Alternative Briquette Material Made from Palm Stem Biomass Mediated by Glycerol Crude of Biodiesel Byproducts as a Natural Adhesive
Abstract
:1. Introduction
2. Materials and Methods
3. Experiment
4. Central Composite Design (CCD)
5. Results and Discussion
5.1. Raw Material and Product Characterization
5.2. Heating Value Analysis
5.3. Compressive Strength Analysis
5.4. Briquette Density
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dewan Energi Nasional. Buku Ketahanan Energi Nasional; Dewan Energi Nasional: Jakarta, Indonesia, 2014. [Google Scholar]
- Kurniawan, R.; Managi, S. Coal consumption, urbanization, and trade openness linkage in Indonesia. Energy Policy 2018, 121, 576–583. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Alatas, M.; Subhan; Lala, A.; Saiful; Suhendra, R.; Idroes, G.M. Marwan Geochemistry of hot springs in the Ie Seu’um hydrothermal areas at Aceh Besar district, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012002. [Google Scholar] [CrossRef]
- Marwan; Syukri, M.; Idroes, R.; Ismail, N. Deep and Shallow Structures of Geothermal Seulawah Agam Based on Electromagnetic and Magnetic Data. Int. J. GEOMATE 2019, 16, 141–147. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Alatas, M.; Subhan; Lala, A.; Muslem; Suhendra, R.; Idroes, G.M.; Suhendrayatna; Marwan; et al. Geochemistry of warm springs in the Ie Brôuk hydrothermal areas at Aceh Besar district. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012010. [Google Scholar] [CrossRef]
- Putri, D.R.; Nanda, M.; Rizal, S.; Idroes, R.; Ismail, N. Interpretation of gravity satellite data to delineate structural features connected to geothermal resources at Bur Ni Geureudong geothermal field. IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012003. [Google Scholar] [CrossRef] [Green Version]
- Idroes, R.; Yusuf, M.; Alatas, M.; Subhan; Lala, A.; Muhammad; Suhendra, R.; Idroes, G.M. Marwan Geochemistry of Sulphate spring in the Ie Jue geothermal areas at Aceh Besar district, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012012. [Google Scholar] [CrossRef]
- Marwan; Yanis, M.; Idroes, R.; Ismail, N. 2D inversion and static shift of MT and TEM data for imaging the geothermal resources of Seulawah Agam Volcano, Indonesia. Int. J. GEOMATE 2019, 17, 173–180. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Saiful, S.; Alatas, M.; Subhan, S.; Lala, A.; Muslem, M.; Suhendra, R.; Idroes, G.M.; Marwan, M.; et al. Geochemistry Exploration and Geothermometry Application in the North Zone of Seulawah Agam, Aceh Besar District, Indonesia. Energies 2019, 12, 4442. [Google Scholar] [CrossRef] [Green Version]
- Erinofiardi; Gokhale, P.; Date, A.; Akbarzadeh, A.; Bismantolo, P.; Suryono, A.F.; Mainil, A.K.; Nuramal, A. A Review on Micro Hydropower in Indonesia. Energy Procedia 2017, 110, 316–321. [Google Scholar] [CrossRef]
- Biddinika, M.K.; Indrawan, B.; Yoshikawa, K.; Tokimatsu, K.; Takahashi, F. Renewable Energy on the Internet: The Readability of Indonesian Biomass Websites. Energy Procedia 2014, 61, 1376–1379. [Google Scholar] [CrossRef] [Green Version]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview properties of biodiesel diesel blends from edible and non-edible feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Handayani, N.A.; Ariyanti, D. Potency of Solar Energy Applications in Indonesia. Int. J. Renew. Energy Dev. 2012, 1, 33. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Characterization of PV panel and global optimization of its model parameters using genetic algorithm. Energy Convers. Manag. 2013, 73, 10–25. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Techno-economic analysis of an optimized photovoltaic and diesel generator hybrid power system for remote houses in a tropical climate. Energy Convers. Manag. 2013, 69, 163–173. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.-T.; Indra Mahlia, T.M.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Martosaputro, S.; Murti, N. Blowing the Wind Energy in Indonesia. Energy Procedia 2014, 47, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Dirjen Energi Baru dan Terbarukan dan Konservasi Energi Kementrian ESDM. Statistika EBTKE; EBTKE: Jakarta, Indonesia, 2015. [Google Scholar]
- Silitonga, A.S.; Atabani, A.E.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Mekhilef, S. A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- Hedwig, R.; Lahna, K.; Idroes, R.; Karnadi, I.; Tanra, I.; Iqbal, J.; Kwaria, D.; Kurniawan, D.P.; Kurniawan, K.H.; Tjia, M.O.; et al. Food analysis employing high energy nanosecond laser and low pressure He ambient gas. Microchem. J. 2019, 147, 356–364. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef]
- Hasanah, U.; Setyowati, M.; Edwarsyah; Efendi, R.; Safitri, E.; Idroes, R.; Heng, L.Y.; Sani, N.D. Isolation of Pectin from coffee pulp Arabica Gayo for the development of matrices membrane. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 12014. [Google Scholar] [CrossRef]
- Hasanah, U.; Sani, N.D.M.; Heng, L.Y.; Idroes, R.; Safitri, E. Construction of a Hydrogel Pectin-Based Triglyceride Optical Biosensor with Immobilized Lipase Enzymes. Biosensors 2019, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Hasanah, U.; Setyowati, M.; Efendi, R.; Muslem, M.; Md Sani, N.D.; Safitri, E.; Yook Heng, L.; Idroes, R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Direktorat Jenderal Perkebunan. Statistika Perkebunan Indonesia Komoditas Kelapa Sawit 2013–2015; Direktorat Jenderal Perkebunan: Jakarta, Indonesia, 2014. [Google Scholar]
- Wardani, L.; Massijaya, M.Y.; Machdie, M.F. Pemanfaatan Limbah Pelepah Sawit dan Plastik Daur Ulang (RPP) sebagai Papam Komposit Plastik. J. Hutan Trop. 2013, 01, 46–53. [Google Scholar]
- Tumuluru, J.S.; Wright, C.T.; Hess, J.R.; Kenney, K.L. A review of biomass densification systems to develop uniform feedstock commodities for bioenergy application. Biofuels Bioprod. Biorefining 2011, 5, 683–707. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield–A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Muslem, M.; Kuncaka, A.; Himah, T.N.; Roto, R. Preparation of Char-Fe3O4 Composites from Polyvinyl Chloride with Hydrothermal and Hydrothermal-Pyrolysis Carbonization Methods as Co(II) Adsorbents. Indones. J. Chem. 2019, 19, 835. [Google Scholar] [CrossRef]
- Asavatesanupap, C.; Santikunaporn, M. A Feasibility Study on Production of Solid Fuel from Glycerol and Agricultural Wastes. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2012, 01, 43–51. [Google Scholar]
- Umam, M.C. Optimasi Penambahan Limbah Gliserol Hasil Samping Transesterifikasi Minyak Jarak Pagar Dan Perekat Tapioka Pada Pembuatan Biomass Pellets Bungkil Jarak Pagar (Jatropha curcas L.); Institut Pertanian Bogor: Bogor, Indonesia, 2007. [Google Scholar]
- Susanty, W.; Helwani, Z. Zulfansyah Torrefaction of oil palm frond: The effect of process condition to calorific value and proximate analysis. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012016. [Google Scholar] [CrossRef] [Green Version]
- Susanty, W.; Helwani, Z.; Bahruddin, B. Optimization of the Condition of Palm Frond Torrefaction Process by Utilizing Liquid Torrefaction Product as Pre-treatment for Improve Product Quality. J. Rekayasa Kim. Lingkung. 2019, 14, 12–18. [Google Scholar] [CrossRef]
- Helwani, Z.; Zulfansyah; Fatra, W.; Fernando, A.Q.; Idroes, G.M.; Muslem; Idroes, R. Torrefaction of Empty Fruit Bunches: Evaluation of Fuel Characteristics Using Response Surface Methodology. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 012019. [Google Scholar] [CrossRef]
- Montgomery, C.D. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Helwani, Z.; Fatra, W.; Arifin, L.; Othman, M.R. Syapsan Effect of process variables on the calorific value and compressive strength of the briquettes made from high moisture Empty Fruit Bunches (EFB). IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012020. [Google Scholar] [CrossRef] [Green Version]
- Sinaga, S. Identifikasi Sifat Fisis dan Kimia Briket Arang Dari Sabut Kelapa; Institut Pertanian Bogor: Bogor, Indonesia, 2008. [Google Scholar]
- Surono, U.B. Peningkatan Kualitas Pembakaran Biomassa Limbah Jagung sebagai Bahan Bakar Alternatif dengan Karbonisasi dan Pembriketan. J. Rekayasa Proses 2010, 4, 13–18. [Google Scholar]
- Ilham, M.A.; Helwani, Z.; Fatra, W. Proses Densifikasi Produk Karbonisasi Pelepah Sawit menjadi Briket Menggunakan Gliserol Produk Samping Biodiesel sebagai Filler. J. Online Mhs. Fak. Tek. Univ. Riau 2016, 3, 1–4. [Google Scholar]
- Thompson, J.C.; He, B.B. Characterization of Crude Glycerol from Biodiesel Production from Multiple Feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Ali, A.; Fortuna, A.D.; Restuhadi, F. Kajian Pemanfaatan Biomassa Limbah Industri Minyak Picung (Pangium Edule Reinw) Untuk Biobriket Sumber Energi Alternatif Di Desa Pulau Picung. J. Sagu 2013, 11. [Google Scholar]
- Martynis, M.; Sundari, E.; Sari, E. Pembuatan Biobriket dari Limbah Cangkang Kakao. J. Litbang Ind. 2012, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Subroto, H. Sartono Pengaruh Variasi Tekanan Pengepresan Terhadap Karakteristik Mekanik dan Karakteristik Pembakaran Briket Kokas Lokal. J. Tek. Gelagar Univ. Sebel. Maret Surak. 2007, 18, 73–79. [Google Scholar]
- Sudiro, R.; Subroto, S. Karakteristik Briket Campuran Arang Kayu dan Jerami. J. Sainstech Politek. Indonusa Surak. 2008, 2355–5009. [Google Scholar]
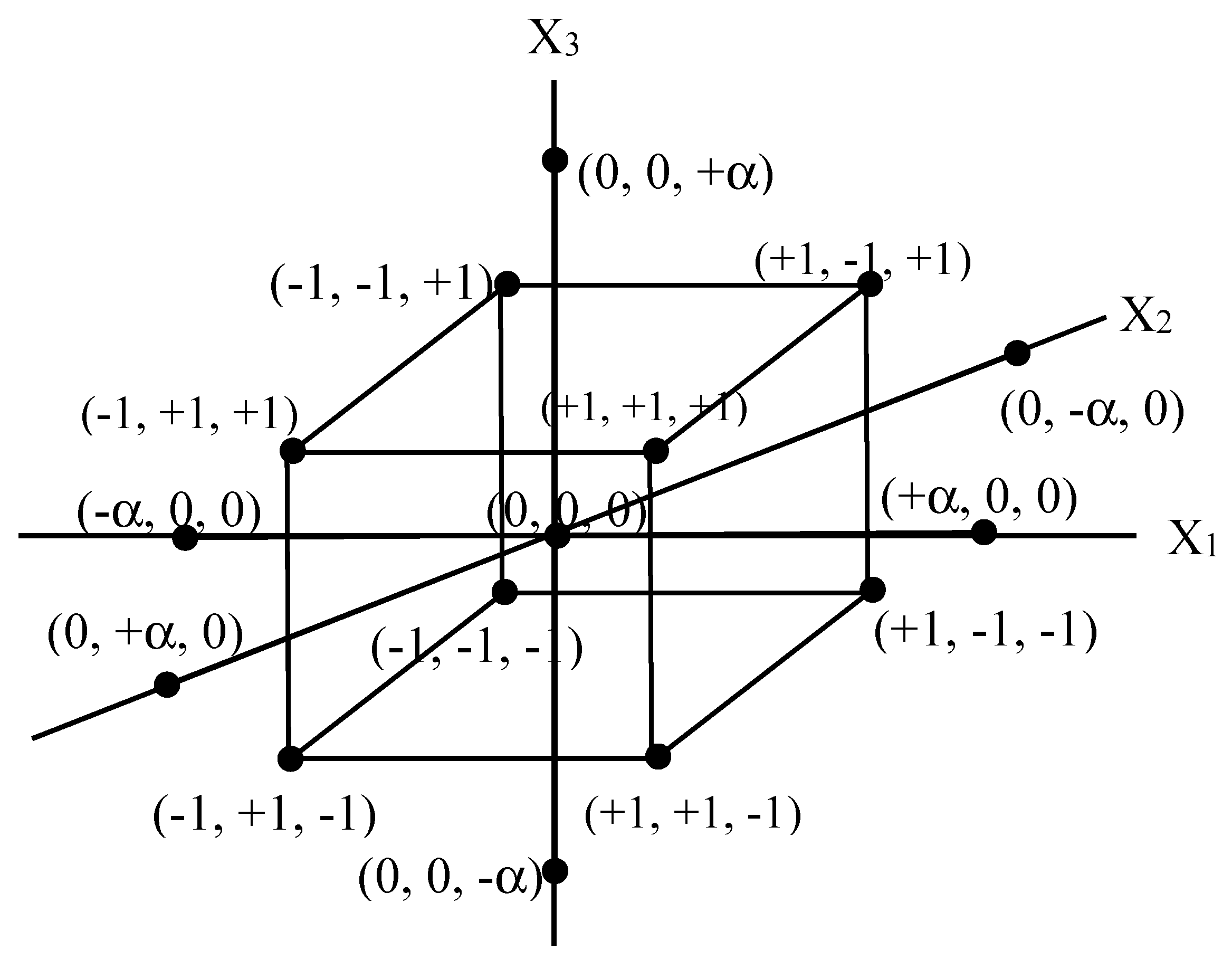

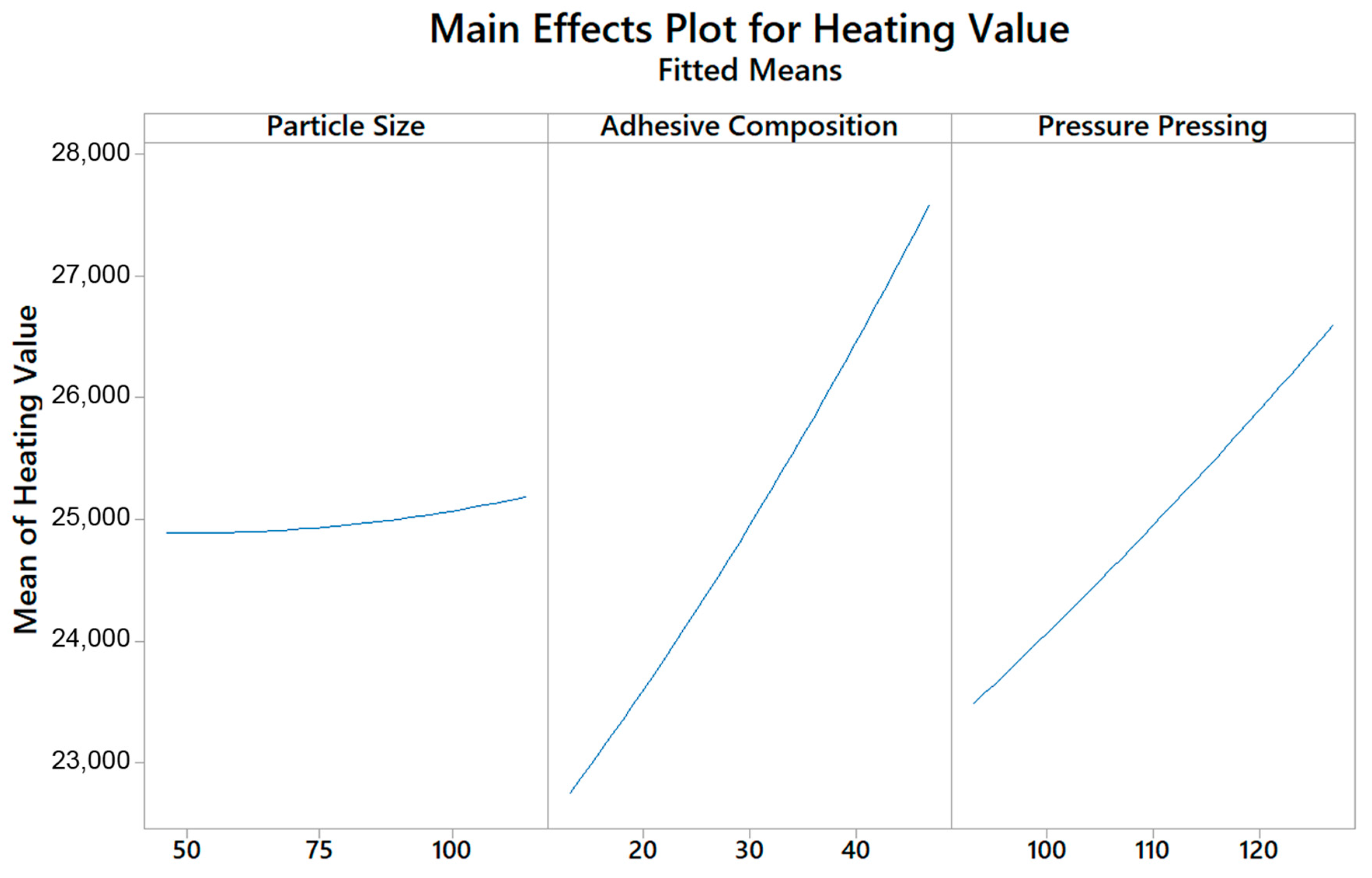

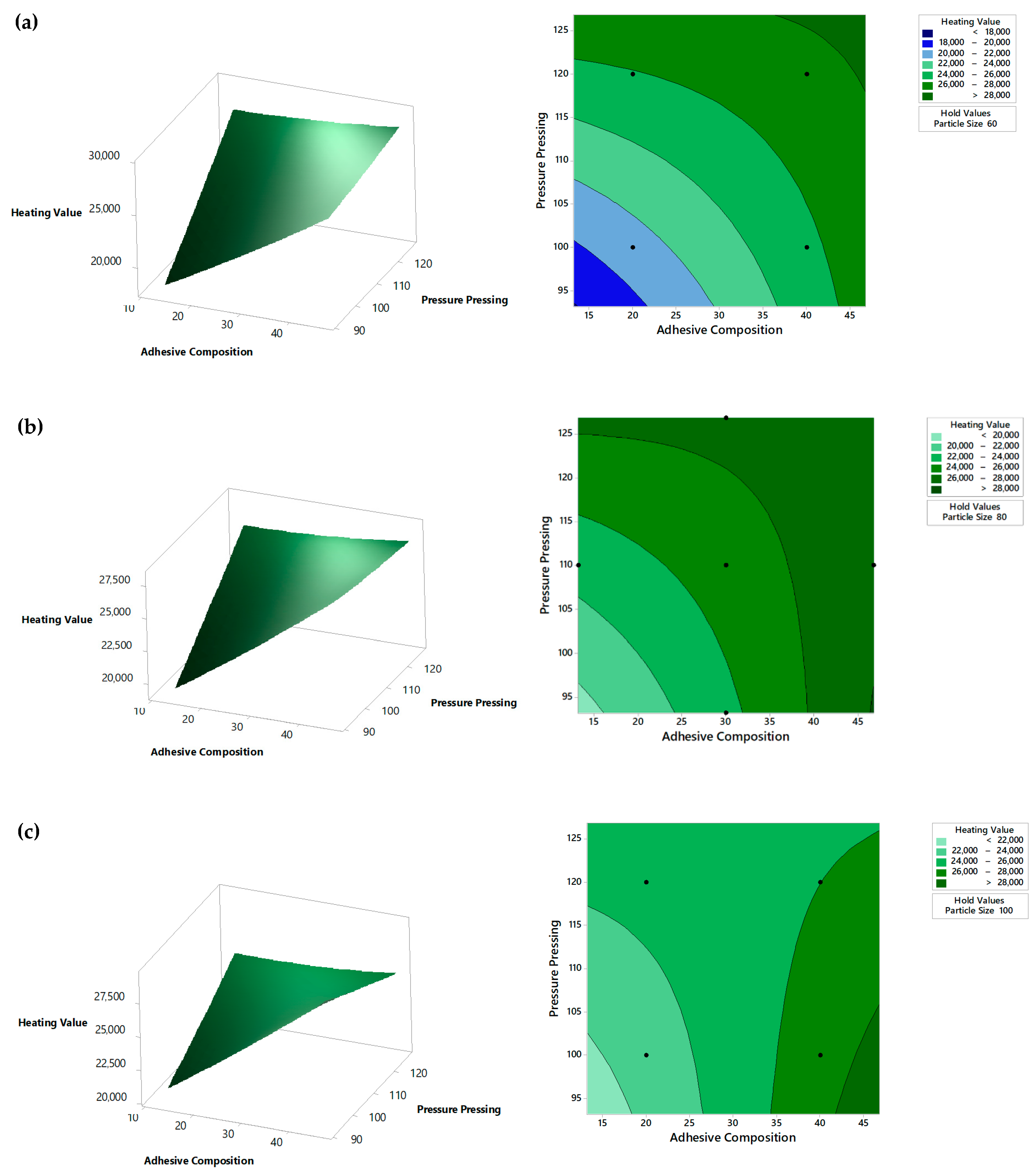
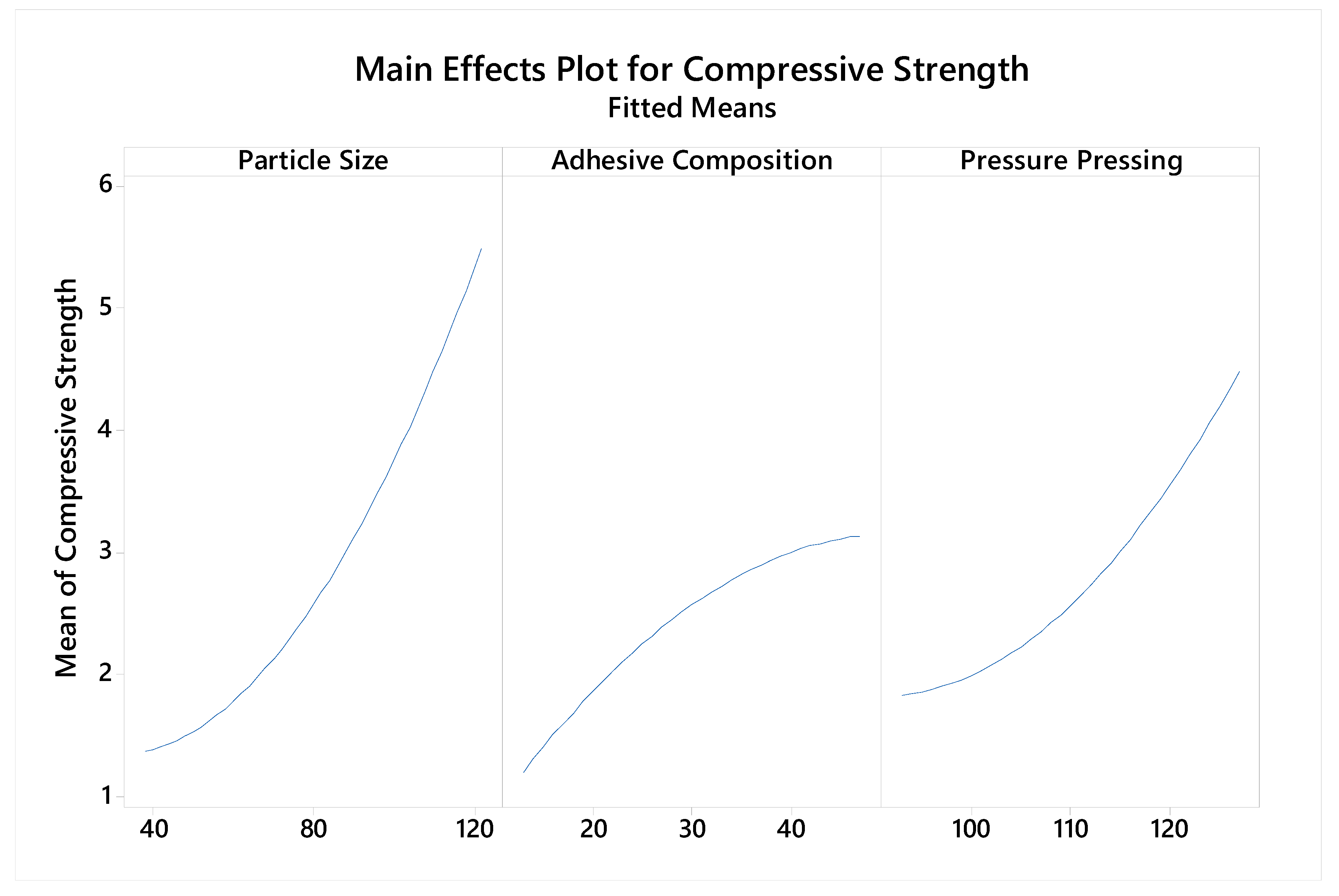


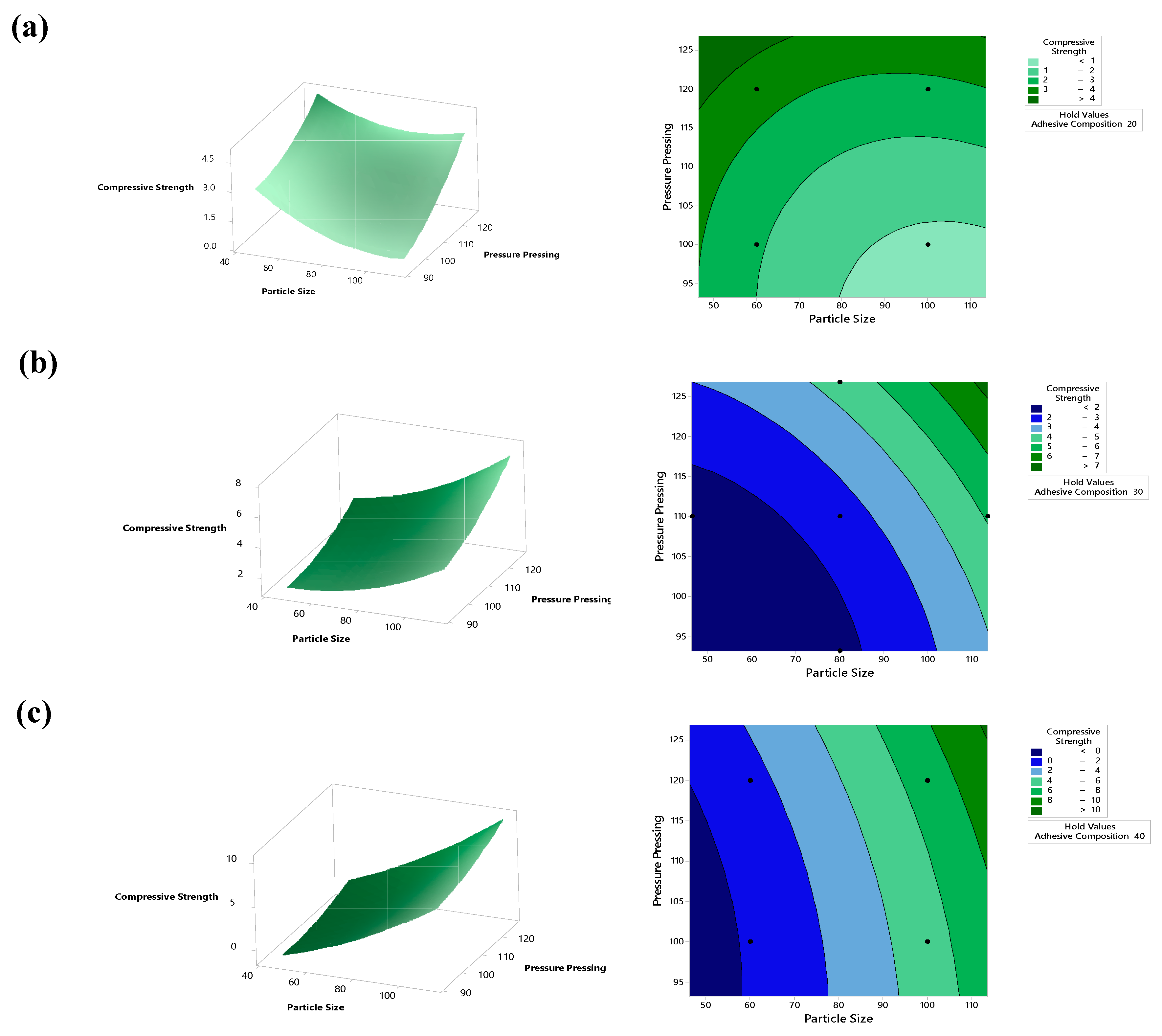
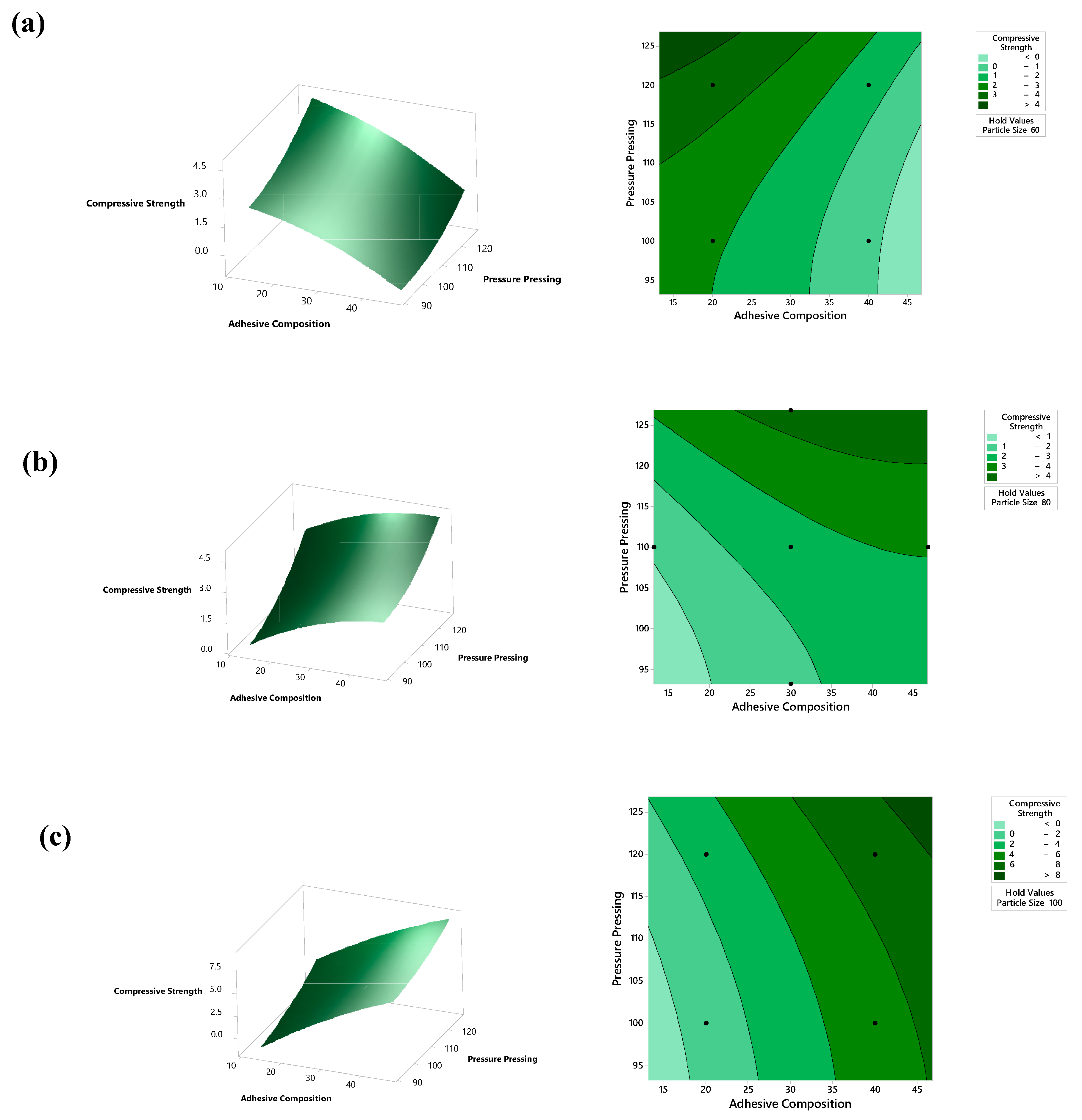
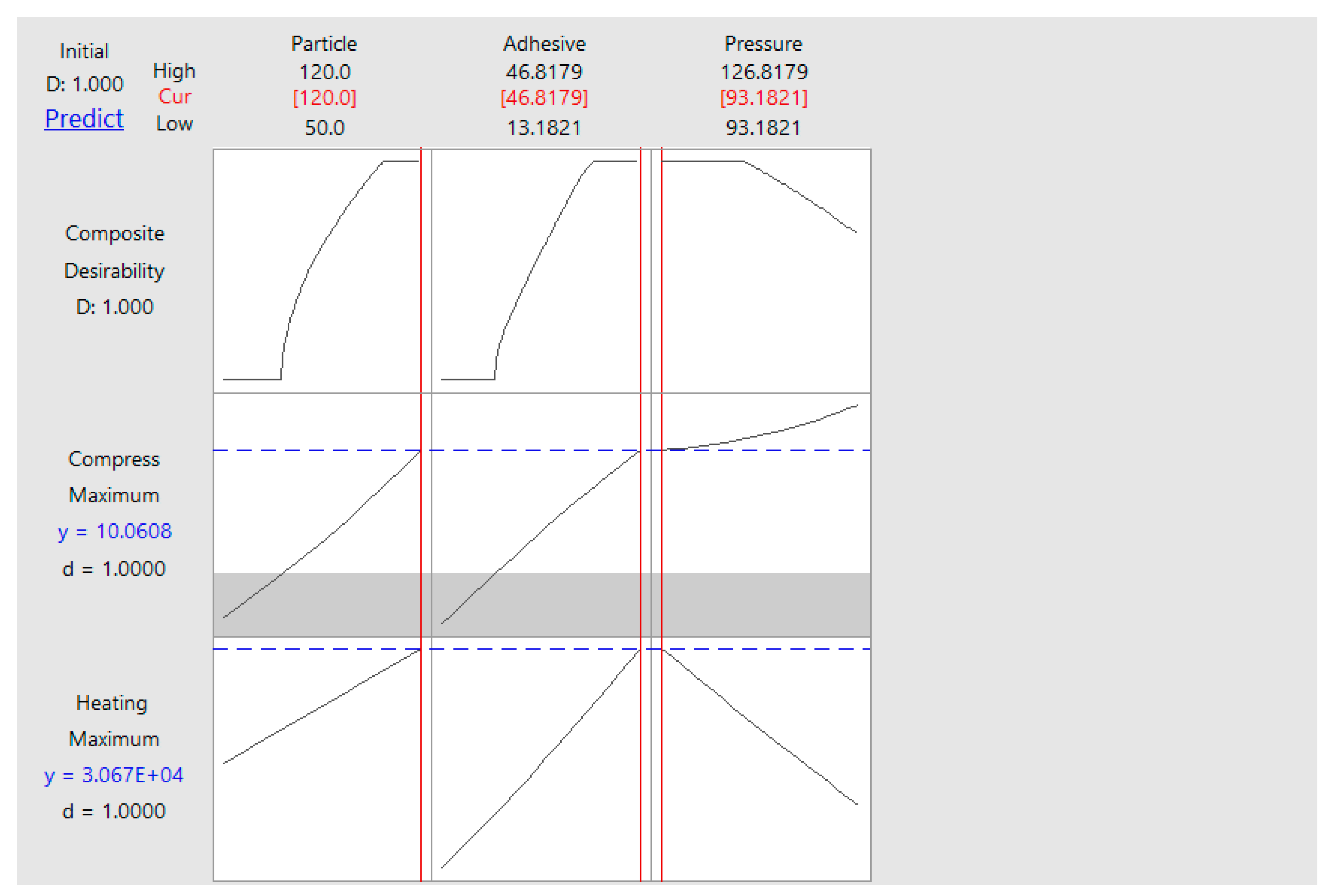
| Variable | Coding | Unit | Levels | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |||
| Particle size | X1 | mesh | 46.4 | 60 | 80 | 100 | 113.6 |
| Adhesive composition | X2 | %wt | 13.2 | 20 | 30 | 40 | 46.8 |
| Pressure pressing | X3 | Kg/cm2 | 93.2 | 100 | 110 | 120 | 126.8 |
| No. | Characteristic | Unit | Palm Stem | Palm Stem Charcoal | Palm Stem Briquettes |
|---|---|---|---|---|---|
| 1 | Heating Value | kJ/kg | 18,123.615 | 21,699.59 | 21,968.2–28,089.6 |
| 2 | Water Content | %-b | 9.10 | 5.03 | 5.5 |
| 3 | Volatile Matter Content | %-b | 76.9 | 22.19 | 19.73 |
| 4 | Ash Content | %-b | 2 | 0.74 | 0,.45 |
| 5 | Carbon bound Content | %-b | 12 | 69 | 71.4 |
| 6 | Compressive Strength | kg/cm2 | - | - | 0.86–7.526 |
| 7 | Density | gr/cm3 | - | - | 0.72–1.06 |
| Std | Run | Natural Variable | Coded Variable | Response | |||||
|---|---|---|---|---|---|---|---|---|---|
| ξ1 | ξ2 | ξ3 | X1 | X2 | X3 | Y1 | Y2 | ||
| 1 | 15 | 60 | 20 | 100 | −1 | −1 | −1 | 21,968.2 | 1.611 |
| 2 | 3 | 100 | 20 | 100 | 1 | −1 | −1 | 22,889.3 | 1.302 |
| 3 | 1 | 60 | 40 | 100 | −1 | 1 | −1 | 25,238.4 | 0.86 |
| 4 | 8 | 100 | 40 | 100 | 1 | 1 | −1 | 27,630.7 | 4.929 |
| 5 | 4 | 60 | 20 | 120 | −1 | −1 | 1 | 25,193.5 | 3.818 |
| 6 | 18 | 100 | 20 | 120 | 1 | −1 | 1 | 25,038.7 | 1.530 |
| 7 | 19 | 60 | 40 | 120 | −1 | 1 | 1 | 27,352.7 | 1.010 |
| 8 | 6 | 100 | 40 | 120 | 1 | 1 | 1 | 25,009.5 | 7.526 |
| 9 | 20 | 46.4 | 30 | 110 | −1.68 | 0 | 0 | 25,093.5 | 1.202 |
| 10 | 9 | 113.6 | 30 | 110 | 1.68 | 0 | 0 | 25,193.5 | 5.346 |
| 11 | 13 | 80 | 13.2 | 110 | 0 | −1.68 | 0 | 22,445.7 | 1.756 |
| 12 | 11 | 80 | 46.8 | 110 | 0 | 1.68 | 0 | 28,089.6 | 2.377 |
| 13 | 7 | 80 | 30 | 93.2 | 0 | 0 | −1.68 | 22,934.1 | 1.205 |
| 14 | 17 | 80 | 30 | 126.8 | 0 | 0 | 1.68 | 27,352.7 | 4.871 |
| 15 | 10 | 80 | 30 | 110 | 0 | 0 | 0 | 24,245.6 | 2.149 |
| 16 | 5 | 80 | 30 | 110 | 0 | 0 | 0 | 25,326.4 | 2.319 |
| 17 | 16 | 80 | 30 | 110 | 0 | 0 | 0 | 24,834.9 | 2.916 |
| 18 | 12 | 80 | 30 | 110 | 0 | 0 | 0 | 24,794.9 | 2.856 |
| 19 | 2 | 80 | 30 | 110 | 0 | 0 | 0 | 25,326.4 | 3.230 |
| 20 | 14 | 80 | 30 | 110 | 0 | 0 | 0 | 25,148.7 | 2.040 |
| Response | Source of Variance | DF | SS | MS | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Heating Value | Regression | 9 | 47,878,095 | 5,319,788 | 10.60 | 0.000 ** |
| Error | 10 | 5,016,949 | 501,695 | |||
| Lack of Fit | 5 | 4,160,719 | 832,144 | 4.86 | 0.054 | |
| Pure Error | 5 | 856,230 | 171,246 | |||
| Total | 19 | 52,895,044 | ||||
| Compressive Strength | Regression | 9 | 49.5564 | 5.5063 | 7.87 | 0.002 ** |
| Error | 10 | 6.9958 | 0.6996 | |||
| Lack of Fit | 5 | 5.8389 | 1.1678 | 5.05 | 0.050 | |
| Pure Error | 5 | 1.1569 | 0.2314 | |||
| Total | 19 | 56.5522 |
| Source | p-Value of Heating Value | p-Value of Compressive Strength |
|---|---|---|
| Constant | 0.000 * | 0.000 * |
| A—Particle Size | 0.671 | 0.002 * |
| B—adhesive composition | 0.000 * | 0.031 * |
| C—Pressure Pressing | 0.001 * | 0.006 * |
| A2 | 0.932 | 0.354 |
| B2 | 0.693 | 0.547 |
| C2 | 0.868 | 0.373 |
| AB | 0.801 | 0.000 * |
| AC | 0.013 * | 0.641 |
| BC | 0.018 * | 0.883 |
| R2 | 0.905 | 0.876 |
| MAPE | 1.681 | 27.575 |
| RMSE | 708.304 | 0.836 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helwani, Z.; Ramli, M.; Rusyana, A.; Marlina, M.; Fatra, W.; Idroes, G.M.; Suhendra, R.; Ashwie, V.; Mahlia, T.M.I.; Idroes, R. Alternative Briquette Material Made from Palm Stem Biomass Mediated by Glycerol Crude of Biodiesel Byproducts as a Natural Adhesive. Processes 2020, 8, 777. https://doi.org/10.3390/pr8070777
Helwani Z, Ramli M, Rusyana A, Marlina M, Fatra W, Idroes GM, Suhendra R, Ashwie V, Mahlia TMI, Idroes R. Alternative Briquette Material Made from Palm Stem Biomass Mediated by Glycerol Crude of Biodiesel Byproducts as a Natural Adhesive. Processes. 2020; 8(7):777. https://doi.org/10.3390/pr8070777
Chicago/Turabian StyleHelwani, Zuchra, Muliadi Ramli, Asep Rusyana, Marlina Marlina, Warman Fatra, Ghazi Mauer Idroes, Rivansyah Suhendra, Viqha Ashwie, Teuku Meurah Indra Mahlia, and Rinaldi Idroes. 2020. "Alternative Briquette Material Made from Palm Stem Biomass Mediated by Glycerol Crude of Biodiesel Byproducts as a Natural Adhesive" Processes 8, no. 7: 777. https://doi.org/10.3390/pr8070777










