Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Catalytic Activity Studies
3. Results and Discussion
3.1. Textural/Structural Characterization
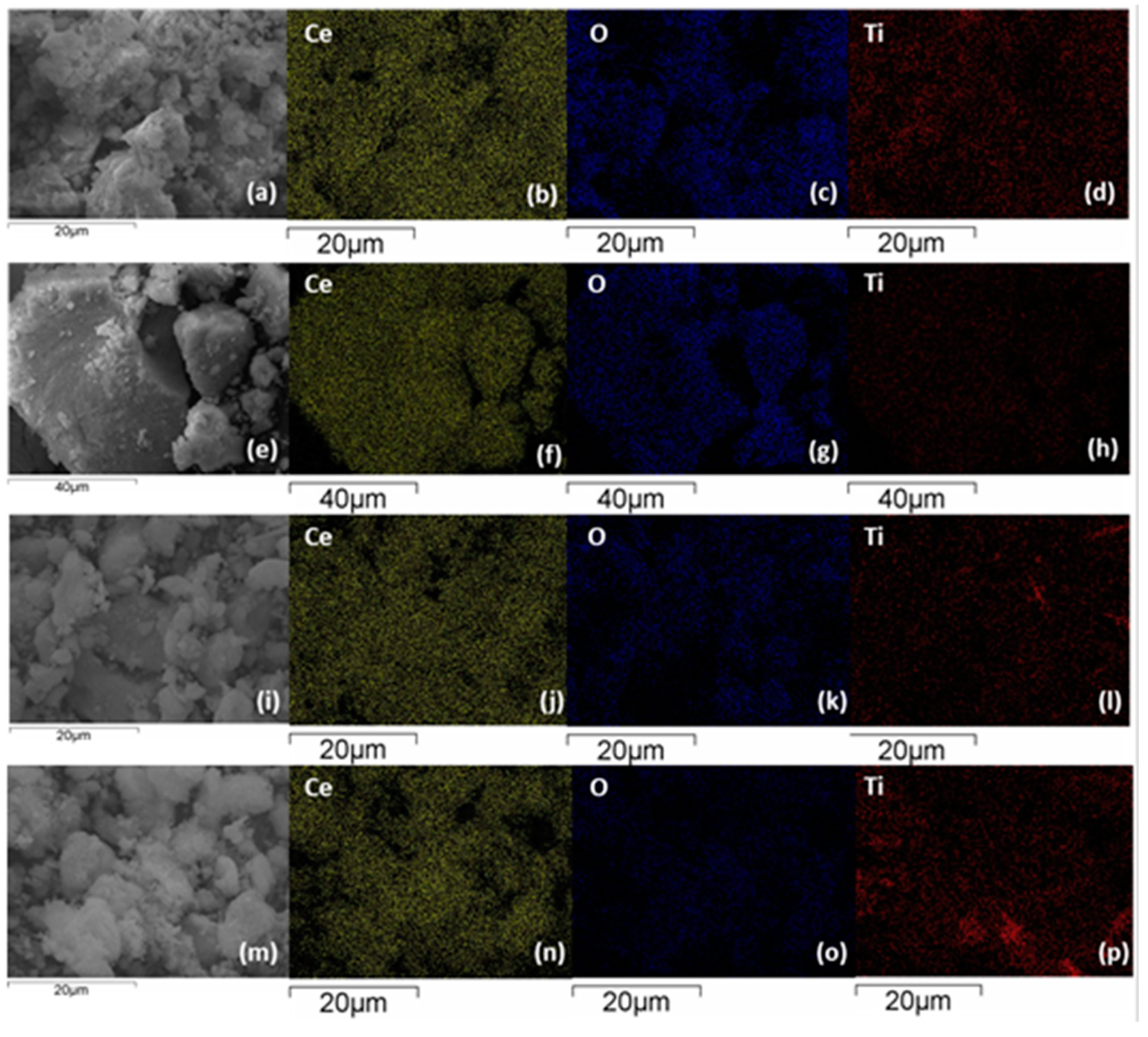
3.2. Morphological Characterization (TEM, SEM-EDS)
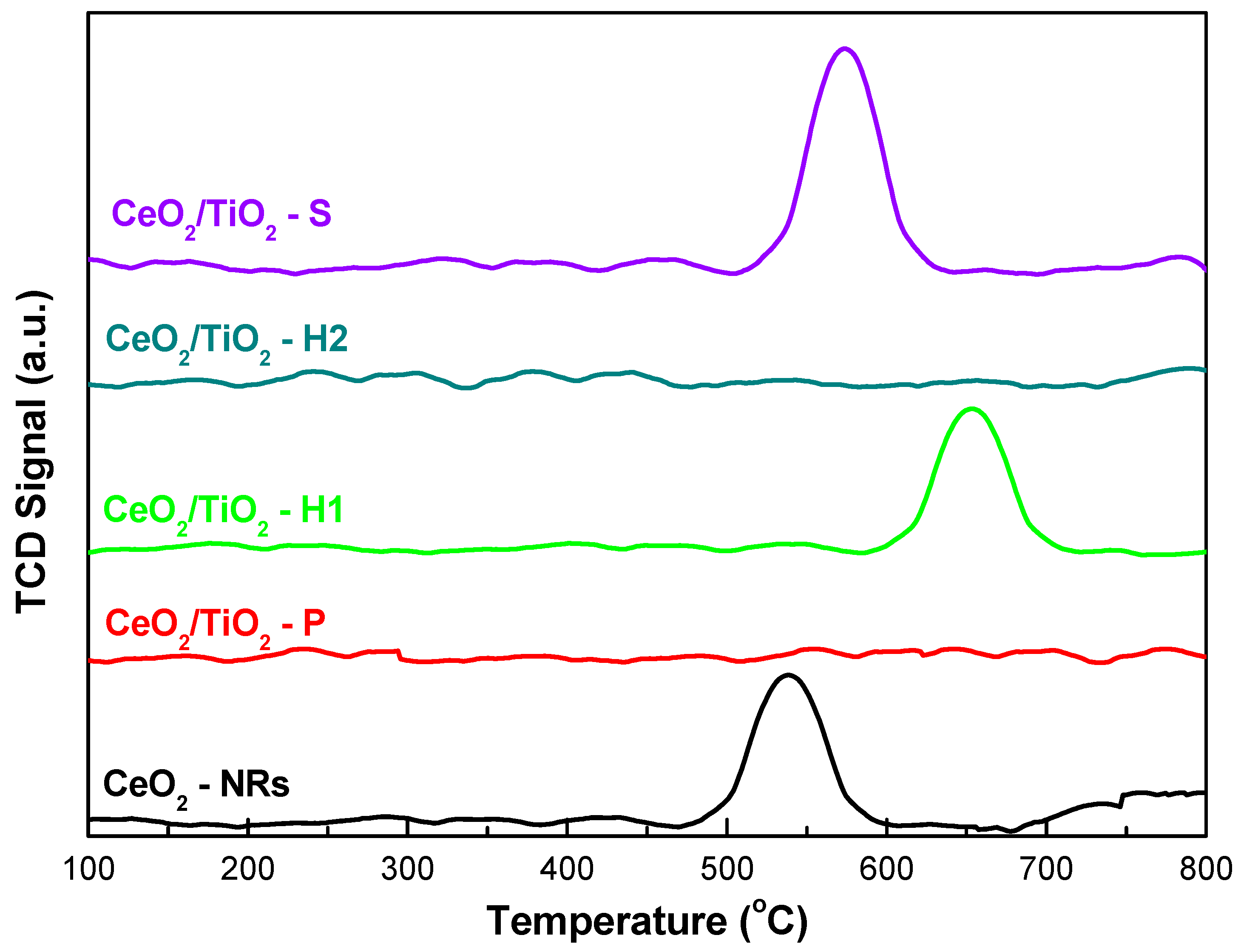
3.3. Redox Properties (H2-TPR)
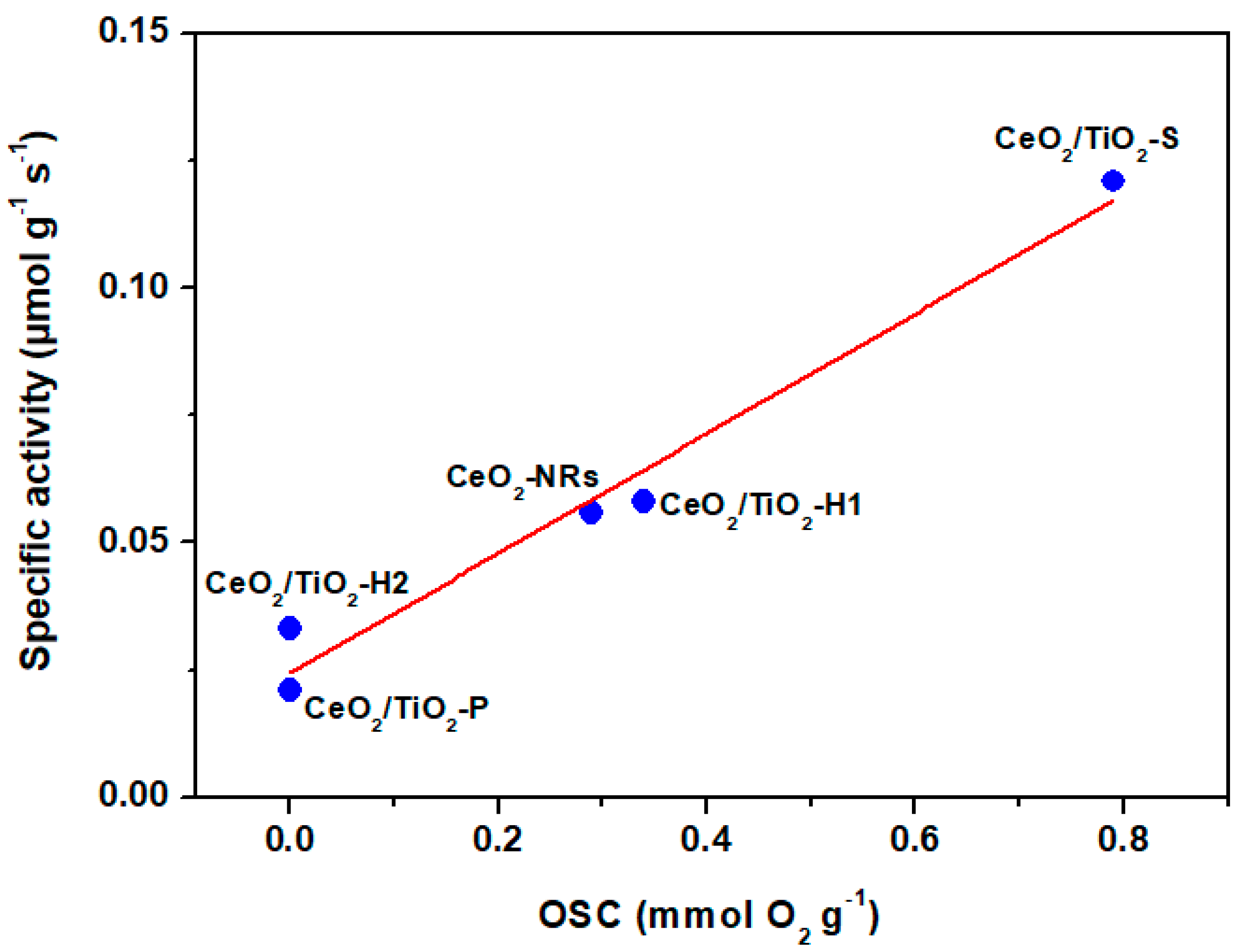
3.4. Catalytic Evaluation Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Konsolakis, M. The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M. Recent Advances on the Rational Design of Non-Precious Metal Oxide Catalysts Exemplified by CuOx/CeO2 Binary System: Implications of Size, Shape and Electronic Effects on Intrinsic Reactivity and Metal-Support Interactions. Catalysts 2020, 10, 160. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. The role of ceria-based nanostructured materials in energy applications. Mater. Today 2014, 17, 349–357. [Google Scholar] [CrossRef]
- Tang, W.-X.; Gao, P.-X. Nanostructured cerium oxide: Preparation, characterization, and application in energy and environmental catalysis. MRS Commun. 2016, 6, 311–329. [Google Scholar] [CrossRef]
- Cortés Corberán, V.; Rives, V.; Stathopoulos, V. Chapter 7—Recent Applications of Nanometal Oxide Catalysts in Oxidation Reactions. In Advanced Nanomaterials for Catalysis and Energy; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 227–293. [Google Scholar] [CrossRef]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen Defects and Surface Chemistry of Ceria: Quantum Chemical Studies Compared to Experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-Controlled Ceria-based Nanostructures for Catalysis Applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. Size-dependent oxygen storage ability of nano-sized ceria. Phys. Chem. Chem. Phys. 2013, 15, 14414–14419. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; De Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-Dependent Activity of Ceria in Soot Combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Li, L.; Han, W.; Tang, Z.; Zhang, J.; Lu, G. Hard-template synthesis of three-dimensional mesoporous Cu-Ce based catalysts with tunable architectures and their application in the CO catalytic oxidation. RSC Adv. 2016, 6, 64247–64257. [Google Scholar] [CrossRef]
- Zhu, W.; Tang, K.; Li, J.; Liu, W.; Niu, X.; Zhao, G.; Ma, X.; Liu, Z.; Wei, H.; Yang, Y. The effect of copper species in copper-ceria catalysts: Structure evolution and enhanced performance in CO oxidation. RSC Adv. 2016, 6, 46966–46971. [Google Scholar] [CrossRef]
- Gu, Z.; Li, K.; Qing, S.; Zhu, X.; Wei, Y.; Li, Y.; Wang, H. Enhanced reducibility and redox stability of Fe2O3 in the presence of CeO2 nanoparticles. RSC Adv. 2014, 4, 47191–47199. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Vasiliades, M.A.; Stathopoulos, V.N.; Efstathiou, A.M. The Effect of CeO2 Preparation Method on the Carbon Pathways in the Dry Reforming of Methane on Ni/CeO2 Studied by Transient Techniques. Catalysts 2019, 9, 621. [Google Scholar] [CrossRef]
- Konsolakis, M. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Zou, W.; Ge, C.; Lu, M.; Wu, S.; Wang, Y.; Sun, J.; Pu, Y.; Tang, C.; Gao, F.; Dong, L. Engineering the NiO/CeO2 interface to enhance the catalytic performance for CO oxidation. RSC Adv. 2015, 5, 98335–98343. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Varvoutis, G.; Papista, E.; Marnellos, G.E. CO2 Hydrogenation over Nanoceria-Supported Transition Metal Catalysts: Role of Ceria Morphology (Nanorods versus Nanocubes) and Active Phase Nature (Co versus Cu). Nanomaterials 2019, 9, 1739. [Google Scholar] [CrossRef]
- Konsolakis, M.; Sgourakis, M.; Carabineiro, S.A.C. Surface and redox properties of cobalt-ceria binary oxides: On the effect of Co content and pretreatment conditions. Appl. Surf. Sci. 2015, 341, 48–54. [Google Scholar] [CrossRef]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G.E. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts 2016, 6, 39. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Wang, Z.; Liu, W.; Wang, L. Catalytic oxidation of CO over mesoporous copper-doped ceria catalysts via a facile CTAB-assisted synthesis. RSC Adv. 2018, 8, 14888–14897. [Google Scholar] [CrossRef]
- Qiu, N.; Zhang, J.; Wu, Z. Peculiar surface-interface properties of nanocrystalline ceria-cobalt oxides with enhanced oxygen storage capacity. Phys. Chem. Chem. Phys. 2014, 16, 22659–22664. [Google Scholar] [CrossRef] [PubMed]
- Konsolakis, M.; Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E.; Tavares, P.B.; Agostinho Moreira, J.; Romaguera-Barcelay, Y.; Figueiredo, J.L. Effect of preparation method on the solid state properties and the deN2O performance of CuO-CeO2 oxides. Catal. Sci. Technol. 2015, 5, 3714–3727. [Google Scholar] [CrossRef]
- Wu, K.; Sun, L.-D.; Yan, C.-H. Ceria-Based Nanocatalysts: Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Devaiah, D.; Thrimurthulu, G.; Smirniotis, P.G.; Reddy, B.M. Nanocrystalline alumina-supported ceria-praseodymia solid solutions: Structural characteristics and catalytic CO oxidation. RSC Adv. 2016, 6, 44826–44837. [Google Scholar] [CrossRef]
- Chen, L.; Weng, D.; Si, Z.; Wu, X. Synergistic effect between ceria and tungsten oxide on WO3–CeO2–TiO2 catalysts for NH3-SCR reaction. Prog. Nat. Sci. Mater. Int. 2012, 22, 265–272. [Google Scholar] [CrossRef][Green Version]
- Chen, F.; Ho, P.; Ran, R.; Chen, W.; Si, Z.; Wu, X.; Weng, D.; Huang, Z.; Lee, C. Synergistic effect of CeO2 modified TiO2 photocatalyst on the enhancement of visible light photocatalytic performance. J. Alloys Compd. 2017, 714, 560–566. [Google Scholar] [CrossRef]
- Lamallem, M.; El Ayadi, H.; Gennequin, C.; Cousin, R.; Siffert, S.; Aïssi, F.; Aboukaïs, A. Effect of the preparation method on Au/Ce-Ti-O catalysts activity for VOCs oxidation. Catal. Today 2008, 137, 367–372. [Google Scholar] [CrossRef]
- Chang, K.; Wang, T.; Chen, J.G. Hydrogenation of CO2 to methanol over CuCeTiOx catalysts. Appl. Catal. B Environ. 2017, 206, 704–711. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, Q.; Zhang, S. A New Insight into Catalytic Ozonation with Nanosized Ce–Ti Oxides for NOx Removal: Confirmation of Ce–O–Ti for Active Sites. Ind. Eng. Chem. Res. 2015, 54, 2012–2022. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce-Ti Amorphous Oxides for Selective Catalytic Reduction of NO with NH3: Confirmation of Ce-O-Ti Active Sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kattel, S.; Senanayake, S.D.; Anibal Boscoboinik, J.; Nie, X.; Graciani, J.; Rodriguez, J.A.; Liu, P.; Stacchiola, D.J.; Chen, J.G. Low Pressure CO2 Hydrogenation to Methanol over Gold Nanoparticles Activated on a CeOx/TiO2 Interface. J. Am. Chem. Soc. 2015, 137, 10104–10107. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Dai, Q.; Lao, Y.; Shi, B.; Wang, X. Low temperature catalytic combustion of 1,2-dichlorobenzene over CeO2–TiO2 mixed oxide catalysts. Appl. Catal. B Environ. 2016, 181, 848–861. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Qin, Z.; Wang, G.; Zhang, Y.; Wu, Z.; Li, Z.; Chen, G.; Dong, W.; Wu, Z.; et al. Morphologic effects of nano CeO2-TiO2 on the performance of Au/CeO2-TiO2 catalysts in low-temperature CO oxidation. Appl. Catal. B Environ. 2014, 144, 498–506. [Google Scholar] [CrossRef]
- Vieira, G.B.; José, H.J.; Peterson, M.; Baldissarelli, V.Z.; Alvarez, P.; De Fátima Peralta Muniz Moreira, R. CeO2/TiO2 nanostructures enhance adsorption and photocatalytic degradation of organic compounds in aqueous suspension. J. Photochem. Photobiol. A Chem. 2018, 353, 325–336. [Google Scholar] [CrossRef]
- Qu, X.; Xie, D.; Gao, L.; Du, F. Synthesis and photocatalytic activity of TiO2/CeO2 core-shell nanotubes. Mater. Sci. Semicond. Process. 2014, 26, 657–662. [Google Scholar] [CrossRef]
- Ameen, S.; Shaheer Akhtar, M.; Seo, H.-K.; Shin, H.-S. Solution-processed CeO2/TiO2 nanocomposite as potent visible light photocatalyst for the degradation of bromophenol dye. Chem. Eng. J. 2014, 247, 193–198. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Xiao, G.; Wang, T.-S.; Zhang, F.; Ma, Y.; Gao, P.; Zhu, C.-L.; Zhang, E.; Xu, Z.; Li, Q. Synthesis and enhanced gas sensing properties of crystalline CeO2/TiO2 core/shell nanorods. Sens. Actuators B Chem. 2011, 156, 867–874. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Zhao, Z.; Zhou, W.; Wang, D.; Kang, X.; Liu, H.; Wang, J.; Chen, S.; Cai, H.; et al. Enhanced Photocatalytic Performances of CeO2/TiO2 Nanobelt Heterostructures. Small 2013, 9, 3864–3872. [Google Scholar] [CrossRef]
- Fan, Z.; Meng, F.; Gong, J.; Li, H.; Hu, Y.; Liu, D. Enhanced photocatalytic activity of hierarchical flower-like CeO2/TiO2 heterostructures. Mater. Lett. 2016, 175, 36–39. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, T.; Li, Y.; Li, X.; Yin, J.; Wang, C. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 2016, 337, 293–302. [Google Scholar] [CrossRef]
- Samadi, S.; Asadi Cordshooli, G.; Yousefi, M.; Kalateh, K.; Zakaria, S. CeO2/TiO2 core/shell nanoparticles as quantitative gas sensor at room temperature. Sens. Rev. 2018, 38, 458–466. [Google Scholar] [CrossRef]
- Miah, A.T.; Malakar, B.; Saikia, P. Gold over Ceria-Titania Mixed Oxides: Solar Light Induced Catalytic Activity for Nitrophenol Reduction. Catal. Lett. 2016, 146, 291–303. [Google Scholar] [CrossRef]
- Gao, X.; Du, X.; Cui, L.; Fu, Y.; Luo, Z.; Cen, K. A Ce-Cu-Ti oxide catalyst for the selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 12, 255–258. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Chen, Y.; Gao, X.; Zhang, L. Supported metal sulfates on Ce–TiOx as catalysts for NH3–SCR of NO: High resistances to SO2 and potassium. J. Ind. Eng. Chem. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, P.; Tao, F.; Zhou, R. New insight into the structure of CeO2-TiO2 mixed oxides and their excellent catalytic performances for 1,2-dichloroethane oxidation. Chem. Eng. J. 2016, 295, 99–108. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Zhong, Y.; Luo, Z.; Cen, K. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2010, 174, 734–739. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Fernández-García, M.; Kubacka, A. Promotion of CeO2-TiO2 photoactivity by g-C3N4: Ultraviolet and visible light elimination of toluene. Appl. Catal. B Environ. 2015, 164, 261–270. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, R.; Chen, L.; Du, J.; Tao, C.; Yang, F.; Dong, L. Selective catalytic reduction of NOx by NH3 over CeO2 supported on TiO2: Comparison of anatase, brookite, and rutile. Appl. Catal. B Environ. 2017, 208, 82–93. [Google Scholar] [CrossRef]
- Fang, Q.; Liang, X. CeO2–Al2O3, CeO2–SiO2, CeO2–TiO2 core-shell spheres: Formation mechanisms and UV absorption. RSC Adv. 2012, 2, 5370–5375. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Fu, Y.; Zhong, Y.; Luo, Z.; Cen, K. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 11, 465–469. [Google Scholar] [CrossRef]
- Soler, L.; Casanovas, A.; Urrich, A.; Angurell, I.; Llorca, J. CO oxidation and COPrOx over preformed Au nanoparticles supported over nanoshaped CeO2. Appl. Catal. B Environ. 2016, 197, 47–55. [Google Scholar] [CrossRef]
- Piumetti, M.; Andana, T.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Study on the CO Oxidation over Ceria-Based Nanocatalysts. Nanoscale Res. Lett. 2016, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Mock, S.A.; Sharp, S.E.; Stoner, T.R.; Radetic, M.J.; Zell, E.T.; Wang, R. CeO2 nanorods-supported transition metal catalysts for CO oxidation. J. Colloid Interface Sci. 2016, 466, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.S.; Risch, M.; Giordano, L.; Mansour, A.N.; Shao-Horn, Y. Structure, Bonding, and Catalytic Activity of Monodisperse, Transition-Metal-Substituted CeO2 Nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Du Fresne von Hohenesche, C.; Stathopoulos, V.; Unger, K.K.; Lind, A.; Lindén, M. Formation of hierarchically ordered silicas prepared by spray drying of nanosized spheres. Stud. Surf. Sci. Catal. 2002, 144, 339–346. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Belessi, V.C.; Costa, C.N.; Neophytides, S.; Falaras, P.; Efstathiou, A.M.; Pomonis, P.J. Catalytic activity of high surface area mesoporous Mn-based mixed oxides for the deep oxidation of methane and lean-NOx reduction. Stud. Surf. Sci. Catal. 2000, 130, 1529–1534. [Google Scholar] [CrossRef]
- Salmas, C.E.; Stathopoulos, V.N.; Pomonis, P.J.; Androutsopoulos, G.P. Pore Structure-Chemical Composition Interactions of New High Surface Area Manganese Based Mesoporous Materials. Materials Preparation, Characterization, and Catalytic Activity. Langmuir 2002, 18, 423–432. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Barthos, R.; Hegyessy, A.; Klébert, S.; Valyon, J. Vanadium dispersion and catalytic activity of Pd/VOx/SBA-15 catalysts in the Wacker oxidation of ethylene. Microporous Mesoporous Mater. 2015, 207, 1–8. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Sebastiammal, S.S.; Shally, V.; Priyadharshini, M.; Jayam, S.G. Structural and optical properties of Cerium oxide nanoparticles. Int. J. Eng. Trends Technol. 2017, 49, 69–73. [Google Scholar] [CrossRef]
- Babitha, K.K.; Sreedevi, A.; Priyanka, K.P.; Sabu, B.; Varghese, T. Structural characterization and optical studies of CeO2 nanoparticles synthesized by chemical precipitation. Indian J. Pure Appl. Phys. 2015, 53, 596–603. [Google Scholar]
- Ali, M.M.; Mahdi, H.S.; Parveen, A.; Azam, A. Optical properties of cerium oxide (CeO2) nanoparticles synthesized by hydroxide mediated method. AIP Conf. Proc. 2018, 1953, 030044. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J. Highly Dispersed CeO2 on TiO2 Nanotube: A Synergistic Nanocomposite with Superior Peroxidase-Like Activity. ACS Appl. Mater. Interfaces 2015, 7, 6451–6461. [Google Scholar] [CrossRef]
- Fang, J.; Bao, H.; He, B.; Wang, F.; Si, D.; Jiang, Z.; Pan, Z.; Wei, S.; Huang, W. Interfacial and Surface Structures of CeO2−TiO2 Mixed Oxides. J. Phys. Chem. C 2007, 111, 19078–19085. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.R.; Pudukudy, M.; Yaakob, Z.; Ismail, N.A. CeO2-TiO2 as a visible light active catalyst for the photoreduction of CO2 to methanol. J. Rare Earths 2015, 33, 1155–1161. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Qian, J.; Miao, N.; Yao, C.; Chen, Z. Synthesis and characterization of CeO2/TiO2 nanotube arrays and enhanced photocatalytic oxidative desulfurization performance. J. Alloys Compd. 2016, 661, 363–371. [Google Scholar] [CrossRef]
- Ansari, A.A.; Labis, J.; Alam, M.; Ramay, S.M.; Ahmad, N.; Mahmood, A. Physicochemical and Redox Characteristics of Fe Ion-doped CeO2 Nanoparticles. J. Chin. Chem. Soc. 2015, 62, 925–932. [Google Scholar] [CrossRef]
- Bespalko, Y.; Kuznetsova, T.; Kriger, T.; Chesalov, Y.; Lapina, O.; Ishchenko, A.; Larina, T.; Sadykov, V.; Stathopoulos, V. La2Zr2O7/LaAlO3 composite prepared by mixing precipitated precursors: Evolution of its structure under sintering. Mater. Chem. Phys. 2020, 251, 123093. [Google Scholar] [CrossRef]
- Castanet, U.; Feral-Martin, C.; Demourgues, A.; Neale, R.L.; Sayle, D.C.; Caddeo, F.; Flitcroft, J.M.; Caygill, R.; Pointon, B.J.; Molinari, M.; et al. Controlling the {111}/{110} Surface Ratio of Cuboidal Ceria Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 11384–11390. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Zhang, S.; Yan, S.; Cao, B.; Wang, Z.; Fu, Y. Enhanced NH3 gas-sensing performance of silica modified CeO2 nanostructure based sensors. Sens. Actuators B Chem. 2018, 255, 862–870. [Google Scholar] [CrossRef]
- Phokha, S.; Pinitsoontorn, S.; Maensiri, S. Structure and Magnetic Properties of Monodisperse Fe3+-doped CeO2 Nanospheres. Nano-Micro Lett. 2013, 5, 223–233. [Google Scholar] [CrossRef]
- Mukherjee, D.; Rao, B.G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B Environ. 2016, 197, 105–115. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Johnston-Peck, A.C.; Senanayake, S.D.; Plata, J.J.; Kundu, S.; Xu, W.; Barrio, L.; Graciani, J.; Sanz, J.F.; Navarro, R.M.; Fierro, J.L.G.; et al. Nature of the Mixed-Oxide Interface in Ceria-Titania Catalysts: Clusters, Chains, and Nanoparticles. J. Phys. Chem. C 2013, 117, 14463–14471. [Google Scholar] [CrossRef]
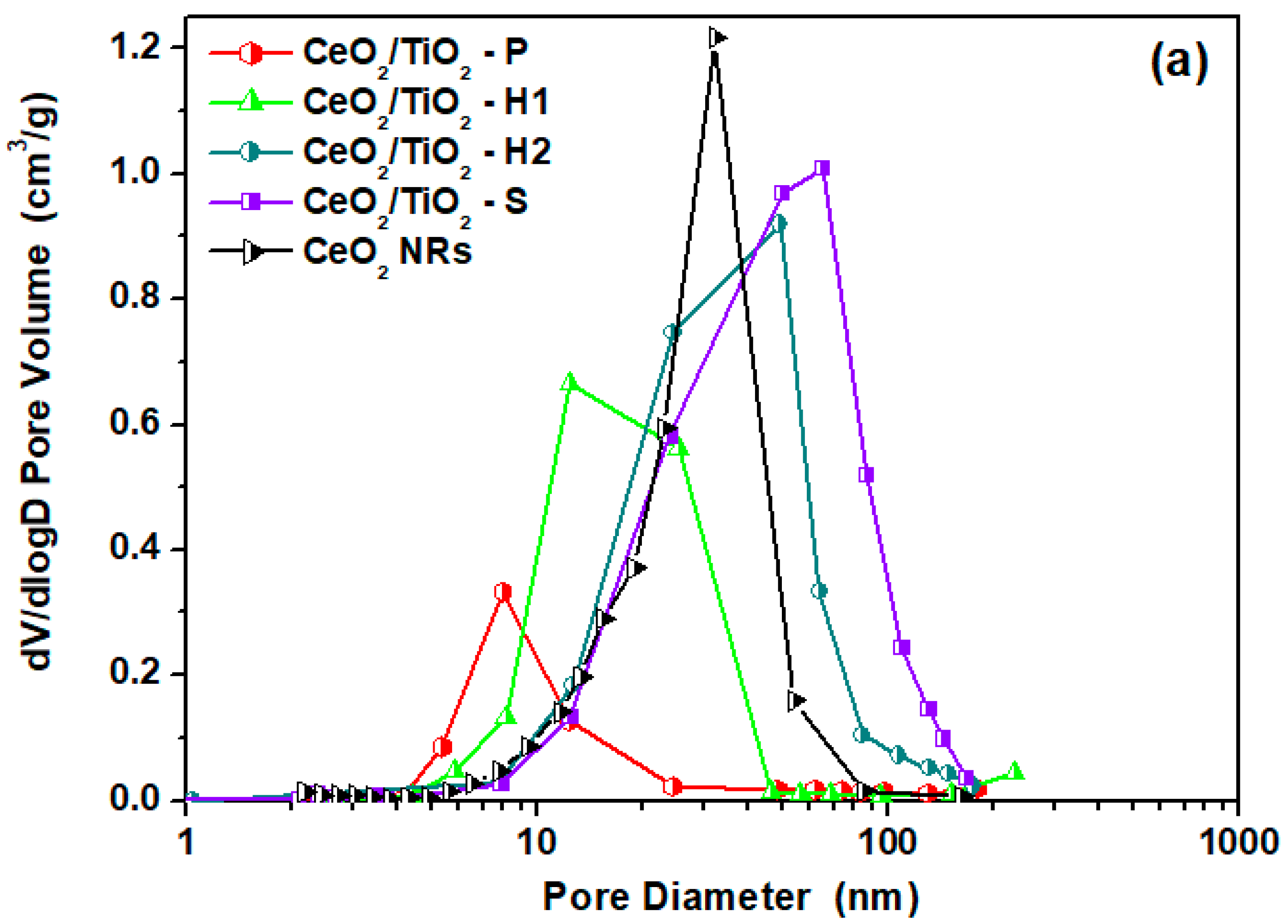
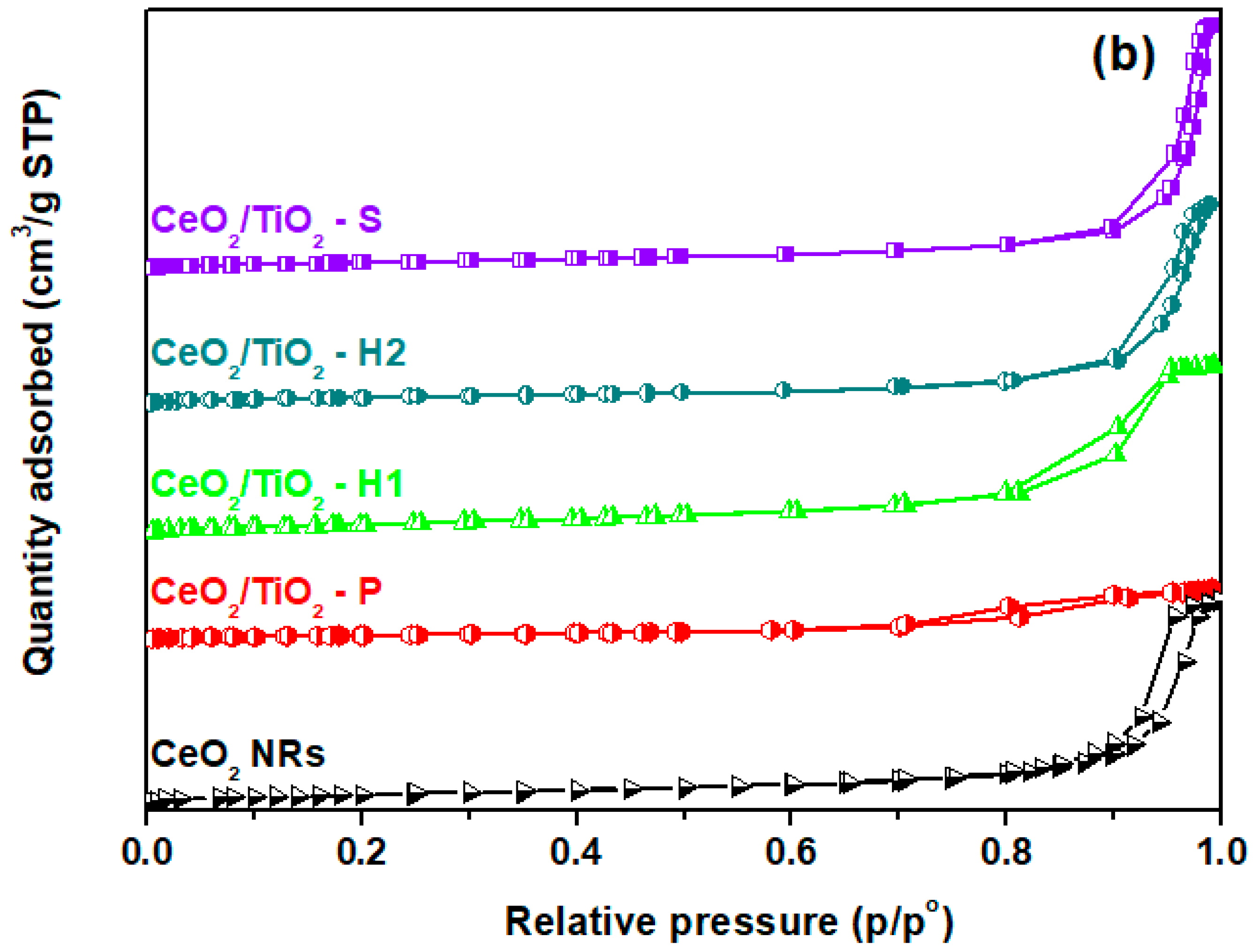
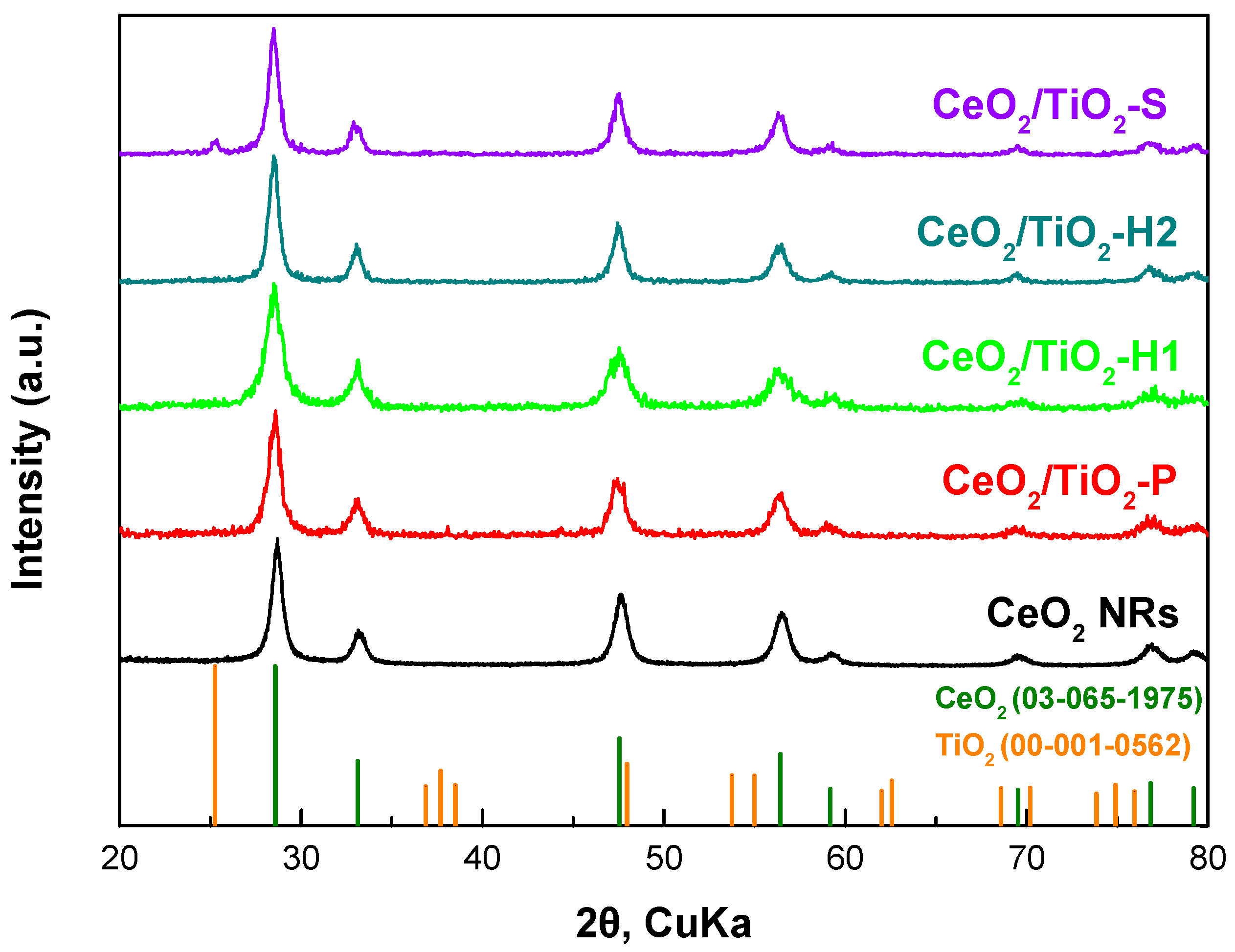
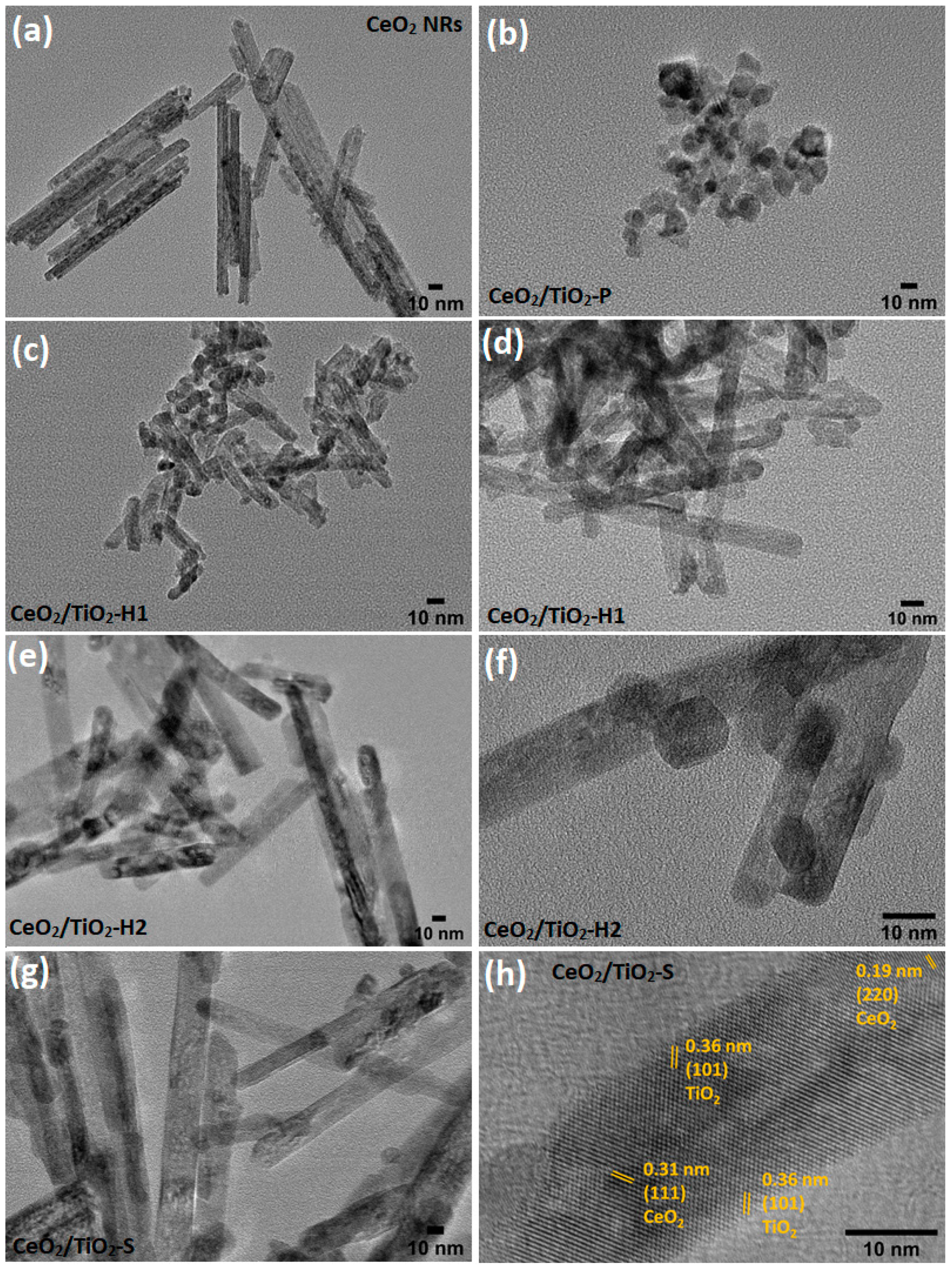

| Sample | BET Analysis | XRD Analysis | H2-TPR | |||
|---|---|---|---|---|---|---|
| BET Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Crystallite Size, DXRD (nm) CeO2 | Oxygen Storage Capacity (mmol O2 g−1) | Temperature of Peak Maximum (°C) | |
| CeO2 NRs | 73.9 | 0.48 | 24.2 | 13 | 0.29 | 545 |
| CeO2/TiO2-P | 41.5 | 0.13 | 9.3 | 12 | - | - |
| CeO2/TiO2-H1 | 100.9 | 0.41 | 15.0 | 9 | 0.34 | 654 |
| CeO2/TiO2-H2 | 63.2 | 0.48 | 24.1 | 14 | - | - |
| CeO2/TiO2-S | 72.0 | 0.58 | 32.0 | 12 | 0.79 | 573 |
| Sample | CO Conversion (%) | Specific Rate | |
|---|---|---|---|
| r (μmol g−1 s−1) | r (×100) (μmol m−2 s−1) | ||
| CeO2-NRs | 5.1 | 0.056 | 0.075 |
| CeO2/TiO2-P | 1.9 | 0.021 | 0.050 |
| CeO2/TiO2-H1 | 5.3 | 0.058 | 0.057 |
| CeO2/TiO2-H2 | 3.0 | 0.033 | 0.052 |
| CeO2/TiO2-S | 11.1 | 0.121 | 0.168 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides. Processes 2020, 8, 847. https://doi.org/10.3390/pr8070847
Stefa S, Lykaki M, Fragkoulis D, Binas V, Pandis PK, Stathopoulos VN, Konsolakis M. Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides. Processes. 2020; 8(7):847. https://doi.org/10.3390/pr8070847
Chicago/Turabian StyleStefa, Sofia, Maria Lykaki, Dimitrios Fragkoulis, Vasileios Binas, Pavlos K. Pandis, Vassilis N. Stathopoulos, and Michalis Konsolakis. 2020. "Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides" Processes 8, no. 7: 847. https://doi.org/10.3390/pr8070847
APA StyleStefa, S., Lykaki, M., Fragkoulis, D., Binas, V., Pandis, P. K., Stathopoulos, V. N., & Konsolakis, M. (2020). Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides. Processes, 8(7), 847. https://doi.org/10.3390/pr8070847










