Application of a Modeling Tool to Describe Fly Ash Generation, Composition, and Melting Behavior in a Wheat Straw Fired Commercial Power Plant
Abstract
1. Introduction
2. Materials and Methods
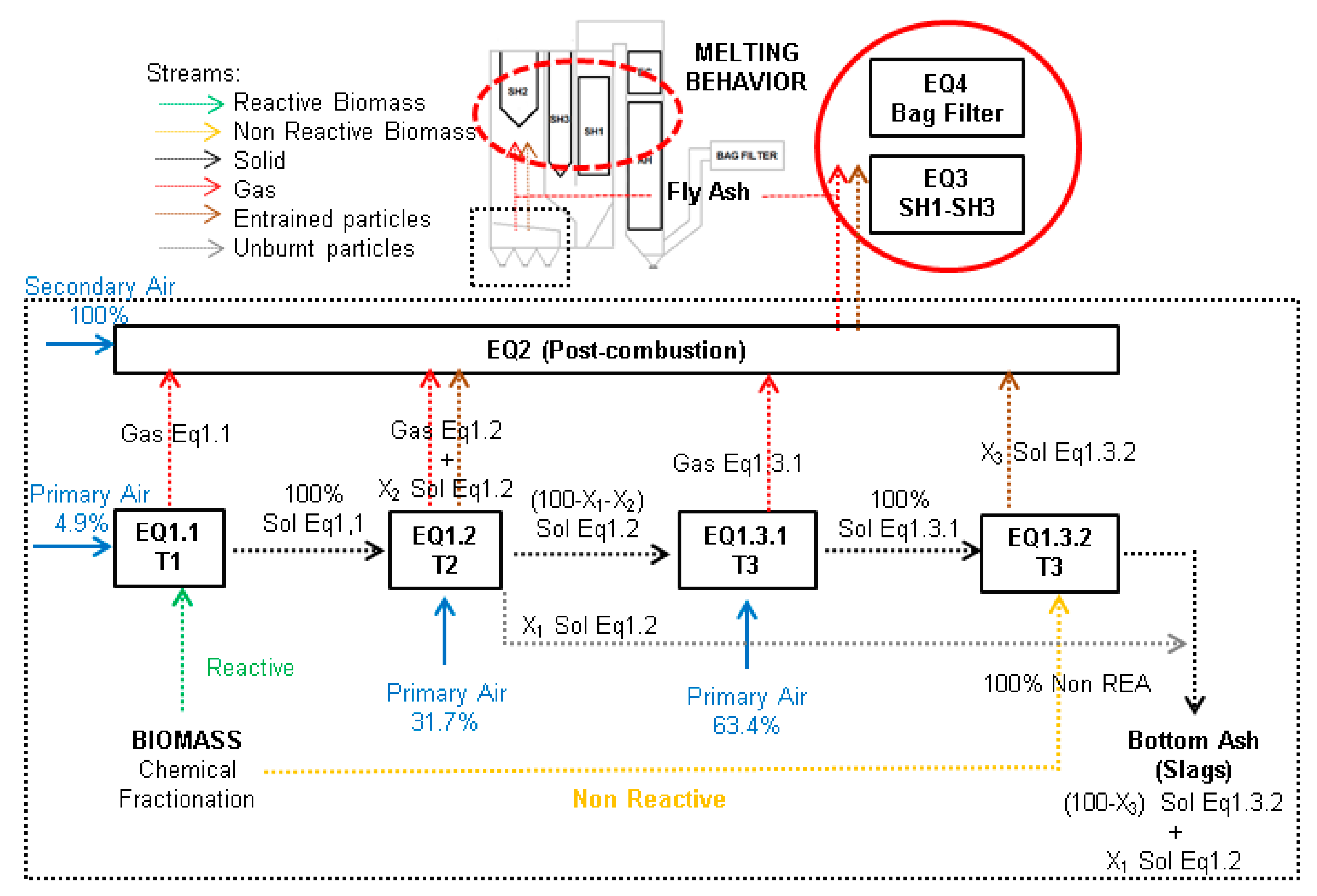
2.1. Plant Characteristics and Sampling Procedures
- Post combustion section (PC, see Figure 1): 900–1300 °C
- SH2: 700–1150 °C
- SH3: 700–900 °C
2.2. Samples Characterization
3. Thermochemical Modeling and Simulation
- CF100: AF and NF fractions are fully reactive.
- CF102: similar to CF100, but including a 2% of reactivity from the HF fraction and solid residue.
- CF110: same as CF102, but increasing up to 10% the reactivity of the partially reactive fractions.
- N°CF: no chemical fractionation considered; the biomass bulk composition is considered as input data.
- In the way that chemical fractionation is applied.
- In the way that the temperatures of each sub-stage on the grate are calculated.
- In the way that the entrained streams are applied based on data from the elemental analyses of the fly ashes.
- The boiler design temperature values have been assumed when solving the post-combustion stage (EQ2), and in the SH2 and SH3 heat exchangers of EQ3.
- Temperatures in the rest of the stages have been established according to process data measured on the site for SH1, as well as in the economizer/air pre-heater (EC-AH) and in the bag filter (EQ4).
- A stream of unburnt species (X1) has been systematically applied between EQ1.2 and EQ1.3.1 (slags flow rate calculation) stages for the entrained streams from pyrolysis and char combustion based on the results of the TGA analyses of the slags samples performed under air atmosphere. Calculations were done using the normalized percentage of mass loss, i.e., without accounting for the mass losses below 300–350 °C. The contribution of each fraction was calculated as a function of temperature. Slags mass losses above 600–700 °C and up to 900–1000 °C were assumed to be due to matter that had not reached the maximum grate temperature. This stream is removed from the solids stream when shifting from EQ1.2 to EQ1.3.1 (X1), being led to the final slags stream. The streams of entrained solids from EQ1.2 and EQ1.3 (X2 and X3, respectively) were established by means of the composition of the fly ash determined from the analyses in a specific way for each complete model tested.
3.1. Global Entrainment Model (GE)
3.2. Individual Entrainment Model (IE)
3.3. Temperature Effect Model (TE)
4. Results and Discussion
4.1. Mass Balances
4.2. Fly Ashes Flow Rates
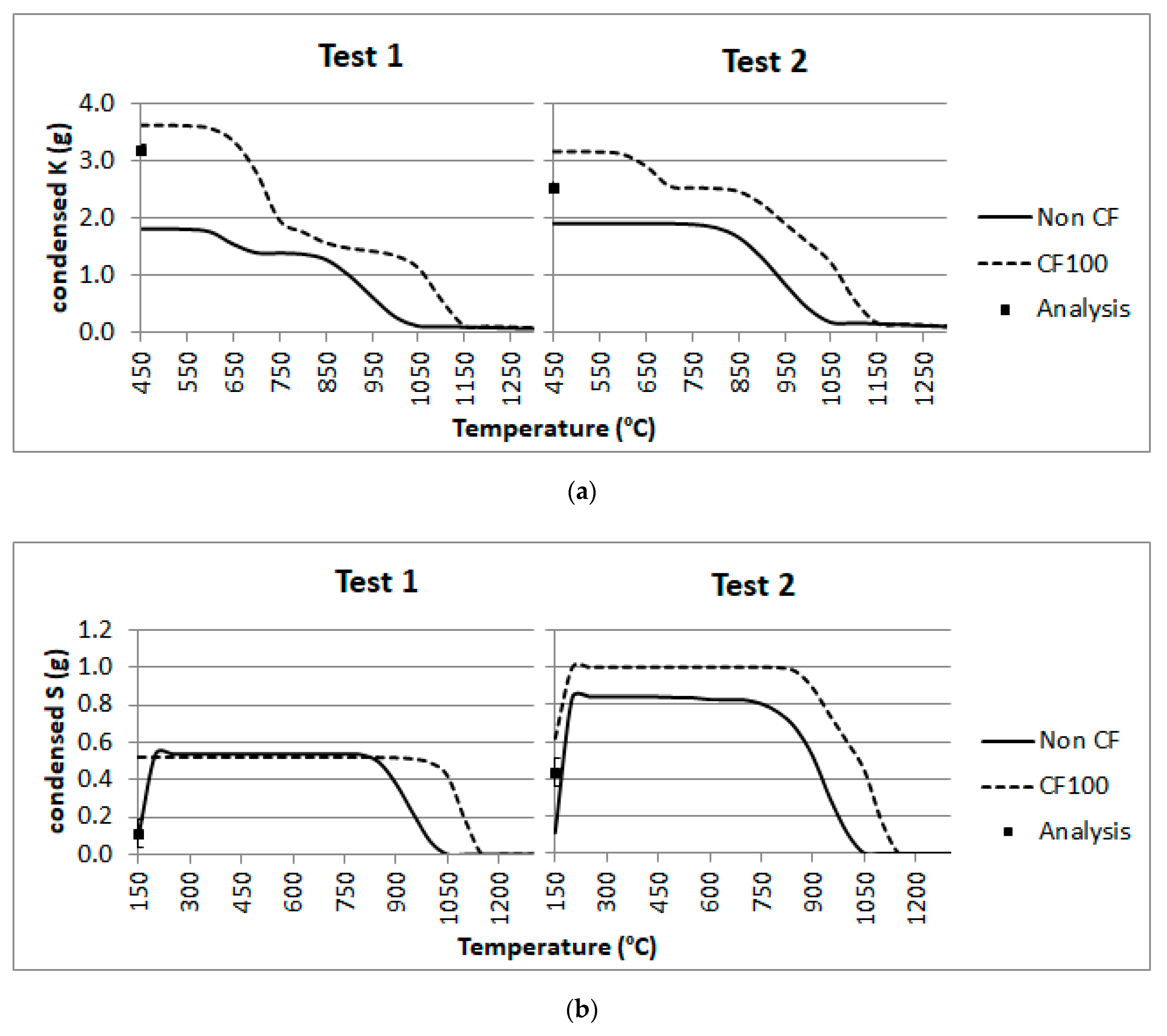
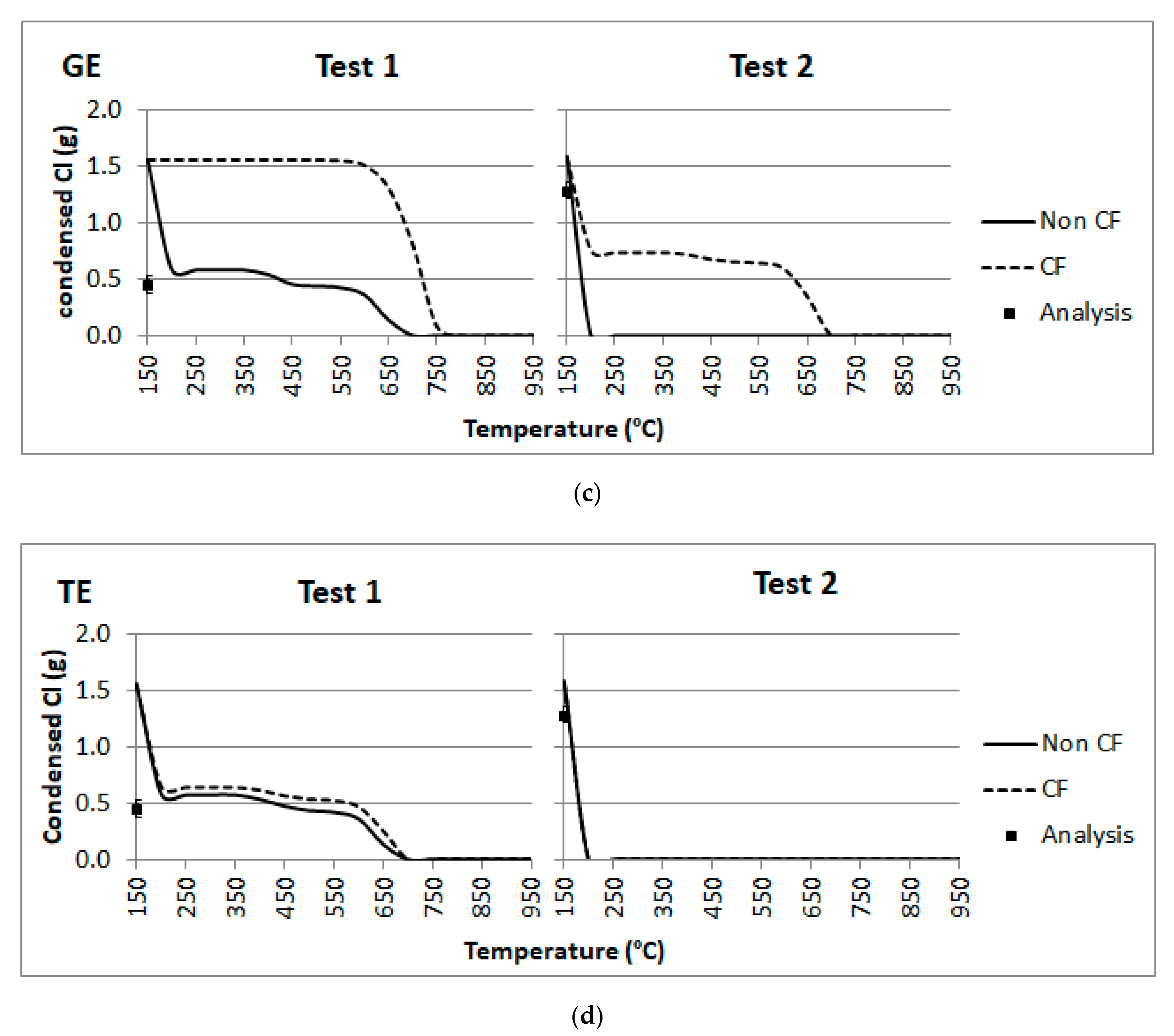
4.3. Fly Ashes Composition: K, S and Cl Behavior
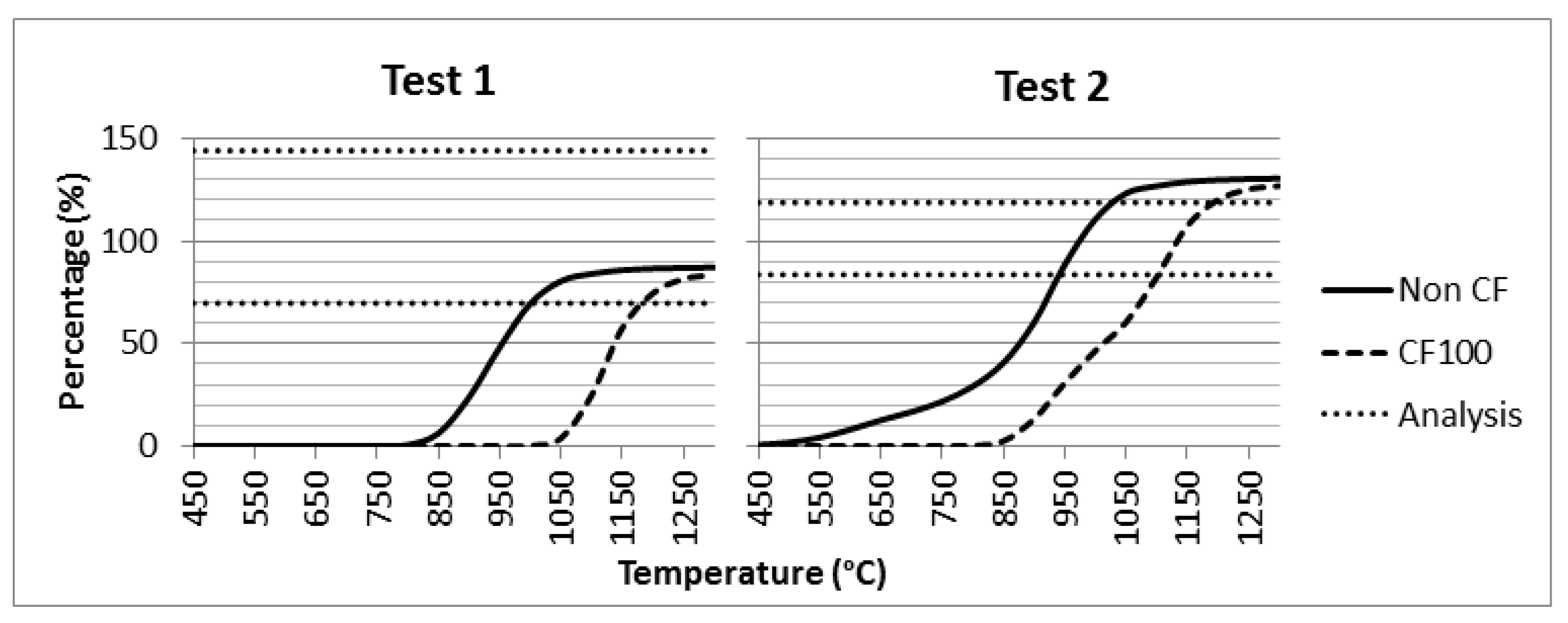
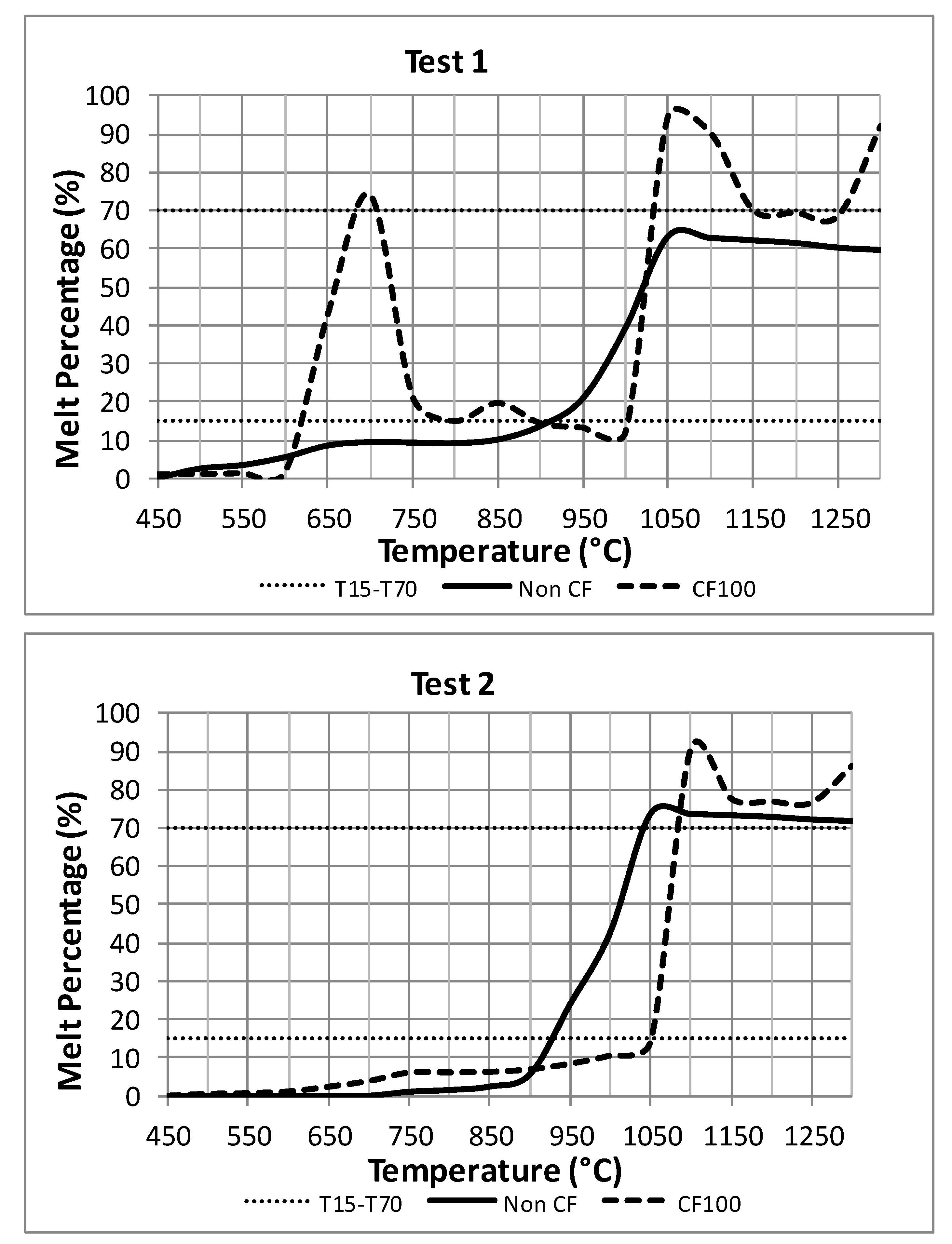
4.4. Ash Melting Curves
5. Conclusions
- The calculations for equilibrium compositions in the simulations are somewhat unrealistic as concerns S and Cl contents, which in turn affect the predicted species formed, and in particular, the distribution of those containing K.
- The differences found in the various simulations carried out to establish fly ashes flow rate reveal that simulations are highly affected by the type of chemical fractionation considered, except for the Temperature Effect (TE) model, in which the grate temperatures considered are so low that they impede the volatilization of elements that would further generate condensates downstream. The Global Entrainment (GE) and Individual Entrainment (IE) models provide very similar results, showing, in general, good predictions for the annual average fly ash flow rates (between 92–98% and 112–125% depending on the chemical fractionation considered). In contrast, the TE model predicts a much lower value in comparison to the actual values obtained from the onsite measurements (around 65% below).
- Regarding fly ashes composition and formation of smelted phases, the best models for the gas cooling stages are both GE and IE when considering chemical fractionation cases CF100 and CF102. Overall, the compositions obtained in the equilibria calculations for the major elements in ash show good agreement with the analytical values obtained in the analyses of fly ash samples, except for K and Na. Below 950–1000 °C, all the models predict values that match closely the analytical ones. In the case of Si, the IE model allows for slightly better predictions owing to the entrained streams applied in that model.
- Calculations carried out for the composition of fly ash in terms of K, S and Cl revealed a match with the gas cooling effects in the superheater sections SH2 (1050–1100 °C), SH3 (850 °C) and SH1 (600 °C). In addition, there seems to be an entrainment effect: S content in fly ash would coincide with the condensate in SH2, whilst Cl would match that of SH3-SH1.
- As concerns the Ash Melting Curves (AMCs), the thermodynamic simulation of the chemical equilibrium at the characteristic temperatures found in different sections of the boiler allowed for the calculation of T15 and the corresponding condensed fractions. It could be established that the transition from T15 to T70 above 1050 °C is fast. This would cause smelted species to downflow on the wall after the post-combustion stage without causing fouling. However, at the outlet gas temperature in the SH3 heat exchanger (700 °C), the values of smelted solid percentage may fall within the T15 and T70 values, thus indicating a high risk of particles adhesion. This fact, combined with the presence of KCl and K2SO4 condensates, would point out at possible corrosion phenomena in the SH3 heat exchange at temperatures around 700–750 °C.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IRENA. Renewable Energy Prospects for the European Union; Remap 2030. 2018. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Feb/IRENA_REmap_EU_2018.pdf (accessed on 15 September 2020).
- Yin, C.; Li, S. Advancing Grate-Firing for Greater Environmental Impacts and Efficiency for Decentralized Biomass/Wastes Combustion. Energy Procedia 2017, 120, 373–379. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.A.; Kær, S.K. Grate-Firing of Biomass for Heat and Power Production. Prog. Energy Combust. Sci. 2008, 34, 725–754. [Google Scholar] [CrossRef]
- Dodson, J.R.; Hunt, A.J.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. The Chemical Value of Wheat Straw Combustion Residues. RSC Adv. 2011, 1, 523–530. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and Disadvantages of Composition and Properties of Biomass in Comparison with Coal: An Overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Biomass Combustion Systems: A Review on the Physical and Chemical Properties of the Ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical Review of Predictive Coefficients for Biomass Ash Deposition Tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Khodaei, H.; Al-Abdeli, Y.M.; Guzzomi, F.; Yeoh, G.H. An Overview of Processes and Considerations in the Modelling of Fixed-Bed Biomass Combustion. Energy 2015, 88, 946–972. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An Overview of the Behaviour of Biomass during Combustion: Part I. Phase-Mineral Transformations of Organic and Inorganic Matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- Forstner, M.; Hofmeister, G.; Joller, M.; Dahl, J.; Braun, M.; Kleditzsch, S.; Scharler, R.; Obernberger, I. CFD Simulation of Ash Deposit Formation in Fixed Bed Biomass Furnaces and Boilers. Prog. Comput. Fluid Dyn. Int. J. 2006, 6, 248–261. [Google Scholar] [CrossRef]
- Frandsen, F.J.; van Lith, S.C.; Korbee, R.; Yrjas, P.; Backman, R.; Obernberger, I.; Brunner, T.; Jöller, M. Quantification of the Release of Inorganic Elements from Biofuels. Fuel Process. Technol. 2007, 88, 1118–1128. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.; Kær, S.K.; Clausen, S.; Hvid, S.L.; Hille, T. Mathematical Modeling and Experimental Study of Biomass Combustion in a Thermal 108 MW Grate-Fired Boiler. Energy Fuels 2008, 22, 1380–1390. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.; Clausen, S.; Hvid, S.L. Characterizing and Modeling of an 88 MW Grate-Fired Boiler Burning Wheat Straw: Experience and Lessons. Energy 2012, 41, 473–482. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, X.; Liao, Y. Mathematical Modeling of Combustion in a Grate-Fired Boiler Burning Straw and Effect of Operating Conditions Under Air- and Oxygen-Enriched Atmospheres. Renew. Energy 2010, 35, 895–903. [Google Scholar] [CrossRef]
- Kaer, S.; Rosendahl, L.; Baxter, L. Towards a CFD-Based Mechanistic Deposit Formation Model for Straw-Fired Boilers. Fuel 2006, 85, 833–848. [Google Scholar] [CrossRef]
- Garba, M.U.; Ingham, D.B.; Ma, L.; Porter, R.T.J.; Pourkashnian, M.; Tan, H.Z.; Williams, A. Prediction of Potassium Chloride Sulfation and Its Effect on Deposition in Biomass-Fired Boilers. Energy Fuels 2012, 26, 6501–6508. [Google Scholar] [CrossRef]
- Zhou, H.; Jensen, P.A.; Frandsen, F.J. Dynamic Mechanistic Model of Superheater Deposit Growth and Shedding in a Biomass Fired Grate Boiler. Fuel 2007, 86, 1519–1533. [Google Scholar] [CrossRef]
- Zbogar, A.; Frandsen, F.J.; Jensen, P.A.; Glarborg, P. Heat Transfer in Ash Deposits: A Modelling Tool-Box. Prog. Energy Combust. Sci. 2005, 31, 371–421. [Google Scholar] [CrossRef]
- Zbogar, A.; Frandsen, F.; Jensen, P.A.; Glarborg, P. Shedding of Ash Deposits. Prog. Energy Combust. Sci. 2009, 35, 31–56. [Google Scholar] [CrossRef]
- Hansen, S.B.; Jensen, P.A.; Frandsen, F.J.; Wu, H.; Bashir, M.S.; Wadenbäck, J.; Sander, B.; Glarborg, P. Deposit Probe Measurements in Large Biomass-Fired Grate Boilers and Pulverized-Fuel Boilers. Energy Fuels 2014, 28, 3539–3555. [Google Scholar] [CrossRef]
- Bashir, M.S.; Jensen, P.A.; Frandsen, F.; Wedel, S.; Dam-Johansen, K.; Wadenbäck, J.; Pedersen, S.T. Ash Transformation and Deposit Build-Up during Biomass Suspension and Grate Firing: Full-Scale Experimental Studies. Fuel Process. Technol. 2012, 97, 93–106. [Google Scholar] [CrossRef]
- Žbogar, A.; Jensen, P.A.; Frandsen, F.J.; Hansen, J.; Glarborg, P. Experimental Investigation of Ash Deposit Shedding in a Straw-Fired Boiler. Energy Fuels 2006, 20, 512–519. [Google Scholar] [CrossRef]
- Lindberg, D.; Backman, R.; Chartrand, P.; Hupa, M. Towards a Comprehensive Thermodynamic Database for Ash-Forming Elements in Biomass and Waste Combustion—Current Situation and Future Developments. Fuel Process. Technol. 2013, 105, 129–141. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An Overview of the Behaviour of Biomass during Combustion: Part II. Ash Fusion and Ash Formation Mechanisms of Biomass Types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Hansen, L.A.; Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Hørlyck, S.; Karlsson, A. Influence of Deposit Formation on Corrosion at a Straw-Fired Boiler. Fuel Process. Technol. 2000, 64, 189–209. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N. Use of Chemical Fractionation to Understand Partitioning of Biomass Ash Constituents during Co-Firing in Fluidized Bed Combustion. Fuel 2012, 101, 215–227. [Google Scholar] [CrossRef]
- Skrifvars, B.-J.; Hupa, M.; Moilanen, A.; Lundqvist, R. Applications of Advanced Technology to Ash-Related Problems in Boilers; Baxter, L., DeSollar, R., Eds.; Springer US: Boston, MA, USA, 1996; pp. 383–398. ISBN 978-1-4757-9223-2. [Google Scholar]
- Hansen, L.A.; Frandsen, F.J.; Dam-Johansen, K. Quantification of Fusion in Ashes from Solid Fuel Combustion. Thermochim. Acta 1999, 326, 105–117. [Google Scholar] [CrossRef]
- Vainikka, P.; Lindberg, D.; Moilanen, A.; Ollila, H.; Tiainen, M.; Silvennoinen, J.; Hupa, M. Trace Elements Found in the Fuel and in-Furnace Fine Particles Collected from 80MW BFB Combusting Solid Recovered Fuel. Fuel Process. Technol. 2013, 105, 202–211. [Google Scholar] [CrossRef]
- Jung, C.H.; Matsuto, T.; Tanaka, N.; Okada, T. Metal Distribution in Incineration Residues of Municipal Solid Waste (MSW) in Japan. Waste Manag. 2004, 24, 381–391. [Google Scholar] [CrossRef]
- Coda, B.; Jacobs, J.M.; Kiel, J.H.A. Impact of Biomass Co-Firing on Ash Formation and Ash Behaviour in IGCC Plants. ECN Rep. 2005. Available online: https://www.researchgate.net/profile/J_Kiel/publication/265008208_IMPACT_OF_BIOMASS_CO-FIRING_ON_ASH_FORMATION_AND_ASH_BEHAVIOUR_IN_IGCC_PLANTS/links/5493307d0cf22d7925da1df9.pdf (accessed on 15 September 2020).
- Jenkins, B.M.; Miles, T.R.J.; Miles, T.R.; Baxter, L.L. Combustion Properties of Biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N. Uncertainty Estimation to Evaluate Mass Balances on a Combustion System. Accredit. Qual. Assur. 2012, 17, 159–166. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. Implications of Chlorine-Associated Corrosion on the Operation of Biomass-Fired Boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]
- Iisa, K.; Lu, Y.; Salmenoja, K. Sulfation of Potassium Chloride at Combustion Conditions. Energy Fuels 1999, 13, 1184–1190. [Google Scholar] [CrossRef]
- Henriksson, M.; Warnqvist, B. Kinetics of Formation of HCI(g) by the Reaction between NaCl(s) and SO2, O2, and H2O(g). Ind. Eng. Chem. Process Des. Dev. 1979, 18, 249–254. [Google Scholar] [CrossRef]
- Blomberg, T. A Thermodynamic Study of the Gaseous Potassium Chemistry in the Convection Sections of Biomass Fired Boilers. Mater. Corros. 2011, 62, 635–641. [Google Scholar] [CrossRef]
- Sengeløv, L.W.; Hansen, T.B.; Bartolomé, C.; Wu, H.; Pedersen, K.H.; Frandsen, F.J.; Jensen, A.D.; Glarborg, P. Sulfation of Condensed Potassium Chloride by SO2. Energy Fuels 2013, 27, 3283–3289. [Google Scholar] [CrossRef]
- Li, B.; Sun, Z.; Li, Z.; Aldén, M.; Jakobsen, J.G.; Hansen, S.; Glarborg, P. Post-Flame Gas-Phase Sulfation of Potassium Chloride. Combust. Flame 2013, 160, 959–969. [Google Scholar] [CrossRef]
- Jiménez, S.; Ballester, J. Formation of Alkali Sulphate Aerosols in Biomass Combustion. Fuel 2007, 86, 486–493. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Baxter, L.L.; Sclippab, G.; Morey, C.; Frandsen, F.J.; Dam-Johansen, K. Deposition of Potassium Salts on Heat Transfer Surfaces in Straw-Fired Boilers: A Pilot-Scale Study. Fuel 2000, 79, 131–139. [Google Scholar] [CrossRef]
- Christensen, K.A.; Stenholm, M.; Livbjerg, H. The Formation of Submicron Aerosol Particles, HCl and SO2 in Straw-Fired Boilers. J. Aerosol Sci. 1998, 29, 421–444. [Google Scholar] [CrossRef]
- Christensen, K.A.; Livbjerg, H. A Field Study of Submicron Particles from the Combustion of Straw. Aerosol Sci. Technol. 1996, 25, 185–199. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Tan, H.; Ma, L.; Xu, T. Mechanism Research on the Development of Ash Deposits on the Heating Surface of Biomass Furnaces. Ind. Eng. Chem. Res. 2012, 51, 12984–12992. [Google Scholar] [CrossRef]
- Hindiyarti, L.; Livbjerg, H.; Glarborg, P.; Marshall, P.; Frandsen, F. An Exploratory Study of Alkali Sulfate Aerosol Formation during Biomass Combustion. Fuel 2008, 87, 1591–1600. [Google Scholar] [CrossRef]
- Glarborg, P.; Marshall, P. Mechanism and Modeling of the Formation of Gaseous Alkali Sulfates. Combust. Flame 2005, 141, 22–39. [Google Scholar] [CrossRef]
- Baxter, L.L.; Miles, T.R.; Miles, T.R., Jr.; Jenkins, B.M.; Dayton, D.C.; Milne, T.A.; Bryers, R.W.; Oden, L.L. Alkali Deposits Found in Biomass Boilers. The Behavior of Inorganic Material in Biomass—Fired Power Boilers—Field and Laboratory Experiences; NREL: Golden, CO, USA, 1996; Volume I. [Google Scholar]
- Jöller, M.; Brunner, T.; Obernberger, I. Modeling of Aerosol Formation during Biomass Combustion for Various Furnace and Boiler Types. Fuel Process. Technol. 2007, 88, 1136–1147. [Google Scholar] [CrossRef]
- Glazer, M.P.; Khan, N.A.; de Jong, W.; Spliethoff, H.; Schürmann, H.; Monkhouse, P. Alkali Metals in Circulating Fluidized Bed Combustion of Biomass and Coal: Measurements and Chemical Equilibrium Analysis. Energy Fuels 2005, 19, 1889–1897. [Google Scholar] [CrossRef]
- Evic, N.; Brunner, T.; Obernberger, I. Prediction of Biomass Ash Melting Behaviour—Correlation Between the Data Obtained from Thermodynamic Equilibrium Calculations and Simultaneous Thermal Analysis (STA). In Proceedings of the 20th European Biomass Conference and Exibition, Milan, Italy, 18–22 June 2012; pp. 807–813. [Google Scholar]
- Sommersacher, P.; Brunner, T.; Obernberger, I.; Kienzl, N.; Kanzian, W. Application of Novel and Advanced Fuel Characterization Tools for the Combustion Related Characterization of Different Wood/Kaolin and Straw/Kaolin Mixtures. Energy Fuels 2013, 27, 5192–5206. [Google Scholar] [CrossRef]




| Test | Value | Biomass | Moisture | Primary Air | Secondary Air |
|---|---|---|---|---|---|
| 1 | Average | 13,073 | 13.9 | 32,289 | 54,280 |
| Maximum | 12,888 | 15.9 | 33,394 | 56,054 | |
| Minimum | 13,284 | 11.6 | 30,699 | 51,862 | |
| RSD (%) 1 | 1.4 | 13.5 | 3.3 | 3.4 | |
| 2 | Average | 13,372 | 9.3 | 32,762 | 50,622 |
| Maximum | 13,536 | 9.8 | 34,036 | 51,306 | |
| Minimum | 13,182 | 8.6 | 31,720 | 49,838 | |
| RSD (%) 1 | 1.3 | 6.4 | 3.6 | 1.5 |
| Chemical Fractionation | GE Model | IE Model | TE Model | |
|---|---|---|---|---|
| Cl | No CF | 600 | 600 | 600 |
| CF 110 | 650 | 650 | 600 | |
| CF 102 | 700 | 700 | 600 | |
| CF 100 | 700 | 700 | 600 | |
| S | No CF | 900–1000 | 900–1000 | 950–1000 |
| CF 110 | 950–1050 | 900–1050 | 950–1000 | |
| CF 102 | 1000–1100 | 1000–1100 | 950–1000 | |
| CF 100 | 1050–1050 | 1050–1050 | 950–1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funcia, I.; Bimbela, F.; Gil, J.; Gandía, L.M. Application of a Modeling Tool to Describe Fly Ash Generation, Composition, and Melting Behavior in a Wheat Straw Fired Commercial Power Plant. Processes 2020, 8, 1510. https://doi.org/10.3390/pr8111510
Funcia I, Bimbela F, Gil J, Gandía LM. Application of a Modeling Tool to Describe Fly Ash Generation, Composition, and Melting Behavior in a Wheat Straw Fired Commercial Power Plant. Processes. 2020; 8(11):1510. https://doi.org/10.3390/pr8111510
Chicago/Turabian StyleFuncia, Ibai, Fernando Bimbela, Javier Gil, and Luis M. Gandía. 2020. "Application of a Modeling Tool to Describe Fly Ash Generation, Composition, and Melting Behavior in a Wheat Straw Fired Commercial Power Plant" Processes 8, no. 11: 1510. https://doi.org/10.3390/pr8111510
APA StyleFuncia, I., Bimbela, F., Gil, J., & Gandía, L. M. (2020). Application of a Modeling Tool to Describe Fly Ash Generation, Composition, and Melting Behavior in a Wheat Straw Fired Commercial Power Plant. Processes, 8(11), 1510. https://doi.org/10.3390/pr8111510







