EEG Synchronization Analysis for Seizure Prediction: A Study on Data of Noninvasive Recordings
Abstract
1. Introduction
2. Methods
2.1. A Scalp EEG Database
2.2. Synchronization Measures and Graph Model
2.3. Embedding Synchronization Measures into a Network Model
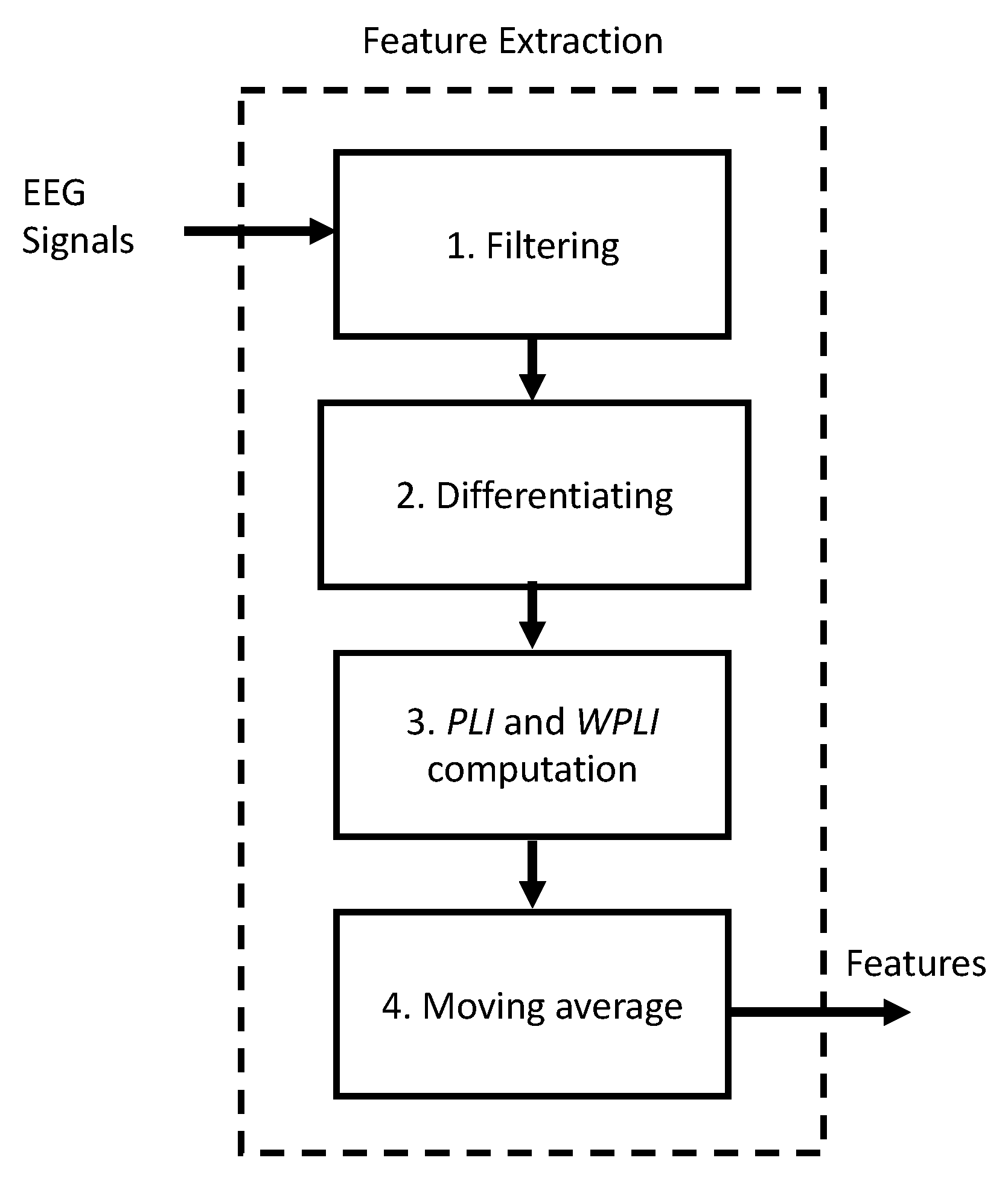
2.4. Employing Synchronization Measures PLI and WPLI for Seizure Prediction
2.5. A Classification Approach for Epileptic Seizures Prediction
Classification and Postprocessing
3. Results
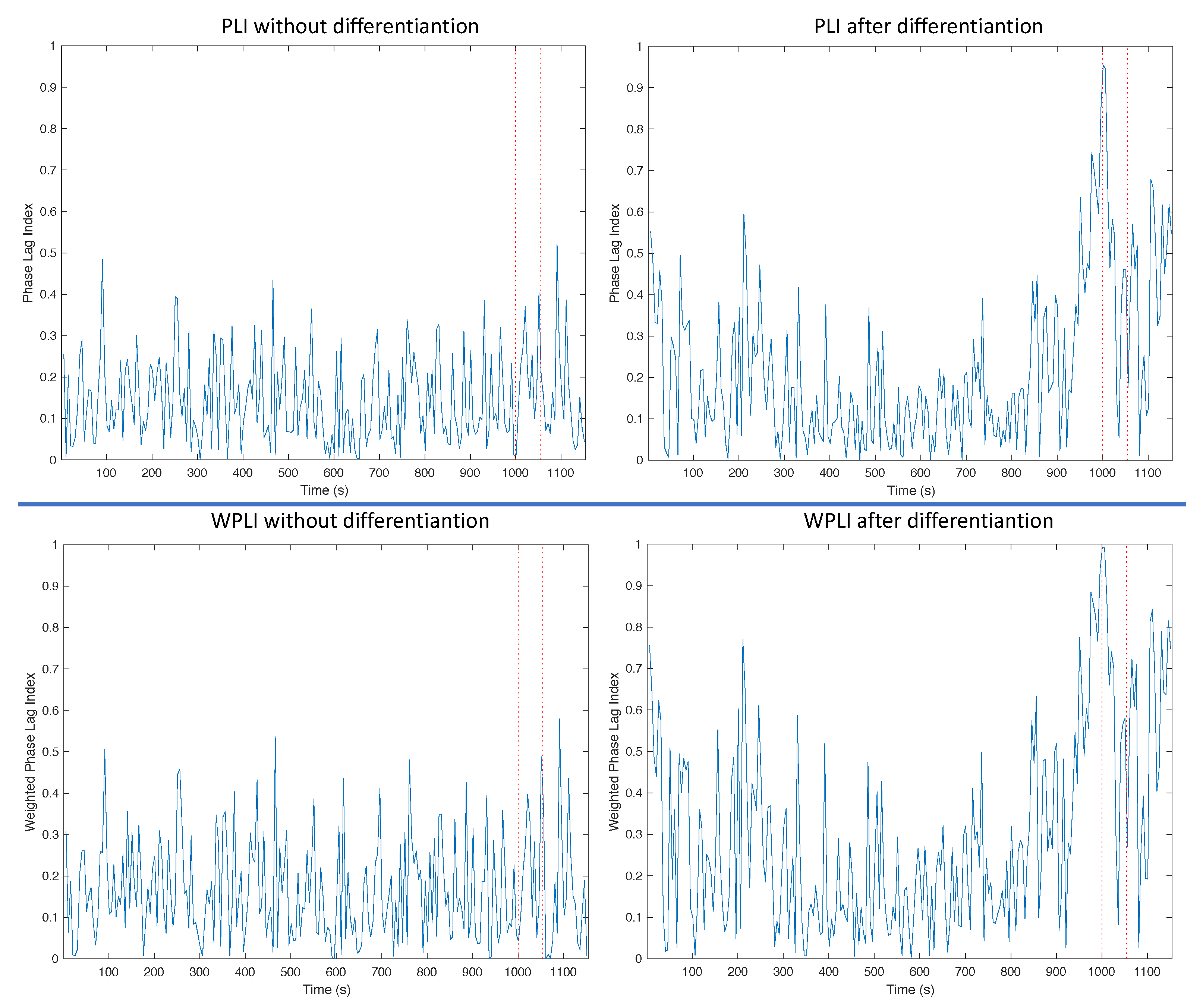
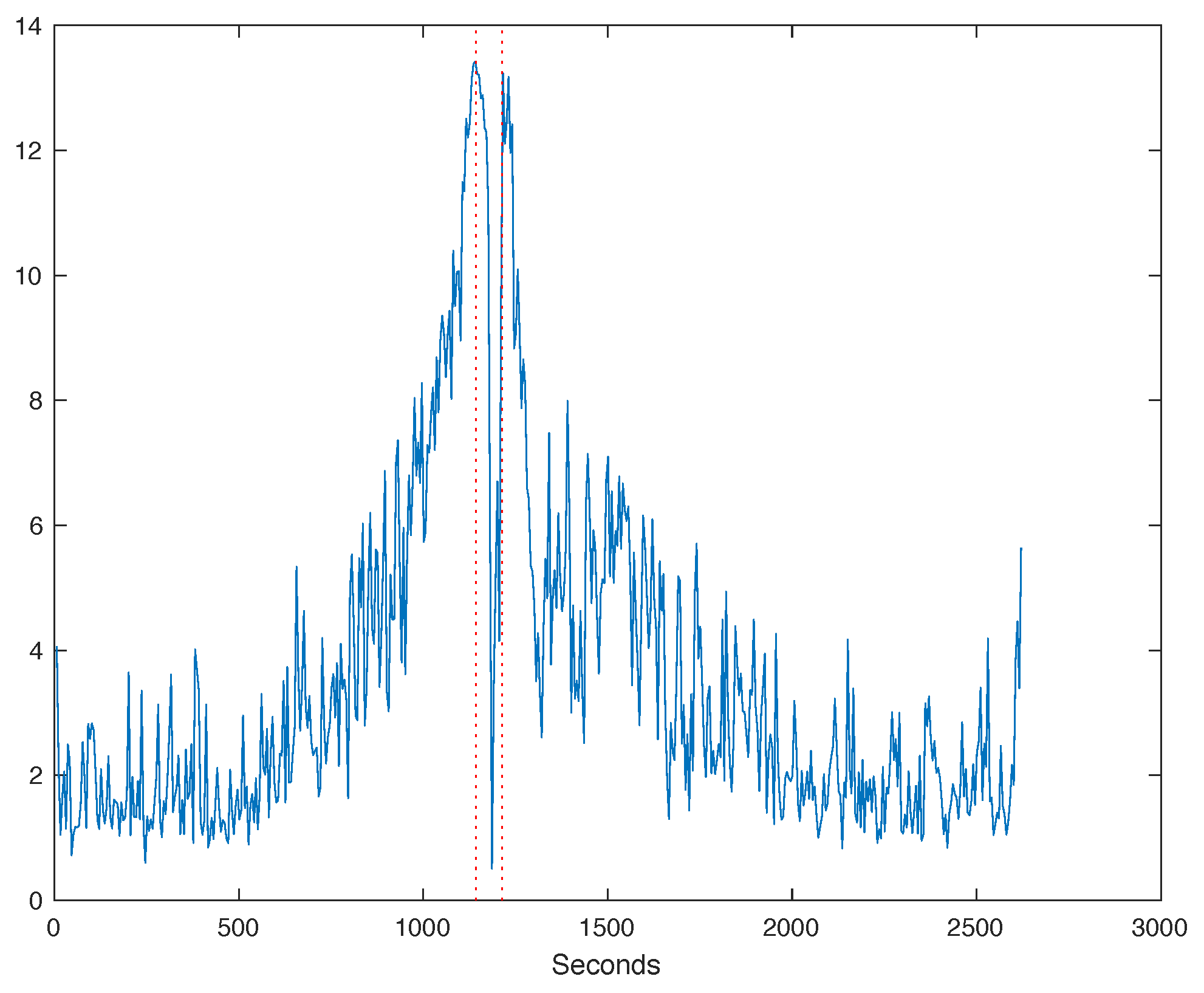
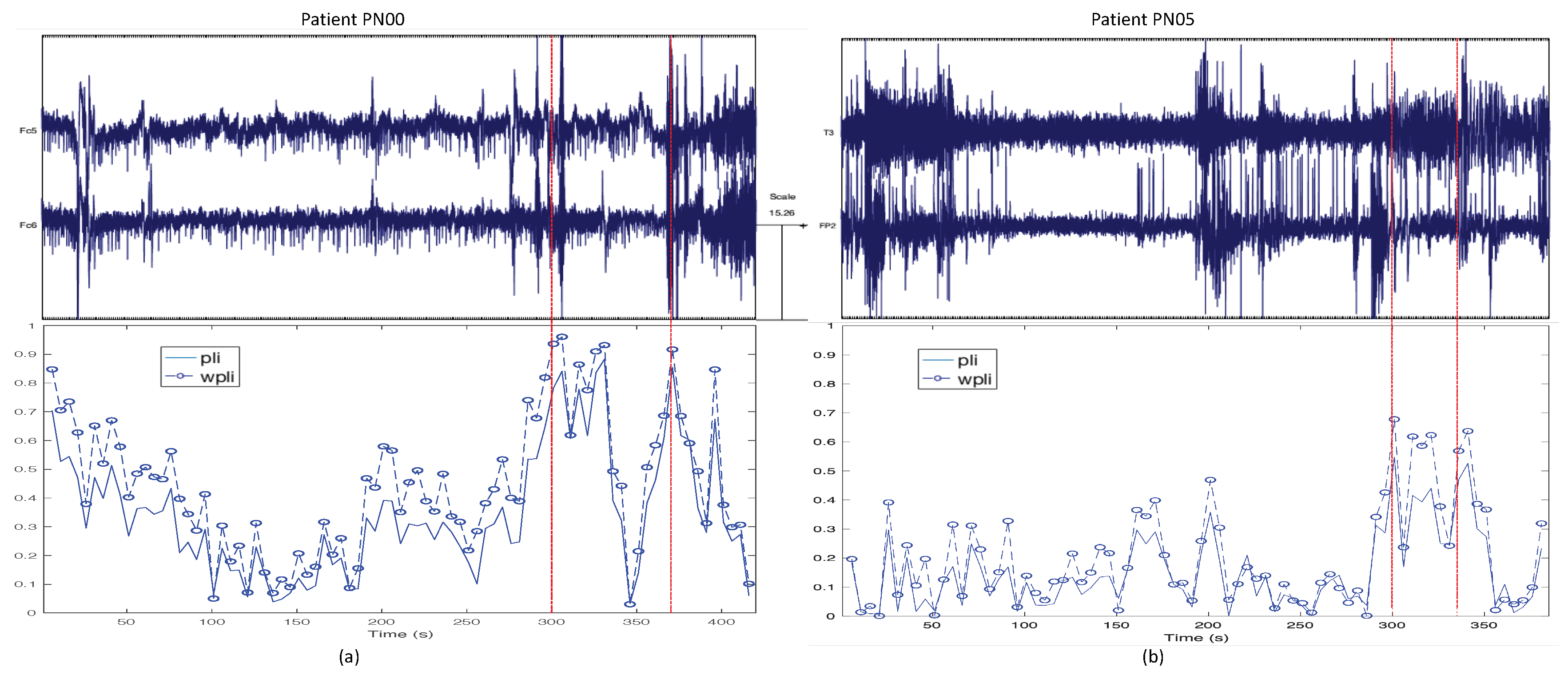
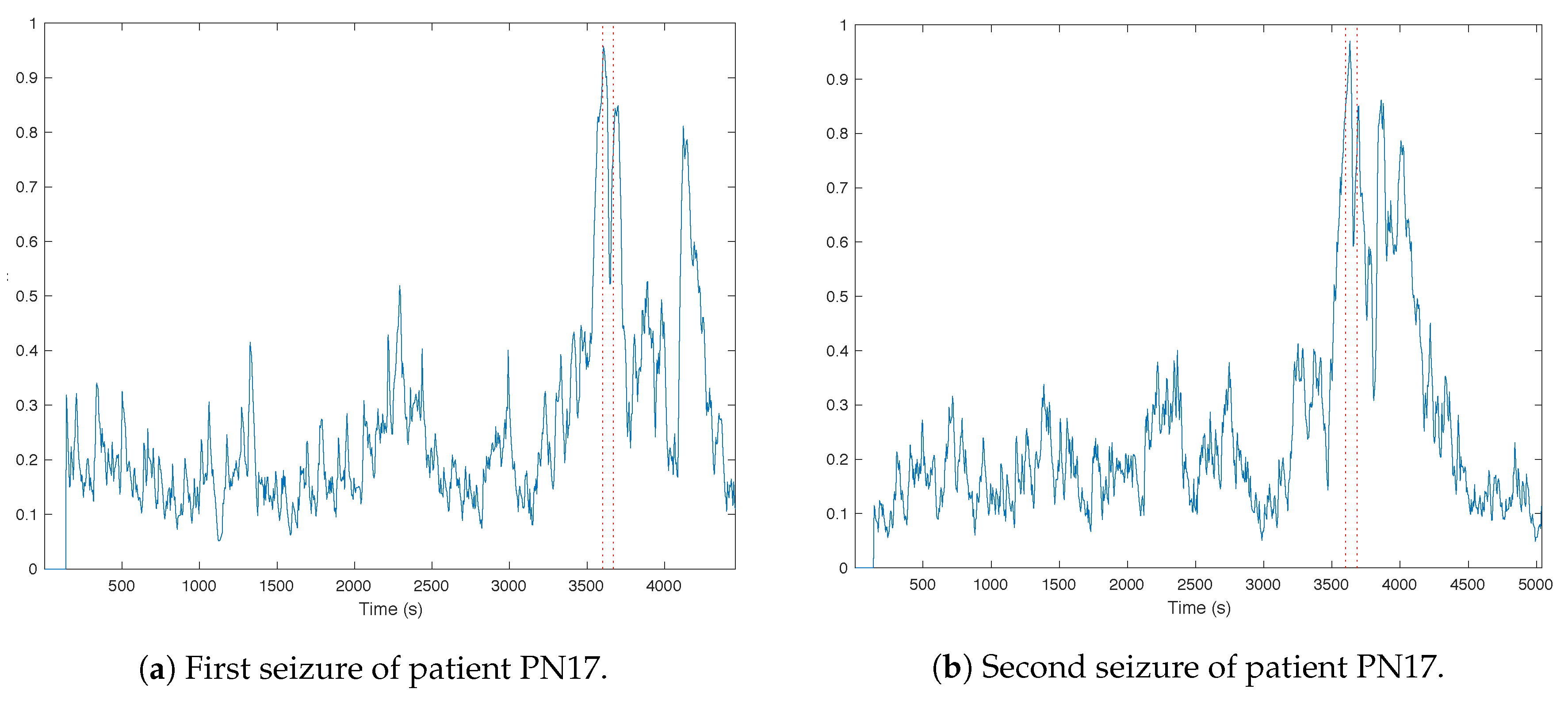
3.1. Trends of the Features for Patient Datasets with One or Two Seizures
3.2. Results of the Classification Algorithm
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Epilepsy Foundations. Available online: https://www.epilepsy.com/ (accessed on 25 January 2019).
- Deckers, C.L.P.; Genton, P.; Sills, G.J.; Schmidt, D. Current limitations of antiepileptic drug therapy: A conference review. Epilepsy Res. 2003, 53, 1–17. [Google Scholar] [CrossRef]
- Assi, E.B.; Nguyen, D.K.; Rihana, S.; Sawan, M. Towards accurate prediction of epileptic seizures: A review. Biomed. Signal Process. Control 2017, 34, 144–157. [Google Scholar] [CrossRef]
- Gadhoumi, K.; Lina, J.M.; Mormann, F.; Gotman, J. Seizure prediction for therapeutic devices: A review. J. Neurosci. Methods 2016, 260, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Chisci, L.; Mavino, A.; Perferi, G.; Sciandrone, M.; Anile, C.; Colicchio, G.; Fuggetta, F. Real-Time Epileptic Seizure Prediction Using AR Models and Support Vector Machines. IEEE Trans. Biomed. Eng. 2010, 57, 1124–1132. [Google Scholar] [CrossRef]
- Mirowski, P.; Madhavan, D.; LeCun, Y.; Kuzniecky, R. Classification of patterns of EEG synchronization for seizure prediction. Clin. Neurophysiol. 2009, 120, 1927–1940. [Google Scholar] [CrossRef]
- Shoeb, A.H. Application of Machine Learning to Epileptic Seizure Onset Detection and Treatment. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2009. [Google Scholar]
- Van Esbroeck, A.; Smith, L.; Syed, Z.; Singh, S.; Karam, Z. Multi-task seizure detection: Addressing intra-patient variation in seizure morphologies. Mach. Learn. 2016, 102, 309–321. [Google Scholar] [CrossRef]
- Bandarabadi, M.; Rasekhi, J.; Teixeira, C.A.; Karami, M.R.; Dourado, A. On the proper selection of preictal period for seizure prediction. Epilepsy Behav. 2015, 46, 158–166. [Google Scholar] [CrossRef]
- Fan, M.; Chou, C. Detecting Abnormal Pattern of Epileptic Seizures via Temporal Synchronization of EEG Signals. IEEE Trans. Biomed. Eng. 2018, 66, 601–608. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Freestone, D.; Lai, A.; Burkitt, A.N.; Fuller, K.; Grayden, D.B.; Seiderer, L.; Vogrin, S.; Mareels, I.M.Y.; Cook, M.J. Patient-specific bivariate-synchrony-based seizure prediction for short prediction horizons. Epilepsy Res. 2010, 91, 214–231. [Google Scholar] [CrossRef]
- Mormann, F.; Lehnertz, K.; David, P.; Elger, C.E. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys. D Nonlinear Phenom. 2000, 144, 358–369. [Google Scholar] [CrossRef]
- Mormann, F.; Kreuz, T.; Andrzejak, R.G.; David, P.; Lehnertz, K.; Elger, C.E. Epileptic seizures are preceded by a decrease in synchronization. Epilepsy Res. 2003, 53, 173–185. [Google Scholar] [CrossRef]
- Myers, M.H.; Padmanabha, A.; Hossain, G.; de Jongh Curry, A.L.; Blaha, C.D. Seizure prediction and detection via phase and amplitude lock values. Front. Hum. Neurosci. 2016, 10, 80. [Google Scholar] [CrossRef]
- Kramer, M.A.; Cash, S.S. Epilepsy as a disorder of cortical network organization. Neuroscientist 2012, 18, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, L.; Daunizeau, J.; Walker, M. Concepts of connectivity and human epileptic activity. Front. Syst. Neurosci. 2011, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wendling, F.; Chauvel, P.; Biraben, A.; Bartolomei, F. From intracerebral EEG signals to brain connectivity: Identification of epileptogenic networks in partial epilepsy. Front. Syst. Neurosci. 2010, 4, 154. [Google Scholar] [CrossRef]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef]
- Vinck, M.; Oostenveld, R.; Van Wingerden, M.; Battaglia, F.; Pennartz, C.M. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef]
- Detti, P.; de Lara, G.Z.M.; Bruni, R.; Pranzo, M.; Sarnari, F.; Vatti, G. A Patient-specific Approach for Short-term Epileptic Seizures Prediction through the Analysis of EEG synchronization. IEEE Trans. Biomed. Eng. 2018, 66, 1494–1504. [Google Scholar] [CrossRef]
- PANACEE Project. Available online: https://panacee.diism.unisi.it/ (accessed on 25 June 2020).
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Perez, E.R.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Siena Scalp Database. Available online: https://drive.google.com/drive/folders/1Xk0de2pMJhilrCul72QXUSOQ8DWuxT53?usp=sharing (accessed on 25 June 2020).
- Zheng, Y.; Wang, G.; Li, K.; Bao, G.; Wang, J. Epileptic seizure prediction using phase synchronization based on bivariate empirical mode decomposition. Clin. Neurophysiol. 2014, 125, 1104–1111. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Majumdar, K.K.; Vardhan, P. Automatic seizure detection in ECoG by differential operator and windowed variance. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 356–365. [Google Scholar] [CrossRef]
- Parvez, M.Z.; Manoranjan, P. Epileptic seizure prediction by exploiting spatiotemporal relationship of EEG signals using phase correlation. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 158–168. [Google Scholar] [CrossRef]

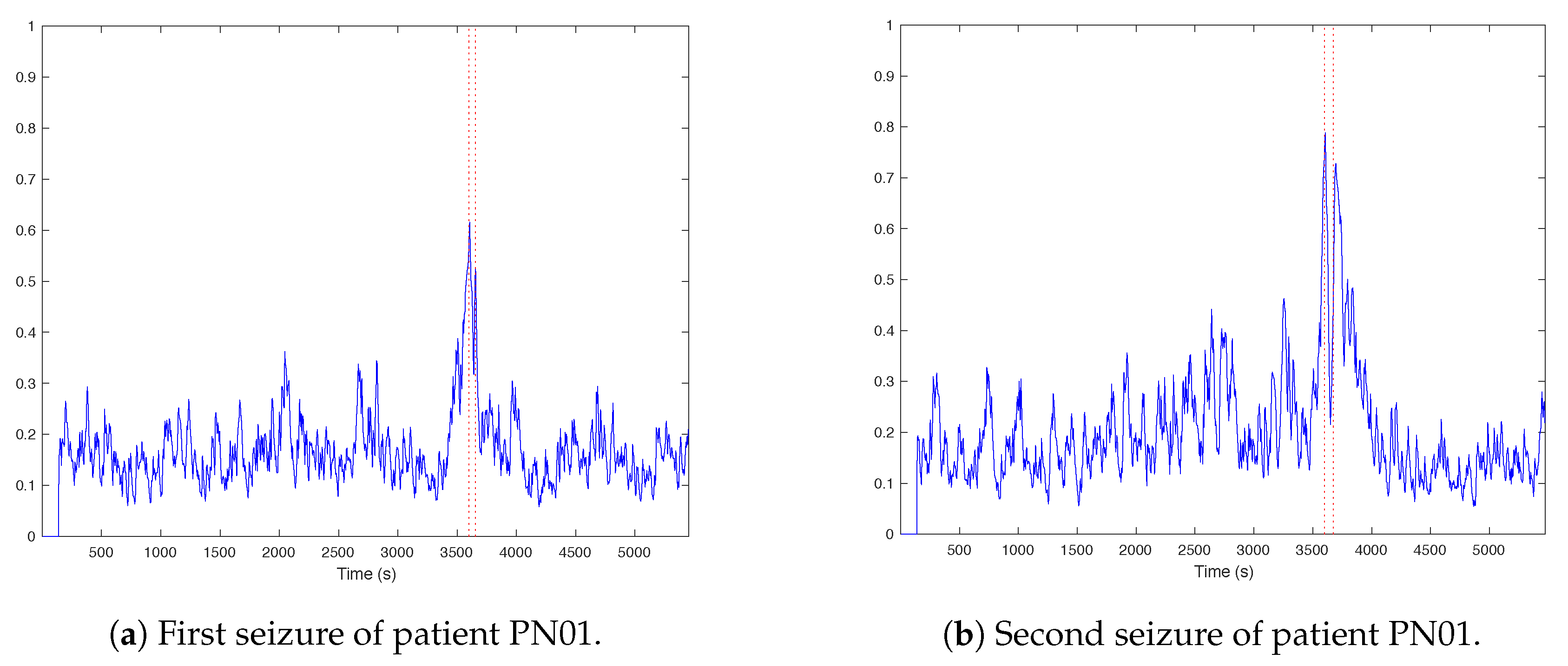

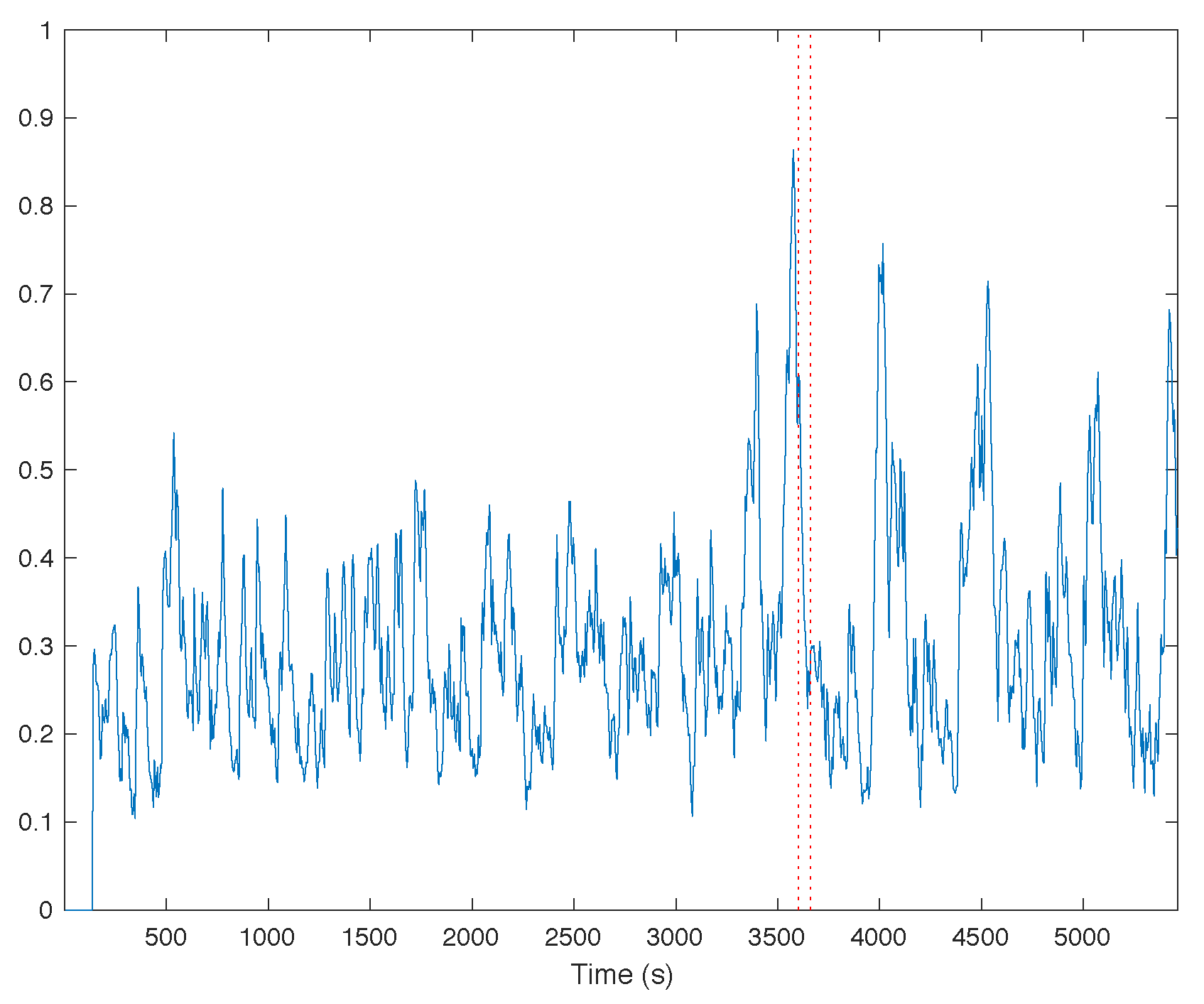
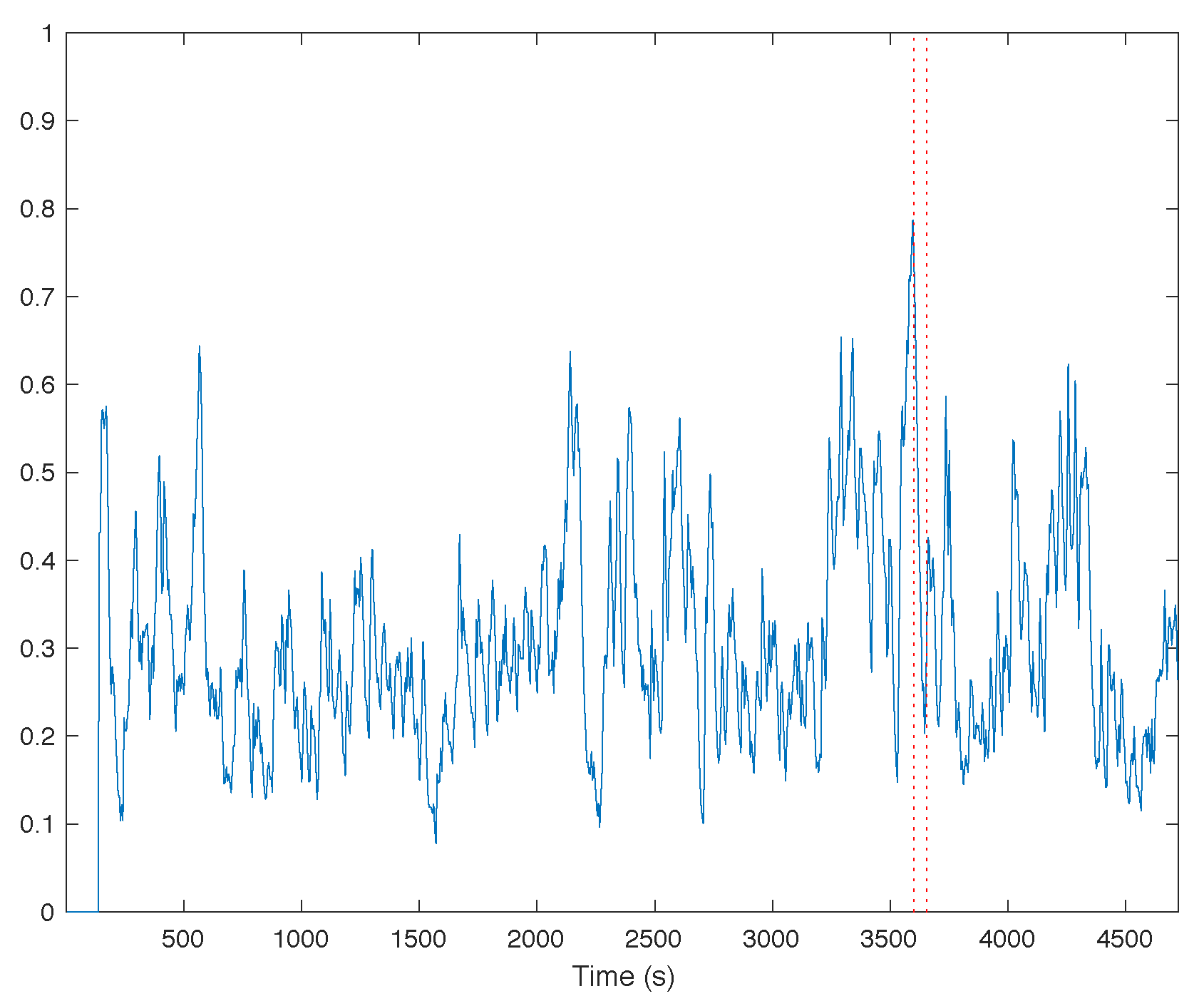
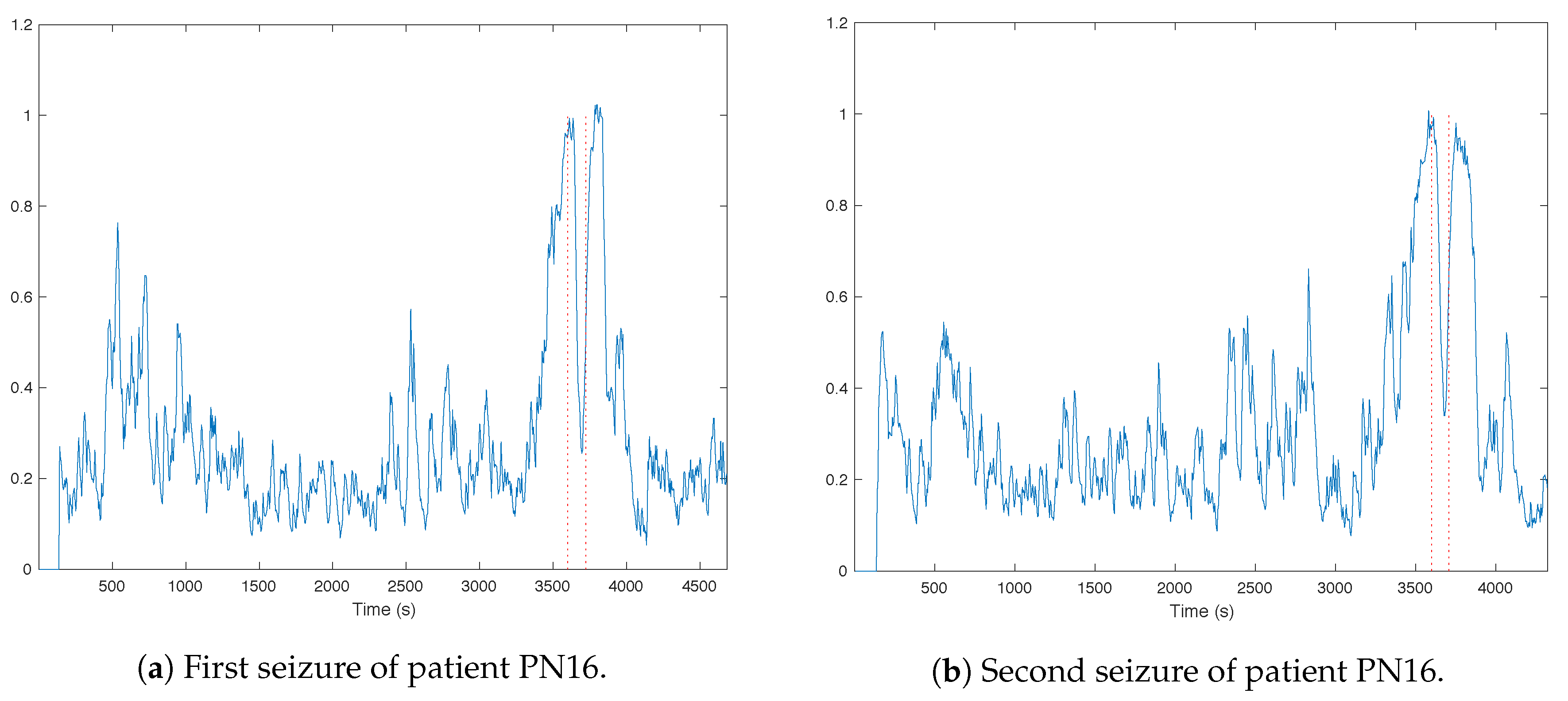
| Pat. Id | Age (Years) | Gender | Seizure | Localization | Lateralization | # EEG Chan. | # Seiz. | Time |
|---|---|---|---|---|---|---|---|---|
| PN00 | 55 | Male | IAS | T | R | 29 | 5 | 198 |
| PN01 | 46 | Male | IAS | T | L | 29 | 2 | 809 |
| PN03 | 54 | Male | IAS | T | R | 29 | 2 | 752 |
| PN05 | 51 | Female | IAS | T | L | 29 | 3 | 359 |
| PN06 | 36 | Male | IAS | T | L | 29 | 5 | 722 |
| PN07 | 20 | Female | IAS | T | L | 29 | 1 | 523 |
| PN09 | 27 | Female | IAS | T | L | 29 | 3 | 410 |
| PN10 | 25 | Male | FBTC | F | Bilateral | 21 | 10 | 1002 |
| PN11 | 58 | Female | IAS | T | R | 29 | 1 | 145 |
| PN12 | 71 | Male | IAS | T | L | 29 | 4 | 246 |
| PN13 | 34 | Female | IAS | T | L | 29 | 3 | 519 |
| PN14 | 49 | Male | WIAS | T | L | 29 | 4 | 1408 |
| PN16 | 41 | Female | IAS | T | L | 29 | 2 | 303 |
| PN17 | 42 | Male | IAS | T | R | 29 | 2 | 308 |
| Pat. Id | Seiz. inst. | Nonseiz. inst. | Train Seiz. | Test Seiz. | Train Hours | Test Hours |
|---|---|---|---|---|---|---|
| PN00 | 5 | 0 | 3 | 2 | 0.86 | 0.54 |
| PN05 | 3 | 2 | 2 | 1 | 2.93 | 1.96 |
| PN06 | 5 | 7 | 3 | 2 | 5.45 | 4.05 |
| PN09 | 3 | 3 | 2 | 1 | 2.99 | 2.98 |
| PN10 | 8 | 4 | 4 | 4 | 6.69 | 4.44 |
| PN12 | 4 | 3 | 2 | 2 | 3.15 | 2.17 |
| PN13 | 3 | 4 | 2 | 1 | 4.08 | 3.09 |
| PN14 | 4 | 12 | 2 | 2 | 8.84 | 6.92 |
| ThAlgo [20] | ThAlgo [20] with Post Processing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pred% | spec% | pred% | spec% | |||||||
| PN00 | 100 | 100 | 142.80 | 29 | 213 | 100 | 100 | 142.8 | 29 | 213 |
| PN05 | 100 | 98.20 | 29.67 | 15 | 37 | 100 | 98.94 | 24.67 | 10 | 32 |
| PN06 | 100 | 95.46 | 152.50 | 0 | 294 | 100 | 97.09 | 157.60 | 0 | 293 |
| PN09 | 100 | 99.98 | 7.67 | 0 | 19 | 100 | 100 | 12.67 | 0 | 29 |
| PN10 | 100 | 96.28 | 94.90 | 0 | 290 | 100 | 96.73 | 131.05 | 0 | 291 |
| PN12 | 90 | 96.63 | 221.67 | 0 | 292 | 100 | 98.78 | 157.40 | 41 | 289 |
| PN13 | 100 | 100 | 67.00 | 1 | 157 | 100 | 100 | 67.00 | 1 | 157 |
| PN14 | 80 | 98.06 | 131.50 | 0 | 289 | 80 | 98.86 | 134.38 | 0 | 269 |
| Av. | 96.25 | 98.08 | 105.96 | 5.63 | 198.88 | 97.50 | 98.80 | 103.44 | 10.13 | 196.63 |
| ModThAlgo with | ModThAlgo with | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pred% | spec% | pred% | spec% | |||||||
| PN00 | 100 | 100 | 166.3 | 64 | 248 | 100 | 100 | 166.3 | 64 | 248 |
| PN05 | 100 | 99.11 | 18 | 2 | 32 | 100 | 99.62 | 598 | 420 | 767 |
| PN06 | 100 | 98.4 | 86.6 | 0 | 202 | 100 | 99.66 | 357.3 | 107 | 649 |
| PN09 | 100 | 100 | 25.6667 | 4 | 64 | 100 | 100 | 25.67 | 4 | 64 |
| PN10 | 100 | 94.11 | 140.25 | 0 | 294 | 100 | 98.69 | 578.15 | 216 | 887 |
| PN12 | 100 | 100 | 58.5 | 0 | 293 | 100 | 100 | 58.5 | 0 | 293 |
| PN13 | 100 | 100 | 58.6667 | 1 | 127 | 100 | 100 | 58.67 | 1 | 127 |
| PN14 | 100 | 99.39 | 212.2 | 33 | 273 | 100 | 99.38 | 341.2 | 33 | 697 |
| Av. | 100 | 98.88 | 95.77 | 13 | 191.63 | 100 | 99.67 | 272.97 | 105.63 | 466.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detti, P.; Vatti, G.; Zabalo Manrique de Lara, G. EEG Synchronization Analysis for Seizure Prediction: A Study on Data of Noninvasive Recordings. Processes 2020, 8, 846. https://doi.org/10.3390/pr8070846
Detti P, Vatti G, Zabalo Manrique de Lara G. EEG Synchronization Analysis for Seizure Prediction: A Study on Data of Noninvasive Recordings. Processes. 2020; 8(7):846. https://doi.org/10.3390/pr8070846
Chicago/Turabian StyleDetti, Paolo, Giampaolo Vatti, and Garazi Zabalo Manrique de Lara. 2020. "EEG Synchronization Analysis for Seizure Prediction: A Study on Data of Noninvasive Recordings" Processes 8, no. 7: 846. https://doi.org/10.3390/pr8070846
APA StyleDetti, P., Vatti, G., & Zabalo Manrique de Lara, G. (2020). EEG Synchronization Analysis for Seizure Prediction: A Study on Data of Noninvasive Recordings. Processes, 8(7), 846. https://doi.org/10.3390/pr8070846





