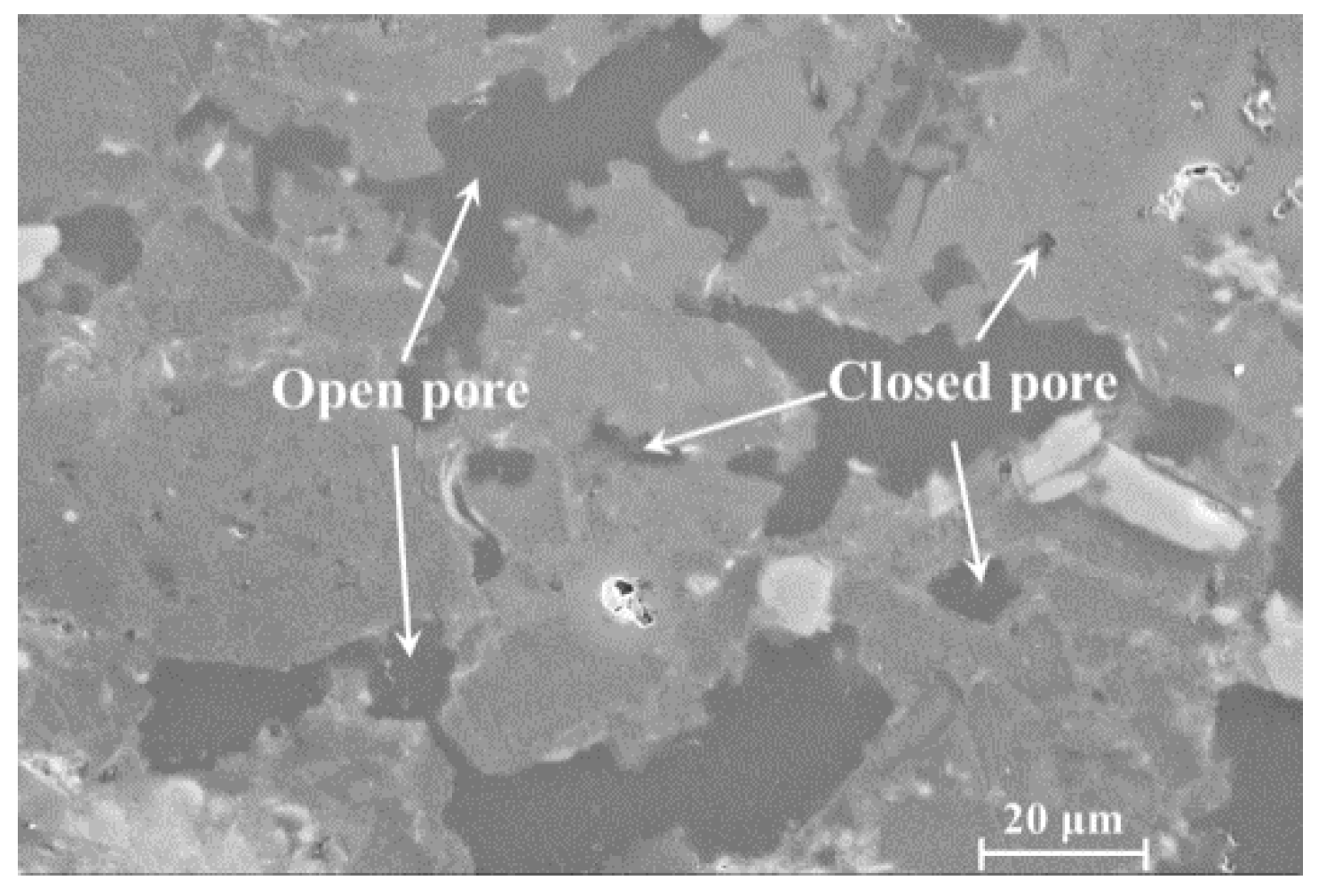

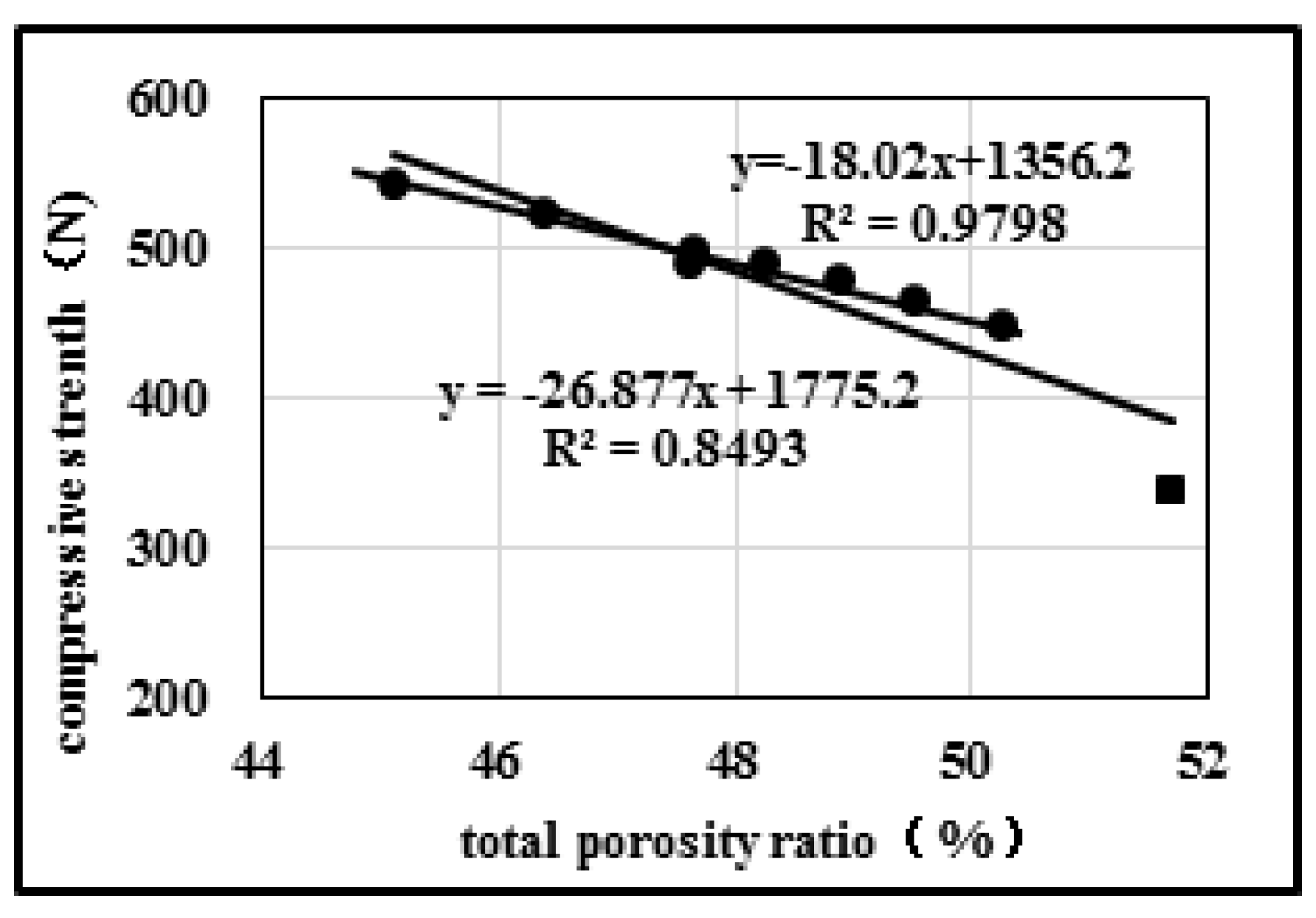
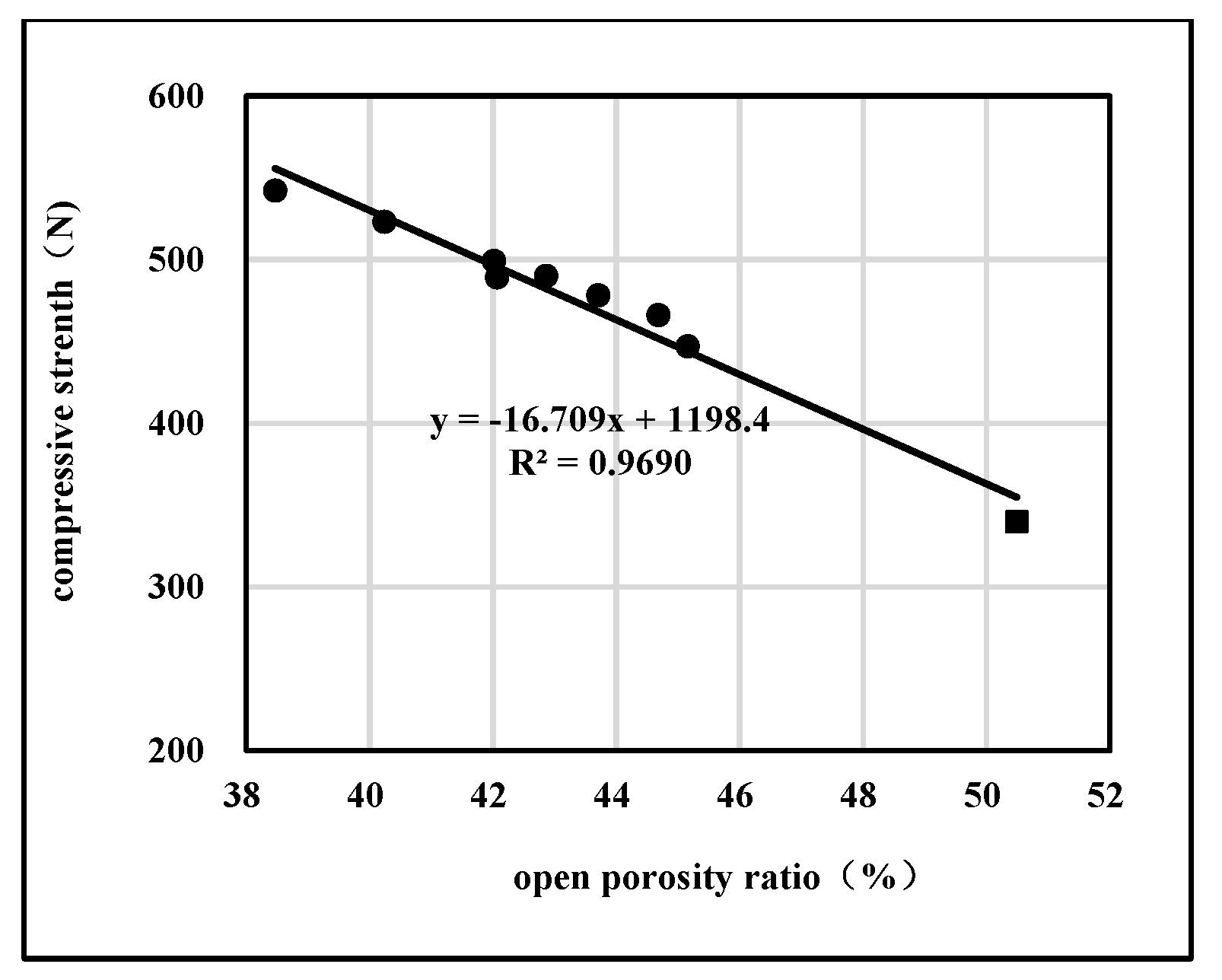
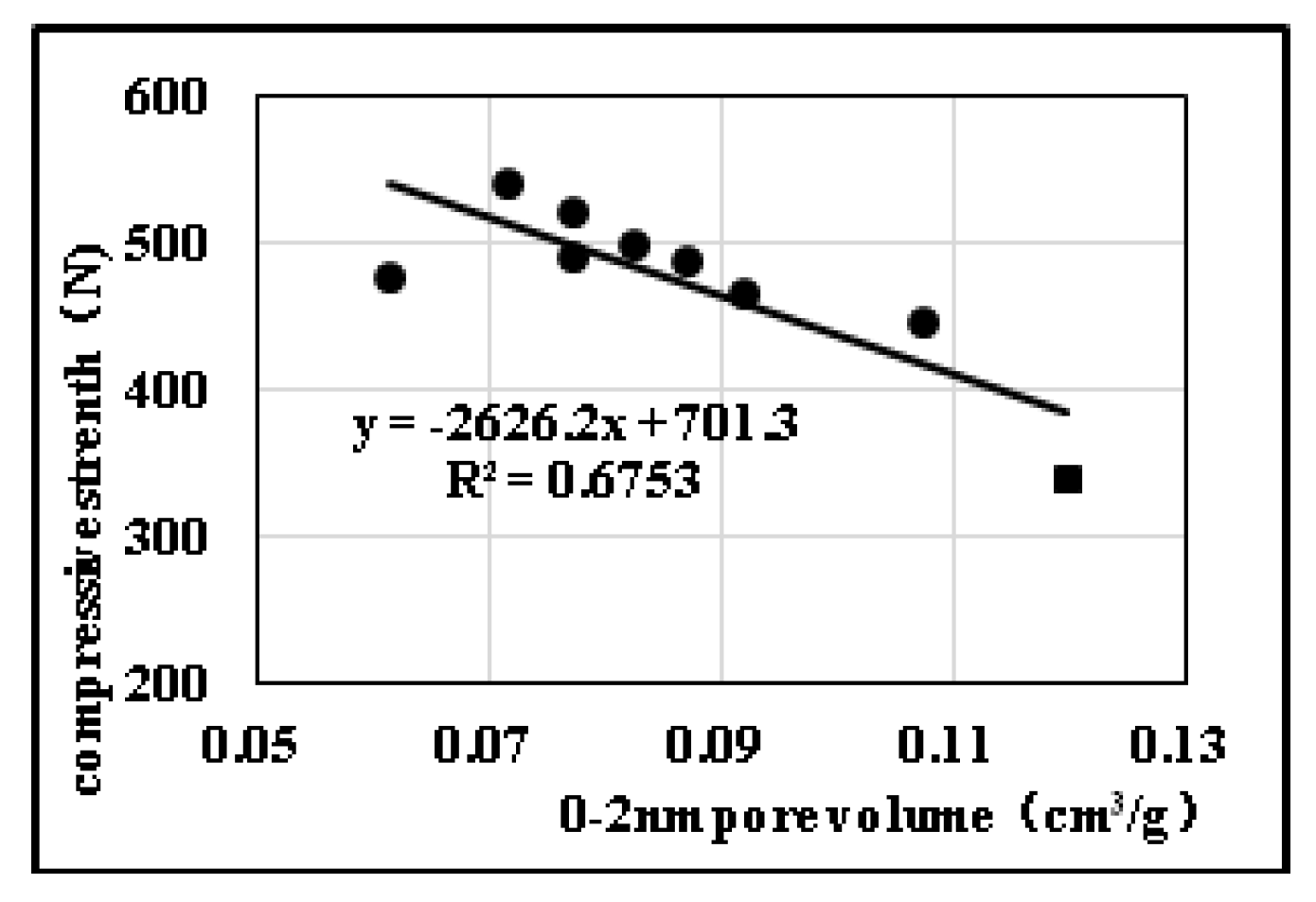
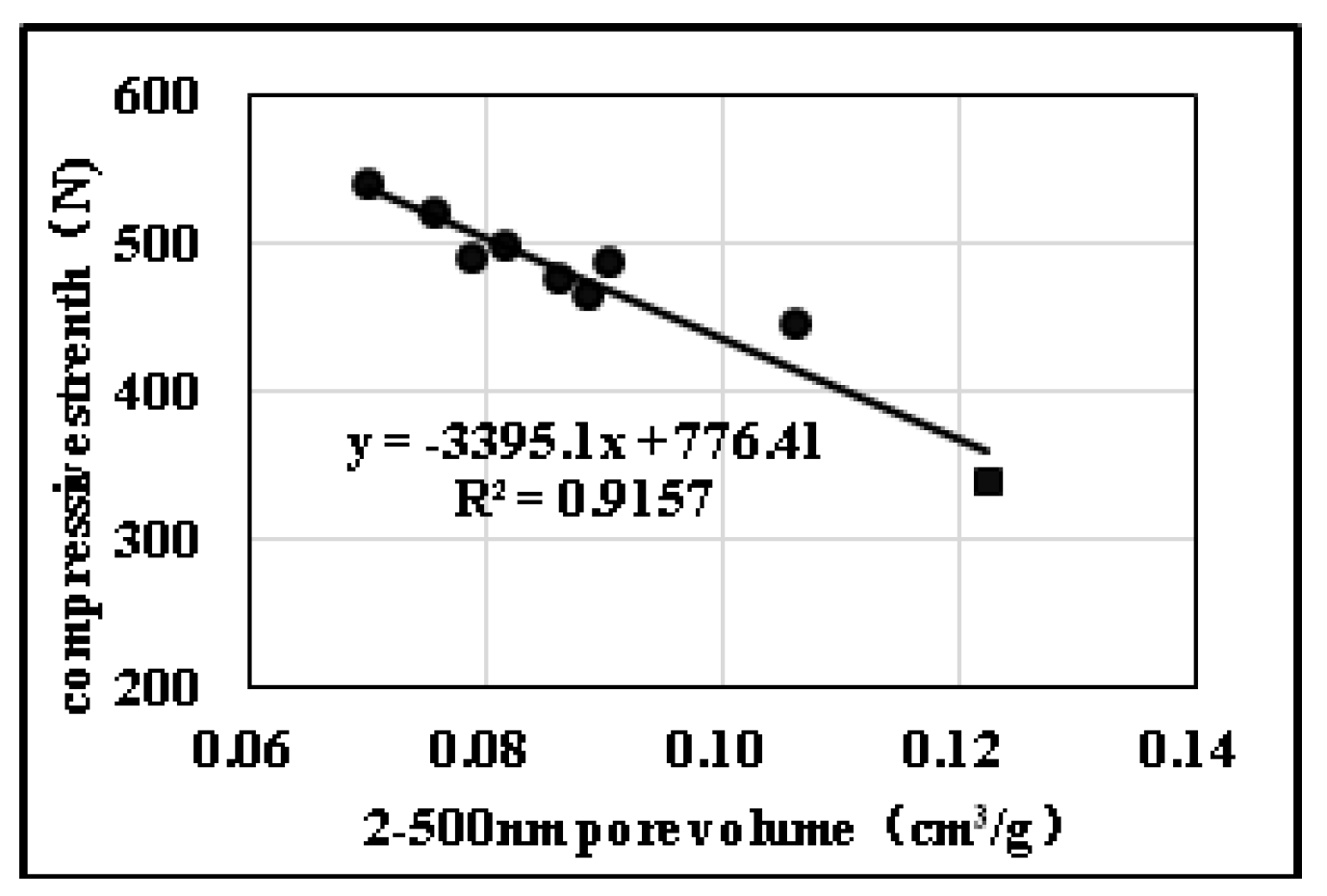

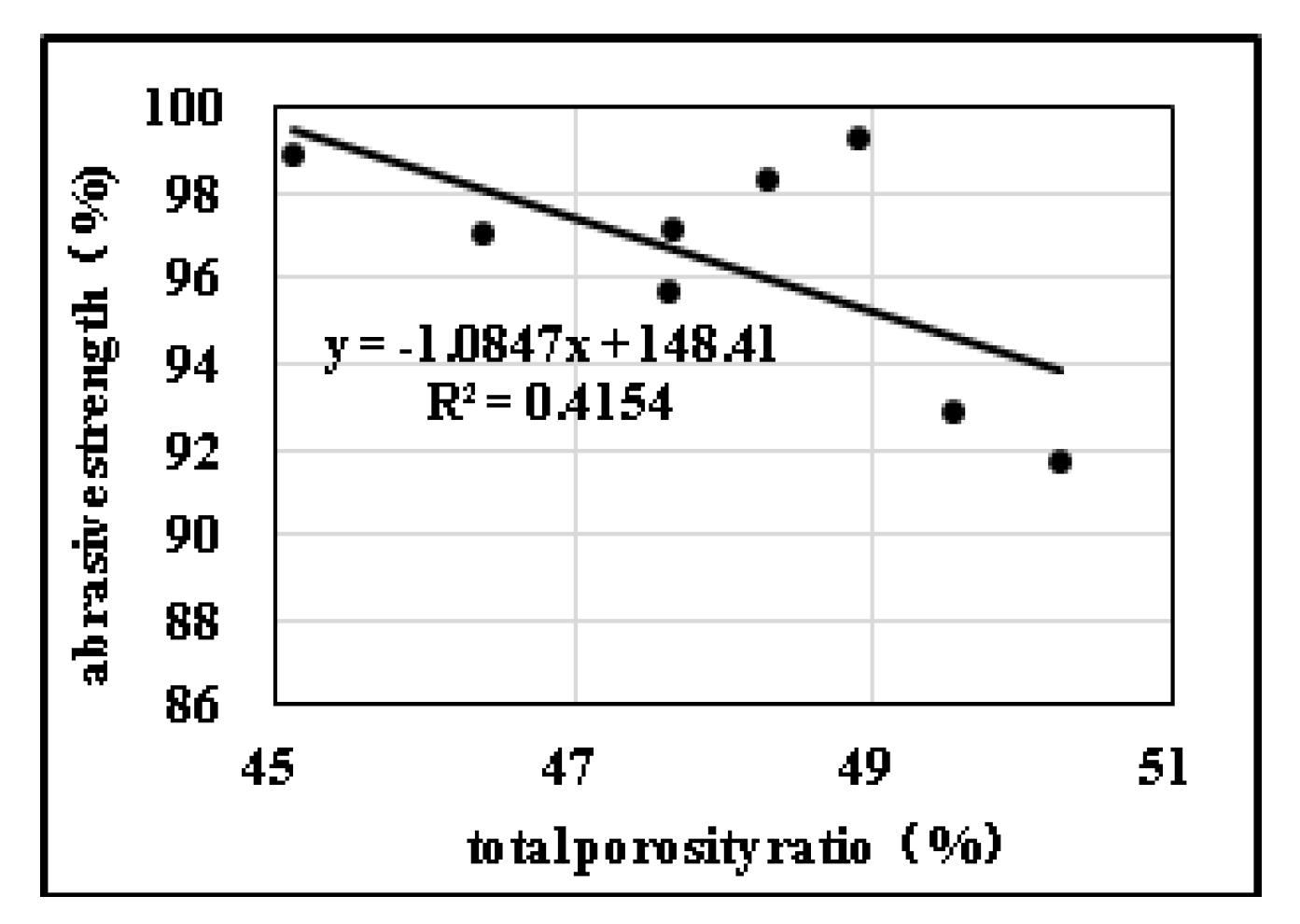

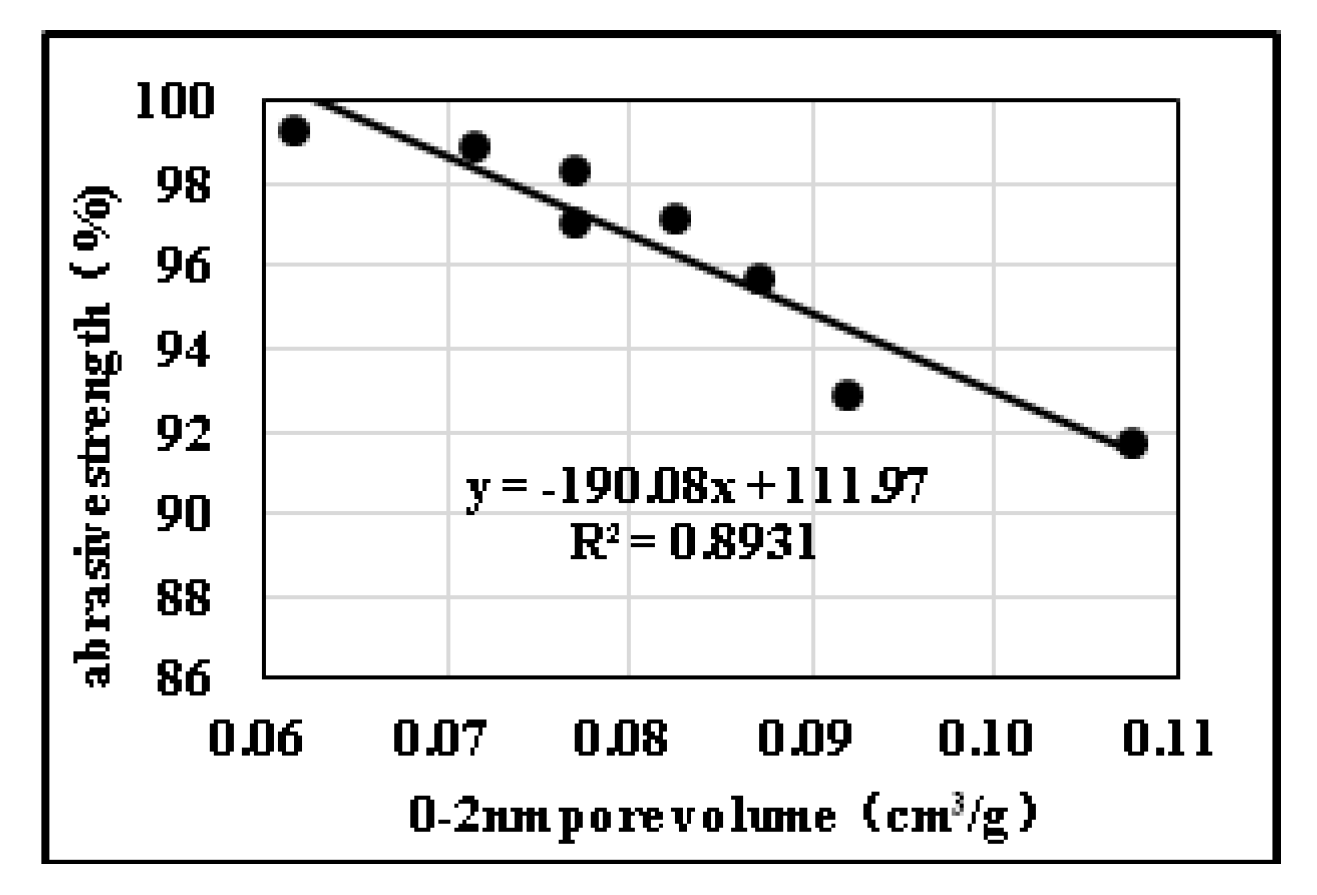
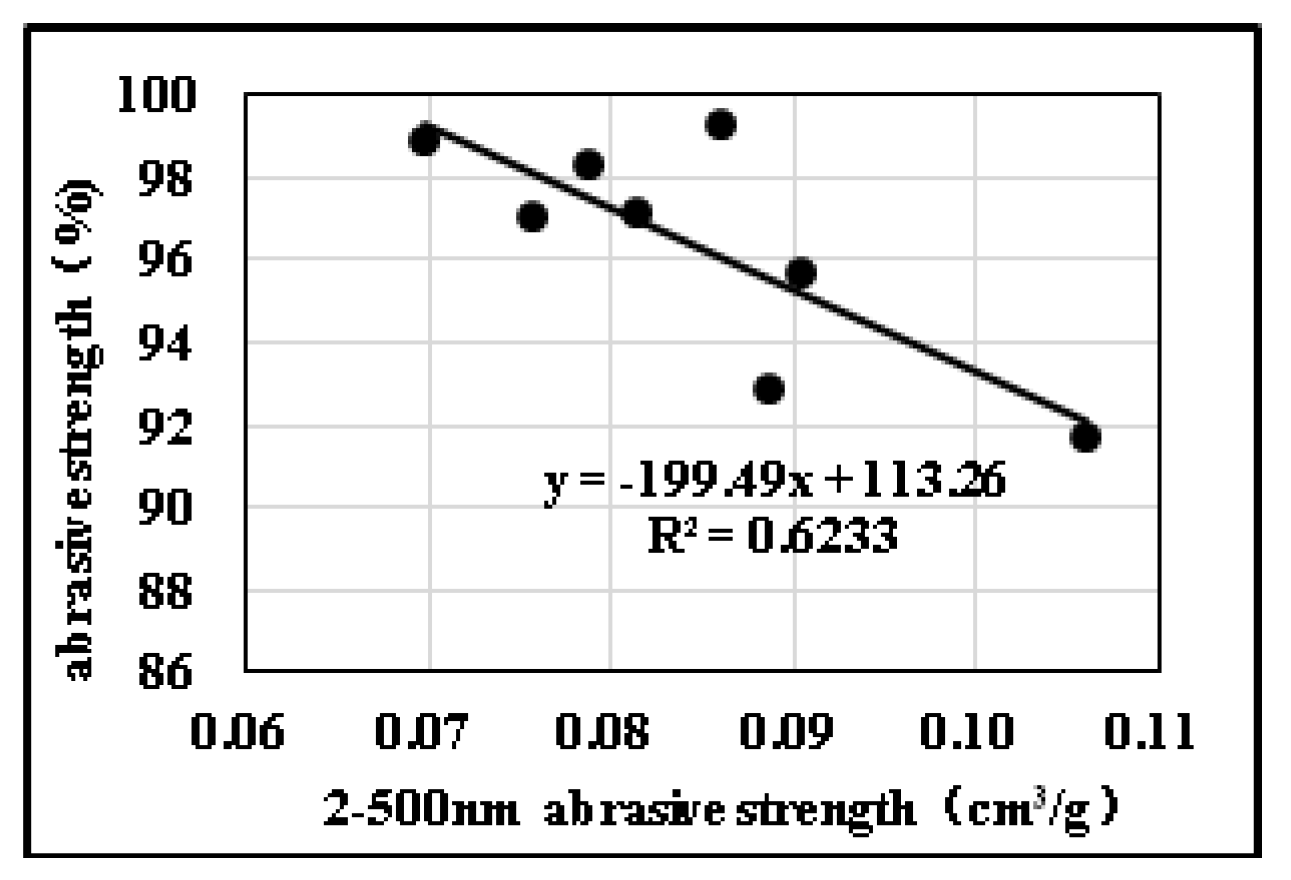
| Sample No. | Open/Close State of Pores | Pore Diameter Distribution | Mechanical Properties | Specific Surface Area (m2) | ||||
|---|---|---|---|---|---|---|---|---|
| Total Porosity Ratio (%) | Open Porosity Ratio (%) | 0–2 nm (cm3/g) | 2–500 nm (cm3/g) | >500 nm (cm3/g) | Compression Strength (N) | Abrasive Resistance (%) | ||
| Fresh-1 | 47.66 | 42.02 | 0.0825 | 0.0814 | 0.1539 | 499 | 97.18 | 198.3 |
| Cycly-x | 51.68 | 50.49 | 0.1199 | 0.1225 | 0.1623 | 340 | 98.88 | 325.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zhou, H.; Zhang, W.; Wu, S. The Influence of the Porous Structure of Activated Coke for the Treatment of Gases from Coal Combustion on Its Mechanical Strength. Processes 2020, 8, 900. https://doi.org/10.3390/pr8080900
Hu Z, Zhou H, Zhang W, Wu S. The Influence of the Porous Structure of Activated Coke for the Treatment of Gases from Coal Combustion on Its Mechanical Strength. Processes. 2020; 8(8):900. https://doi.org/10.3390/pr8080900
Chicago/Turabian StyleHu, Zhongjie, Heng Zhou, Weili Zhang, and Shengli Wu. 2020. "The Influence of the Porous Structure of Activated Coke for the Treatment of Gases from Coal Combustion on Its Mechanical Strength" Processes 8, no. 8: 900. https://doi.org/10.3390/pr8080900
APA StyleHu, Z., Zhou, H., Zhang, W., & Wu, S. (2020). The Influence of the Porous Structure of Activated Coke for the Treatment of Gases from Coal Combustion on Its Mechanical Strength. Processes, 8(8), 900. https://doi.org/10.3390/pr8080900





